Abstract
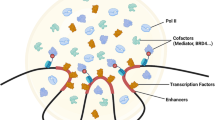
Biomolecular condensates regulate transcription by dynamically compartmentalizing the transcription machinery. Classic models of transcription regulation focus on the recruitment and regulation of RNA polymerase II by the formation of complexes at the 1–10 nm length scale, which are driven by structured and stoichiometric interactions. These complexes are further organized into condensates at the 100–1,000 nm length scale, which are driven by dynamic multivalent interactions often involving domain–ligand pairs or intrinsically disordered regions. Regulation through condensate-mediated organization does not supersede the processes occurring at the 1–10 nm scale, but it provides regulatory mechanisms for promoting or preventing these processes in the crowded nuclear environment. Regulation of transcription by transcriptional condensates is involved in cell state transitions during animal and plant development, cell signalling and cellular responses to the environment. These condensate-mediated processes are dysregulated in developmental disorders, cancer and neurodegeneration. In this Review, we discuss the principles underlying the regulation of transcriptional condensates, their roles in physiology and their dysregulation in human diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levine, M. & Tjian, R. Transcription regulation and animal diversity. Nature 424, 147–151 (2003).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251 (2013).
Roeder, R. G. 50+ years of eukaryotic transcription: an expanding universe of factors and mechanisms. Nat. Struct. Mol. Biol. 26, 783–791 (2019).
Cramer, P. Organization and regulation of gene transcription. Nature 573, 45–54 (2019).
Richter, W. F., Nayak, S., Iwasa, J. & Taatjes, D. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 23, 732–749 (2022).
Wang, H., Schilbach, S., Ninov, M., Urlaub, H. & Cramer, P. Structures of transcription preinitiation complex engaged with the +1 nucleosome. Nat. Struct. Mol. Biol. 30, 226–232 (2023).
Sabari, B. R., Dall’Agnese, A. & Young, R. A. Biomolecular condensates in the nucleus. Trends Biochem. Sci. 45, 961–977 (2020).
Bhat, P., Honson, D. & Guttman, M. Nuclear compartmentalization as a mechanism of quantitative control of gene expression. Nat. Rev. Mol. Cell Biol. 22, 653–670 (2021).
Palacio, M. & Taatjes, D. J. Merging established mechanisms with new insights: condensates, hubs, and the regulation of RNA polymerase II transcription. J. Mol. Biol. 432, 167216 (2021).
Demmerle, J., Hao, S. & Cai, D. Transcriptional condensates and phase separation: condensing information across scales and mechanisms. Nucleus 14, 2213551 (2023).
Sabari, B. R. Biomolecular condensates and gene activation in development and disease. Dev. Cell 55, 84–96 (2020).
Tsai, A., Alves, M. R. P. & Crocker, J. Multi-enhancer transcriptional hubs confer phenotypic robustness. eLife 8, e45325 (2019).
Mir, M. et al. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife 7, e40497 (2018).
Cho, W.-K. et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361, 412–415 (2018).
Boehning, M. et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat. Struct. Mol. Biol. 25, 833–840 (2018).
Lu, H. et al. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 558, 318–323 (2018).
Mir, M. et al. Dense Bicoid hubs accentuate binding along the morphogen gradient. Genes Dev. 31, 1784–1794 (2017).
Tsai, A. et al. Nuclear microenvironments modulate transcription from low-affinity enhancers. eLife 6, e28975 (2017).
Woringer, M. & Darzacq, X. Protein motion in the nucleus: from anomalous diffusion to weak interactions. Biochem. Soc. Trans. 46, 945–956 (2018).
Nguyen, V. Q. et al. Spatiotemporal coordination of transcription preinitiation complex assembly in live cells. Mol. Cell 81, 3560–3575.e6 (2021).
Thomson, J. A., Schurtenberger, P., Thurston, G. M. & Benedek, G. B. Binary liquid phase separation and critical phenomena in a protein/water solution. Proc. Natl Acad. Sci. USA 84, 7079–7083 (1987).
Broide, M. L., Berland, C. R., Pande, J., Ogun, O. O. & Benedek, G. B. Binary-liquid phase separation of lens protein solutions. Proc. Natl Acad. Sci. USA 88, 5660–5664 (1991).
Norledge, B. V., Hay, R. E., Bateman, O. A., Slingsby, C. & Driessen, H. P. C. Towards a molecular understanding of phase separation in the lens: a comparison of the X-ray structures of two highTcγ-crystallins, γE and γF, with two lowTcγ-crystallins, γB and γD. Exp. Eye Res. 65, 609–630 (1997).
Leong, K. W. et al. DNA-polycation nanospheres as non-viral gene delivery vehicles. J. Control. Release 53, 183–193 (1998).
Albertsson, P.-Å. Partition of cell particles and macromolecules in polymer two-phase systems. Adv. Protein Chem. 24, 309–341 (1970).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Mittag, T. & Pappu, R. V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 82, 2201–2214 (2022).
Pappu, R. V., Cohen, S. R., Dar, F., Farag, M. & Kar, M. Phase transitions of associative biomacromolecules. Chem. Rev. 123, 8945–8987 (2023).
Elbaum-Garfinkle, S. et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl Acad. Sci. USA 112, 7189–7194 (2015).
Lin, Y., David, M. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Martin, E. W. et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367, 694–699 (2020).
Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947 (2015).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16 (2018).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
Jain, A. & Vale, R. D. RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017).
Shimobayashi, S. F., Ronceray, P., Sanders, D. W., Haataja, M. P. & Brangwynne, C. P. Nucleation landscape of biomolecular condensates. Nature 599, 503–506 (2021).
Bracha, D. et al. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 175, 1467–1480.e13 (2018).
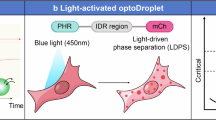
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optodroplets. Cell 168, 159–171.e14 (2017).
Strom, A. R. & Brangwynne, C. P. The liquid nucleome — phase transitions in the nucleus at a glance. J. Cell Sci. 132, jcs235093 (2019).
Riback, J. A. et al. Composition-dependent thermodynamics of intracellular phase separation. Nature 357, eaaf4382–eaaf4386 (2020).
Lyon, A. S., Peeples, W. B. & Rosen, M. K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 18, 215–235 (2020).
Schede, H. H., Natarajan, P., Chakraborty, A. K. & Shrinivas, K. A model for organization and regulation of nuclear condensates by gene activity. Nat. Commun. 14, 4152 (2023).
Banani, S. F. et al. Compositional control of phase-separated cellular bodies. Cell 166, 651–663 (2016).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Gao, Y. et al. Multivalent m6A motifs promote phase separation of YTHDF proteins. Cell Res. 29, 767–769 (2019).
Sun, D., Wu, R., Zheng, J., Li, P. & Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415 (2018).
Shrinivas, K. et al. Enhancer features that drive formation of transcriptional condensates. Mol. Cell 75, 549–561.e7 (2019).
Morin, J. A. et al. Sequence-dependent surface condensation of a pioneer transcription factor on DNA. Nat. Phys. 18, 271–276 (2022).
Banjade, S. & Rosen, M. K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3, e04123 (2014).
Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e21 (2019).
Bhat, P. et al. Genome organization around nuclear speckles drives mRNA splicing efficiency. Nature 629, 1165–1173 (2024).
Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 22, 165–182 (2021).
Larson, A. G. & Narlikar, G. J. The role of phase separation in heterochromatin formation, function, and regulation. Biochemistry 57, 2540–2548 (2018).
Markaki, Y. et al. Xist nucleates local protein gradients to propagate silencing across the X chromosome. Cell 184, 6174–6192.e32 (2021).
Niekamp, S., Marr, S. K., Oei, T. A., Subramanian, R. & Kingston, R. E. Modularity of PRC1 composition and chromatin interaction define condensate properties. Mol. Cell 84, 1651–1666.e12 (2024).
Wiedner, H. J. & Giudice, J. It’s not just a phase: function and characteristics of RNA-binding proteins in phase separation. Nat. Struct. Mol. Biol. 28, 465–473 (2021).
Chen, X. et al. Structural visualization of transcription initiation in action. Science 382, eadi5120 (2023).
Malik, S. & Roeder, R. G. Regulation of the RNA polymerase II pre-initiation complex by its associated coactivators. Nat. Rev. Genet. 24, 767–782 (2023).
Verger, A., Monté, D. & Villeret, V. Take your PIC. Trends Biochem. Sci. 46, 705–707 (2021).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Cook, P. R. The organization of replication and transcription. Science 284, 1790–1795 (1999).
Cook, P. R. A model for all genomes: the role of transcription factories. J. Mol. Biol. 395, 1–10 (2010).
Iborra, F. J., Pombo, A., Jackson, D. A. & Cook, P. R. Active RNA polymerases are localized within discrete transcription ‘factories’ in human nuclei. J. Cell Sci. 109, 1427–1436 (1996).
Chen, J. et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156, 1274–1285 (2014).
Cissé, I. I. et al. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 341, 664–667 (2013).
Liu, Z. et al. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife 3, e04236 (2014).
Izeddin, I. et al. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 3, e02230 (2014).
Cho, W.-K. et al. RNA polymerase II cluster dynamics predict mRNA output in living cells. eLife 5, e13617 (2016).
Li, J. et al. Single-molecule nanoscopy elucidates RNA polymerase II transcription at single genes in live cells. Cell 178, 491–506.e28 (2019).
Zuo, L. et al. Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat. Commun. 12, 1491 (2021).
Lewis, B. A., Das, S. K., Jha, R. K. & Levens, D. Self-assembly of promoter DNA and RNA Pol II machinery into transcriptionally active biomolecular condensates. Sci. Adv. 9, eadi4565 (2023).
Henninger, J. E. et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225.e24 (2021).
Wei, M.-T. et al. Nucleated transcriptional condensates amplify gene expression. Nat. Cell Biol. 22, 1187–1196 (2020).
Kim, Y. J. et al. Light-activated macromolecular phase separation modulates transcription by reconfiguring chromatin interactions. Sci. Adv. 9, eadg1123 (2023).
Schneider, N. et al. Liquid-liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci. Adv. 7, eabd3568 (2021).
Kawasaki, K. & Fukaya, T. Functional coordination between transcription factor clustering and gene activity. Mol. Cell 83, 1605–1622.e09 (2023).
Du, M. et al. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 187, 331–344.e17 (2024).
Gan, P. et al. Coactivator condensation drives cardiovascular cell lineage specification. Sci. Adv. 10, eadk7160 (2024).
Lyons, H. et al. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 186, 327–345.e8 (2023).
Powers, S. K. et al. Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in Arabidopsis thaliana. Mol. Cell 76, 177–190.e5 (2019).
Schibler, U. How the circadian nuclear orphan receptor REV-ERBα represses transcription: temporal and spatial phase separation combined. Mol. Cell 83, 3399–3401 (2023).
Wu, Y. et al. Disrupting the phase separation of KAT8–IRF1 diminishes PD-L1 expression and promotes antitumor immunity. Nat. Cancer 4, 382–400 (2023).
He, Y. et al. MSL1 promotes liver regeneration by driving phase separation of STAT3 and histone H4 and enhancing their acetylation. Adv. Sci. 10, e2301094 (2023).
Rawat, P. et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Mol. Cell 81, 1013–1026 (2021).
Oksuz, O. et al. Transcription factors interact with RNA to regulate genes. Mol. Cell 83, 2449–2463.e13 (2023).
Shao, W. et al. Phase separation of RNA-binding protein promotes polymerase binding and transcription. Nat. Chem. Biol. 18, 70–80 (2022).
Nair, S. J. et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat. Struct. Mol. Biol. 26, 193–203 (2019).
Lee, J.-H. et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell 81, 3368–3385.e9 (2021).
Quinodoz, S. A. et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 184, 5775–5790.e30 (2021).
Song, L. et al. Hotspot mutations in the structured ENL YEATS domain link aberrant transcriptional condensates and cancer. Mol. Cell 82, 4080–4098.e12 (2022).
Chong, S. et al. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell 82, 2084–2097.e5 (2022).
Trojanowski, J. et al. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell 82, 1878–1893.e10 (2022).
Hur, W. et al. CDK-regulated phase separation seeded by histone genes ensures precise growth and function of histone locus bodies. Dev. Cell 54, 379–394.e6 (2020).
Chong, S. et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 361, eaar2555 (2018).
Patil, A. et al. A disordered region controls cBAF activity via condensation and partner recruitment. Cell 186, 4936–4955.e26 (2023).
De La Cruz, N. et al. Disorder-mediated interactions target proteins to specific condensates. Mol. Cell 84, 3497–3512.e9 (2024).
Musacchio, A. On the role of phase separation in the biogenesis of membraneless compartments. EMBO J. 41, e109952 (2022).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855.e16 (2018).
Ferrie, J. J., Karr, J. P., Tjian, R. & Darzacq, X. “Structure”-function relationships in eukaryotic transcription factors: the role of intrinsically disordered regions in gene regulation. Mol. Cell 82, 3970–3984 (2022).
Brodsky, S. et al. Intrinsically disordered regions direct transcription factor in vivo binding specificity. Mol. Cell 79, 459–471.e4 (2020).
Zamudio, A. V. et al. Mediator condensates localize signaling factors to key cell identity genes. Mol. Cell 76, 753–766.e6 (2019).
Lu, F., Portz, B. & Gilmour, D. S. The C-terminal domain of RNA polymerase II is a multivalent targeting sequence that supports Drosophila development with only consensus heptads. Mol. Cell 73, 1232–1242.e4 (2019).
Holehouse, A. S. & Kragelund, B. B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 25, 187–211 (2023).
Choi, J. M., Holehouse, A. S. & Pappu, R. V. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 49, 107–133 (2020).
Malik, S. & Roeder, R. G. The metazoan mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11, 761–772 (2010).
Lu, G. & Li, P. PHF1 compartmentalizes PRC2 via phase separation. Biochem. J. 480, 1833–1844 (2023).
Han, X. et al. Roles of the BRD4 short isoform in phase separation and active gene transcription. Nat. Struct. Mol. Biol. 27, 333–341 (2020).
Wan, L. et al. Impaired cell fate through gain-of-function mutations in a chromatin reader. Nature 577, 121–126 (2020).
Sanulli, S. et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 575, 390–394 (2019).
Tatavosian, R. et al. Nuclear condensates of the polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 294, 1451–1463 (2019).
Wang, L. et al. Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism. Mol. Cell 76, 646–659.e6 (2019).
Ferrie, J. J. et al. p300 is an obligate integrator of combinatorial transcription factor inputs. Mol. Cell 84, 234–243 (2023).
Cermakova, K. et al. A ubiquitous disordered protein interaction module orchestrates transcription elongation. Science 374, 1113–1121 (2021).
Seif, E. et al. Phase separation by the polyhomeotic sterile alpha motif compartmentalizes polycomb group proteins and enhances their activity. Nat. Commun. 11, 5609 (2020).
Plys, A. J. et al. Phase separation of lycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 33, 799–813 (2019).
Knerr, J. et al. Formin-mediated nuclear actin at androgen receptors promotes transcription. Nature 617, 616–622 (2023).
Xie, J. et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat. Chem. Biol. 18, 1341–1350 (2022).
Frank, F., Liu, X. & Ortlund, E. A. Glucocorticoid receptor condensates link DNA-dependent receptor dimerization and transcriptional transactivation. Proc. Natl Acad. Sci. USA 118, e2024685118 (2021).
Jung, J. H. et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585, 256–260 (2020).
Banerjee, P. R., Milin, A. N., Moosa, M. M., Onuchic, P. L. & Deniz, A. A. Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets. Angew. Chem. Int. Ed. Engl. 56, 11354–11359 (2017).
Zug, R. Developmental disorders caused by haploinsufficiency of transcriptional regulators: a perspective based on cell fate determination. Biol. Open 11, bio058896 (2022).
Kuang, J. et al. SS18 regulates pluripotent-somatic transition through phase separation. Nat. Commun. 12, 4090 (2021).
Christou-Kent, M. et al. CEBPA phase separation links transcriptional activity and 3D chromatin hubs. Cell Rep. 42, 112897 (2023).
Quail, T. et al. Force generation by protein–DNA co-condensation. Nat. Phys. 17, 1007–1012 (2021).
Tang, L. Measuring condensation forces. Nat. Methods 18, 986–986 (2021).
Ji, D. et al. FOXA1 forms biomolecular condensates that unpack condensed chromatin to function as a pioneer factor. Mol. Cell 84, 244–260.e7 (2024).
Wang, Y. et al. A prion-like domain in transcription factor EBF1 promotes phase separation and enables B cell programming of progenitor chromatin. Immunity 53, 1151–1167 (2020).
Wei, C. et al. CTCF organizes inter-A compartment interactions through RYBP-dependent phase separation. Cell Res. 32, 744–760 (2022).
Wang, J. et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. Cell Stem Cell 28, 1868–1883.e11 (2021).
Sharma, R. et al. Liquid condensation of reprogramming factor KLF4 with DNA provides a mechanism for chromatin organization. Nat. Commun. 12, 5579 (2021).
Choi, K. J. et al. NANOG prion-like assembly mediates DNA bridging to facilitate chromatin reorganization and activation of pluripotency. Nat. Cell Biol. 24, 737–747 (2022).
He, J. et al. Dual-role transcription factors stabilize intermediate expression levels. Cell 187, 2746–2766.e25 (2024).
Naderi, J. et al. An activity-specificity trade-off encoded in human transcription factors. Nat. Cell Biol. 26, 1309–1321 (2024).
Theis, A. & Harrison, M. M. Reprogramming of three-dimensional chromatin organization in the early embryo. Curr. Opin. Struct. Biol. 81, 102613 (2023).
Du, Z. & Xie, W. A party for transcription: multiway interactions of enhancers and promoters. Mol. Cell 83, 1542–1544 (2023).
Huang, S.-K., Whitney, P. H., Dutta, S., Shvartsman, S. Y. & Rushlow, C. A. Spatial organization of transcribing loci during early genome activation in Drosophila. Curr. Biol. 31, 5102–5110.e05 (2021).
Cho, C.-Y. & O’Farrell, P. H. Stepwise modifications of transcriptional hubs link pioneer factor activity to a burst of transcription. Nat. Commun. 14, 4848 (2023).
Kuznetsova, K. et al. Nanog organizes transcription bodies. Curr. Biol. 33, 164–173.e5 (2023).
Wilkinson, E. G. & Strader, L. C. A prion-based thermosensor in plants. Mol. Cell 80, 181–182 (2020).
Alberti, S. The plant response to heat requires phase separation. Nature 585, 191–192 (2020).
Ruff, K. M., Roberts, S., Chilkoti, A. & Pappu, R. V. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J. Mol. Biol. 430, 4619–4635 (2018).
Nakashima, K. K., Vibhute, M. A. & Spruijt, E. Biomolecular chemistry in liquid phase separated compartments. Front. Mol. Biosci. 6, 21 (2019).
Kim, D.-H., Doyle, M. R., Sung, S. & Amasino, R. M. Vernalization: winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25, 277–299 (2009).
Osman, S. Changing of the seasons — transcriptional buffers against flowering in the cold. Nat. Struct. Mol. Biol. 28, 963 (2021).
Zhu, P., Lister, C. & Dean, C. Cold-induced Arabidopsis FRIGIDA nuclear condensates for FLC repression. Nature 599, 657–661 (2021).
Chen, D. et al. Integration of light and temperature sensing by liquid-liquid phase separation of phytochrome B. Mol. Cell 82, 3015–3029.e6 (2022).
Shi, H. & Zhong, S. Light and temperature perceptions go through a phase separation. Curr. Opin. Plant Biol. 74, 102397 (2023).
Liu, Q., Liu, W., Niu, Y., Wang, T. & Dong, J. Liquid–liquid phase separation in plants: advances and perspectives from model species to crops. Plant Commun. 5, 100663 (2024).
Kim, C. et al. Phytochrome B photobodies are comprised of phytochrome B and its primary and secondary interacting proteins. Nat. Commun. 14, 1708 (2023).
Xie, Y. et al. Phytochrome B inhibits the activity of phytochrome-interacting factor 7 involving phase separation. Cell Rep. 42, 113562 (2023).
Wang, P. et al. Photomorphogenesis in plants: the central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 194, 104704 (2022).
Pardi, S. A. & Nusinow, D. A. Out of the dark and into the light: a new view of phytochrome photobodies. Front. Plant Sci. 12, 732947 (2021).
Pham, V. N., Kathare, P. K. & Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 176, 1025–1038 (2018).
Vicioso-Mantis, M. et al. JMJD3 intrinsically disordered region links the 3D-genome structure to TGFβ-dependent transcription activation. Nat. Commun. 13, 3263 (2022).
Field, S., Jang, G. J., Dean, C., Strader, L. C. & Rhee, S. Y. Plants use molecular mechanisms mediated by biomolecular condensates to integrate environmental cues with development. Plant Cell 35, 3173–3186 (2023).
Wang, Q. & Lin, C. Mechanisms of cryptochrome-mediated photoresponses in plants. Annu. Rev. Plant Biol. 71, 103–129 (2020).
Mo, W. et al. Arabidopsis cryptochrome 2 forms photobodies with TCP22 under blue light and regulates the circadian clock. Nat. Commun. 13, 2631 (2022).
Górska, A. M., Bartrina, I. & Werner, T. Biomolecular condensation: a new player in auxin signaling. Trends Plant Sci. 28, 620–622 (2023).
Jing, H. et al. Regulation of AUXIN RESPONSE FACTOR condensation and nucleo-cytoplasmic partitioning. Nat. Commun. 13, 4015 (2022).
Emenecker, R. J., Holehouse, A. S. & Strader, L. C. Biological phase separation and biomolecular condensates in plants. Annu. Rev. Plant Biol. 72, 17–46 (2021).
Kmiecik, S. W. & Mayer, M. P. Molecular mechanisms of heat shock factor 1 regulation. Trends Biochem. Sci. 47, 218–234 (2022).
Chowdhary, S., Kainth, A. S., Paracha, S., Gross, D. S. & Pincus, D. Inducible transcriptional condensates drive 3D genome reorganization in the heat shock response. Mol. Cell 82, 4386–4399.e7 (2022).
Goenka, A. et al. Human satellite-III non-coding RNAs modulate heat-shock-induced transcriptional repression. J. Cell Sci. 129, 3541–3552 (2016).
Zhang, H. et al. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat. Cell Biol. 24, 340–352 (2022).
Xu, W., Ding, M. & Li, P. Struggle for survival: new insights into NELF condensation for adaptive transcriptional reprogramming. Mol. Cell 81, 896–898 (2021).
Jalihal, A. P. et al. Multivalent proteins rapidly and reversibly phase-separate upon osmotic cell volume change. Mol. Cell 79, 978–990 (2020).
Majumder, S. & Jain, A. Osmotic stress triggers phase separation. Mol. Cell 79, 876–877 (2020).
Liu, S. et al. CPSF6 regulates alternative polyadenylation and proliferation of cancer cells through phase separation. Cell Rep. 42, 113197 (2023).
Wang, B. et al. Condensation of SEUSS promotes hyperosmotic stress tolerance in Arabidopsis. Nat. Chem. Biol. 18, 1361–1369 (2022).
Foyer, C. H. Redox control of flowering. Nat. Chem. Biol. 17, 504–505 (2021).
Huang, X. et al. ROS regulated reversible protein phase separation synchronizes plant flowering. Nat. Chem. Biol. 17, 549–557 (2021).
Huang, S., Zhu, S., Kumar, P. & MacMicking, J. D. A phase-separated nuclear GBPL circuit controls immunity in plants. Nature 594, 424–429 (2021).
Pinheiro, E. D. S., Preato, A. M., Petrucci, T. V. B., dos Santos, L. S. & Glezer, I. Phase-separation: a possible new layer for transcriptional regulation by glucocorticoid receptor. Front. Endocrinol. 14, 1160238 (2023).
Stortz, M., Presman, D. M., Pecci, A. & Levi, V. Phasing the intranuclear organization of steroid hormone receptors. Biochem. J. 478, 443–461 (2021).
Stortz, M., Pecci, A., Presman, D. M. & Levi, V. Unraveling the molecular interactions involved in phase separation of glucocorticoid receptor. BMC Biol. 18, 59 (2020).
Zhang, F. et al. Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression. Nucleic Acids Res. 51, 99–116 (2023).
Klein, I. A. et al. Partitioning of cancer therapeutics in nuclear condensates. Science 368, 1386–1392 (2020).
Li, Z. et al. PPARγ phase separates with RXRα at PPREs to regulate target gene expression. Cell Discov. 8, 37 (2022).
Ma, L. et al. Co-condensation between transcription factor and coactivator p300 modulates transcriptional bursting kinetics. Mol. Cell 81, 1682–1697.e7 (2021).
Ding, M., Xu, W., Pei, G. & Li, P. Long way up: rethink diseases in light of phase separation and phase transition. Protein Cell 15, 475–492 (2023).
Zhang, S., Pei, G., Li, B., Li, P. & Lin, Y. Abnormal phase separation of biomacromolecules in human diseases. Acta Biochim. Biophys. Sin. 55, 1133–1152 (2023).
Gao, Q. et al. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep. 23, 227–238.e3 (2018).
Brien, G. L., Stegmaier, K. & Armstrong, S. A. Targeting chromatin complexes in fusion protein-driven malignancies. Nat. Rev. Cancer 19, 255–269 (2019).
Shirnekhi, H. K., Chandra, B. & Kriwacki, R. W. The role of phase-separated condensates in fusion oncoprotein-driven cancers. Annu. Rev. Cancer Biol. 7, 73–91 (2023).
Tripathi, S. et al. Defining the condensate landscape of fusion oncoproteins. Nat. Commun. 14, 6008 (2023).
Leach, B. I. et al. Leukemia fusion target AF9 is an intrinsically disordered transcriptional regulator that recruits multiple partners via coupled folding and binding. Structure 21, 176–183 (2013).
Shorter, J. Prion-like domains program Ewing’s sarcoma. Cell 171, 30–31 (2017).
Owen, I. et al. The oncogenic transcription factor FUS-CHOP can undergo nuclear liquid–liquid phase separation. J. Cell Sci. 134, jcs258578 (2021).
Davis, R. B., Kaur, T., Moosa, M. M. & Banerjee, P. R. FUS oncofusion protein condensates recruit mSWI/SNF chromatin remodeler via heterotypic interactions between prion‐like domains. Protein Sci. 30, 1454–1466 (2021).
Wang, Y. et al. Dissolution of oncofusion transcription factor condensates for cancer therapy. Nat. Chem. Biol. 19, 1223–1234 (2023).
Boulay, G. et al. Cancer-specific retargeting of BAF complexes by a prion-like domain. Cell 171, 163–178.e19 (2017).
Cheng, Y. et al. Phase transition and remodeling complex assembly are important for SS18-SSX oncogenic activity in synovial sarcomas. Nat. Commun. 13, 2724 (2022).
Terlecki-Zaniewicz, S. et al. Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nat. Struct. Mol. Biol. 28, 190–201 (2021).
Chandra, B. et al. Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. 12, 1152–1169 (2022).
Jevtic, Z. et al. SMARCA5 interacts with NUP98-NSD1 oncofusion protein and sustains hematopoietic cells transformation. J. Exp. Clin. Cancer Res. 41, 34 (2022).
Oka, M. et al. Phase-separated nuclear bodies of nucleoporin fusions promote condensation of MLL1/CRM1 and rearrangement of 3D genome structure. Cell Rep. 42, 112884 (2023).
Ahn, J. H. et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 595, 591–595 (2021).
Rosencrance, C. D. et al. Chromatin hyperacetylation impacts chromosome folding by forming a nuclear subcompartment. Mol. Cell 78, 112–126 (2020).
Kosno, M., Currie, S. L., Kumar, A., Xing, C. & Rosen, M. K. Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct. Sci. Rep. 13, 11907 (2023).
Yu, D. et al. Structural mechanism of BRD4-NUT and p300 bipartite interaction in propagating aberrant gene transcription in chromatin in NUT carcinoma. Nat. Commun. 14, 378 (2023).
Ibrahim, Z. et al. Structural insights into p300 regulation and acetylation-dependent genome organisation. Nat. Commun. 13, 7759 (2022).
Perlman, E. J. et al. MLLT1 YEATS domain mutations in clinically distinctive favourable histology Wilms tumours. Nat. Commun. 6, 10013 (2015).
Erb, M. A. et al. Transcription control by the ENL YEATS domain in acute leukaemia. Nature 543, 270–274 (2017).
Muragaki, Y., Mundlos, S., Upton, J. & Olsen, B. R. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science 272, 548–551 (1996).
Basu, S. et al. Unblending of transcriptional condensates in human repeat expansion disease. Cell 181, 1062–1079 (2020).
Chen, L. et al. Hormone-induced enhancer assembly requires an optimal level of hormone receptor multivalent interactions. Mol. Cell 83, 3438–3456.e12 (2023).
Darling, A. L. & Uversky, V. N. Intrinsic disorder in proteins with pathogenic repeat expansions. Molecules 22, 2027 (2017).
Utsch, B. et al. A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-foot-genital syndrome: proper function of poly(A)-harbouring transcription factors depends on a critical repeat length? Hum. Genet. 110, 488–494 (2002).
Albrecht, A. N. et al. A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum. Mol. Genet. 13, 2351–2359 (2004).
Shi, B. et al. UTX condensation underlies its tumour-suppressive activity. Nature 597, 726–731 (2021).
Greig, J. A. et al. Arginine-enriched mixed-charge domains provide cohesion for nuclear speckle condensation. Mol. Cell 77, 1237–1250.e4 (2020).
Boeynaems, S. et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 65, 1044–1055.e5 (2017).
Mensah, M. A. et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 614, 564–571 (2023).
Yang, J. et al. MYC phase separation selectively modulates the transcriptome. Nat. Struct. Mol. Biol. 31, 1567–1579 (2024).
Wang, X. et al. Prognostic significance of EZH2 expression in non-small cell lung cancer: a meta-analysis. Sci. Rep. 6, 19239 (2016).
Zhang, J. et al. Myristoylation-mediated phase separation of EZH2 compartmentalizes STAT3 to promote lung cancer growth. Cancer Lett. 516, 84–98 (2021).
Zou, Y. et al. New insights into the important roles of phase seperation in the targeted therapy of lung cancer. Cell Biosci. 13, 150 (2023).
Zhong, Z., Jiao, Z. & Yu, F. X. The Hippo signaling pathway in development and regeneration. Cell Rep. 43, 113926 (2024).
Cai, D. et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 21, 1578–1589 (2019).
Shao, Y. et al. A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation. Nat. Cell Biol. 26, 86–99 (2024).
Lu, Y. et al. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 (2020).
Franklin, J. M. & Guan, K. L. YAP/TAZ phase separation for transcription. Nat. Cell Biol. 22, 357–358 (2020).
Zhu, G. et al. Pharmacological inhibition of SRC-1 phase separation suppresses YAP oncogenic transcription activity. Cell Res. 31, 1028–1031 (2021).
Hao, S. et al. YAP condensates are highly organized hubs. iScience 27, 109927 (2024).
Qin, M. et al. LATS2 condensates organize signalosomes for Hippo pathway signal transduction. Nat. Chem. Biol. 20, 710–720 (2024).
Liu, Q. et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184, 5559–5576 (2021).
Hu, X. et al. Nuclear condensates of YAP fusion proteins alter transcription to drive ependymoma tumourigenesis. Nat. Cell Biol. 25, 323–336 (2023).
Mi, Z. et al. cAMP-induced nuclear condensation of CRTC2 promotes transcription elongation and cystogenesis in autosomal dominant polycystic kidney disease. Adv. Sci. 9, e2104578 (2022).
Shrinivas, K. & Brenner, M. P. Phase separation in fluids with many interacting components. Proc. Natl Acad. Sci. USA 118, e2108551118 (2021).
Shin, Y. et al. Liquid nuclear condensates mechanically sense and restructure the genome. Cell 175, 1481–1491.e13 (2018).
Strom, A. R. et al. Condensate interfacial forces reposition DNA loci and probe chromatin viscoelasticity. Cell 187, 5282–5297.e20 (2024).
Rao, S. S. P. et al. Cohesin loss eliminates all loop domains. Cell 171, 305–320.e24 (2017).
Beagrie, R. A. et al. Complex multi-enhancer contacts captured by genome architecture mapping. Nature 543, 519–524 (2017).
Chen, Y. et al. Mapping 3D genome organization relative to nuclear compartments using TSA-Seq as a cytological ruler. J. Cell Biol. 217, 4025–4048 (2018).
Benabdallah, N. S. et al. Decreased enhancer-promoter proximity accompanying enhancer activation. Mol. Cell 76, 473–484.e7 (2019).
Heist, T., Fukaya, T. & Levine, M. Large distances separate coregulated genes in living Drosophila embryos. Proc. Natl Acad. Sci. USA 116, 15062–15067 (2019).
Yang, J. H. & Hansen, A. S. Enhancer selectivity in space and time: from enhancer-promoter interactions to promoter activation. Nat. Rev. Mol. Cell Biol. 25, 574–591 (2024).
Neumayr, C. et al. Differential cofactor dependencies define distinct types of human enhancers. Nature 606, 406–413 (2022).
Conti, B. A. & Oppikofer, M. Biomolecular condensates: new opportunities for drug discovery and RNA therapeutics. Trends Pharmacol. Sci. 43, 820–837 (2022).
Patel, A. et al. Principles and functions of condensate modifying drugs. Front. Mol. Biosci. 9, 1007744 (2022).
Gui, T. et al. Targeted perturbation of signaling-driven condensates. Mol. Cell 83, 4141–4157.e11 (2023).
Basu, S. et al. Rational optimization of a transcription factor activation domain inhibitor. Nat. Struct. Mol. Biol. 30, 1958–1969 (2023).
Li, W. et al. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat. Cell Biol. 22, 960–972 (2020).
Liu, B. & Abdel-Wahab, O. Oncogenic splicing regulated by phase separation. Nat. Cell Biol. 22, 916–918 (2020).
Zhuang, Y. et al. Circadian clocks are modulated by compartmentalized oscillating translation. Cell 186, 3245–3260.e23 (2023).
Dang, L. et al. Nuclear condensation of CDYL links histone crotonylation and cystogenesis in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 33, 1708–1725 (2022).
Lee, R. et al. CTCF-mediated chromatin looping provides a topological framework for the formation of phase-separated transcriptional condensates. Nucleic Acids Res. 50, 207–226 (2022).
Hsieh, T. H. S. et al. Enhancer–promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat. Genet. 54, 1919–1932 (2022).
Fu, H. et al. Poly(ADP-ribosylation) of P-TEFb by PARP1 disrupts phase separation to inhibit global transcription after DNA damage. Nat. Cell Biol. 24, 513–525 (2022).
Pessina, F. et al. Functional transcription promoters at DNA double-strand breaks mediate RNA-driven phase separation of damage-response factors. Nat. Cell Biol. 21, 1286–1299 (2019).
Kang, K., Shi, Q., Wang, X. & Chen, Y. G. Dishevelled phase separation promotes Wnt signalosome assembly and destruction complex disassembly. J. Cell Biol. 221, e202205069 (2022).
Zhu, Y. et al. Oncogenic extrachromosomal DNA functions as mobile enhancers to globally amplify chromosomal transcription. Cancer Cell 39, 694–707 (2021).
Fang, X. et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. Nature 569, 265–269 (2019).
Qin, Z. et al. Deactylation by SIRT1 enables liquid–liquid phase separation of IRF3/IRF7 in innate antiviral immunity. Nat. Immunol. 23, 1193–1207 (2022).
Wang, X. Q. D. et al. Mutant NPM1 hijacks transcriptional hubs to maintain pathogenic gene programs in acute myeloid leukemia. Cancer Discov. 13, 724–745 (2023).
Lu, Y. et al. Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Sci. Transl. Med. 12, eaba3613 (2020).
Ibáñez de Opakua, A. et al. Molecular interactions of FG nucleoporin repeats at high resolution. Nat. Chem. 14, 1278–1285 (2022).
Cheng, S. L. H. et al. Nutrient status regulates MED19a phase separation for ORESARA1‐dependent senescence. New Phytol. 236, 1779–1795 (2022).
Kamagata, K. et al. Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains. Sci. Rep. 10, 580 (2020).
Petronilho, E. C. et al. Phase separation of p53 precedes aggregation and is affected by oncogenic mutations and ligands. Chem. Sci. 12, 7334–7349 (2021).
Chen, C., Fu, G., Guo, Q., Xue, S. & Luo, S. Z. Phase separation of p53 induced by its unstructured basic region and prevented by oncogenic mutations in tetramerization domain. Int. J. Biol. Macromol. 222, 207–216 (2022).
Xu, J. et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 7, 285–295 (2011).
Perez-Schindler, J. et al. RNA-bound PGC-1α controls gene expression in liquid-like nuclear condensates. Proc. Natl Acad. Sci. USA 118, e2105951118 (2021).
Zhu, K. et al. An intrinsically disordered region controlling condensation of a circadian clock component and rhythmic transcription in the liver. Mol. Cell 83, 3457–3469.e7 (2023).
Dejosez, M. et al. Regulatory architecture of housekeeping genes is driven by promoter assemblies. Cell Rep. 42, 112505 (2023).
Xiao, M. et al. Smad4 sequestered in SFPQ condensates prevents TGF-β tumor-suppressive signaling. Dev. Cell 59, 48–63.e8 (2024).
Yan, K. et al. SGF29 nuclear condensates reinforce cellular aging. Cell Discov. 9, 110 (2023).
Cao, X., Du, Q., Guo, Y., Wang, Y. & Jiao, Y. Condensation of STM is critical for shoot meristem maintenance and salt tolerance in Arabidopsis. Mol. Plant 16, 1445–1459 (2023).
Chen, G. et al. Taf14 recognizes a common motif in transcriptional machineries and facilitates their clustering by phase separation. Nat. Commun. 11, 4206 (2020).
Wang, W. et al. A histidine cluster determines YY1-compartmentalized coactivators and chromatin elements in phase-separated enhancer clusters. Nucleic Acids Res. 50, 4917–4937 (2022).
Acknowledgements
The authors thank J. Henninger, S. Bannani, B. Li, Y. Li, Y. Lin and members of the Sabari and Li laboratories for the helpful comments and discussion. This work was supported by the National Natural Science Foundation of China grant 32125010 (P.L.), the Postdoctoral Program of Tsinghua University–Peking University Joint Center for Life Sciences, Shuimu Tsinghua Scholar Program, and Advanced Innovation Fellow programme (G.P.), Cancer Prevention and Research Institute of Texas grant RR190090 (B.R.S.), the National Institutes of Health grant GM147583 (B.R.S.) and the Robert A. Welch Foundation grants I-2163-20230405 and V-I-0004-20230731 (B.R.S.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Critical thresholds
-
Specific concentrations or condition points at which a sudden transition occurs, such as phase separation.
- DNA regulatory elements
-
DNA sequences located either proximal or distal to a gene that regulate its expression by interacting with transcription factors. Prominent examples are promoters and enhancers.
- Domain–ligand pairs
-
Interacting modules between a protein domain and its corresponding ligand.
- Histone locus body
-
A nuclear condensate associated with the active transcription of replication-dependent histone genes, which facilitates the coordination of histone mRNA transcription, processing and export during early S phase.
- Lower critical solution temperature behaviour
-
The phenomenon whereby a protein remains soluble in a solvent below a specific temperature, but above this temperature, it undergoes phase separation into condensates owing to the disruption of stabilizing interactions by increased thermal motion.
- Mediator coactivator complex
-
A multiprotein complex that acts as a bridge between transcription factors and RNA polymerase II to regulate gene activation.
- Multivalency
-
The property of a molecule or a complex that has multiple binding sites that can interact with multiple binding surfaces of another molecule or complex.
- Patterned charge blocks
-
Sequences within proteins wherein charged amino acids are organized in clusters.
- Phase separation
-
A physicochemical process through which molecules demix from the bulk environment to form dense, droplet-like structures.
- Poly-glutamine
-
A sequence motif in proteins consisting of multiple Gln residues. Some poly-glutamine tracts enhance the formation of pathological condensates or aggregates by promoting strong hydrogen bonding and intermolecular interactions often associated with neurodegenerative diseases.
- Re-entrant phase transition
-
A situation in a multi-component system in which increasing the concentration of a component initially favours the formation of condensates, but further increases lead to the disruption of the condensates.
- Skotomorphogenesis
-
The developmental process in plants that occurs in darkness, characterized by elongated stems, closed cotyledons and lack of chlorophyll synthesis.
- Transcription bursting
-
The phenomenon whereby transcription occurs in rapid, transient bursts rather than at a constant rate.
- Transcriptional condensates
-
Biomolecular condensates that concentrate components of the transcription machinery and related factors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, G., Lyons, H., Li, P. et al. Transcription regulation by biomolecular condensates. Nat Rev Mol Cell Biol 26, 213–236 (2025). https://doi.org/10.1038/s41580-024-00789-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-024-00789-x
This article is cited by
-
Two distinct chromatin modules regulate proinflammatory gene expression
Nature Cell Biology (2026)
-
A high-throughput, flow cytometry approach to measure phase behavior and exchange in biomolecular condensates
Nature Communications (2026)
-
Receptor activation via phase separation affects cardiac preservation quality
Nature Cardiovascular Research (2025)
-
Selective RNA sequestration in biomolecular condensates directs cell fate transitions
Nature Biotechnology (2025)
-
Differential conformational expansion of NUP98-HOXA9 oncoprotein from nanosized assemblies to macrophases
Nature Communications (2025)