Abstract
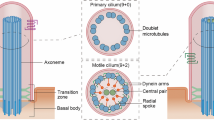
Primary and motile cilia are eukaryotic organelles that perform crucial roles in cellular signalling and motility. Intraflagellar transport (IFT) contributes to the formation of the highly specialized ciliary proteome by active and selective transport of soluble and membrane proteins into and out of cilia. IFT is performed by the IFT-A and IFT-B protein complexes, which together link cargoes to the microtubule motors kinesin and dynein. In this Review, we discuss recent structural and mechanistic insights on how the IFT complexes are first recruited to the base of the cilium, how they polymerize into an anterograde IFT train, and how this complex imports cargoes from the cytoplasm. We will describe insights into how kinesin-driven anterograde trains are carried to the ciliary tip, where they are remodelled into dynein-driven retrograde trains for cargo export. We will also present how the interplay between IFT-A and IFT-B complexes, motor proteins and cargo adaptors is regulated for bidirectional ciliary transport.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Saggese, T., Young, A. A., Huang, C., Braeckmans, K. & McGlashan, S. R. Development of a method for the measurement of primary cilia length in 3D. Cilia 1, 11 (2012).
Mitchell, D. R. Evolution of cilia. Cold Spring Harb. Persp. Biol. 9, a028290 (2017).
Carvalho-Santos, Z., Azimzadeh, J., Pereira-Leal, J. B. & Bettencourt-Dias, M. Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 194, 165–175 (2011).
Hao, K., Chen, Y., Yan, X. & Zhu, X. Cilia locally synthesize proteins to sustain their ultrastructure and functions. Nat. Commun. 12, 6971 (2021).
Kozminski, K. G., Johnson, K. A., Forscher, P. & Rosenbaum, J. L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. USA 90, 5519–5523 (1993).
Kozminski, K. G., Beech, P. L. & Rosenbaum, J. L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131, 1517–1527 (1995).
Pazour, G. J., Wilkerson, C. G. & Witman, G. B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141, 979–992 (1998).
Pigino, G. et al. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 187, 135–148 (2009).
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998).
Pazour, G. J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Huangfu, D. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003).
Hilgendorf, K. I., Myers, B. R. & Reiter, J. F. Emerging mechanistic understanding of cilia function in cellular signalling. Nat. Rev. Mol. Cell Biol. 25, 555–573 (2024).
Mill, P., Christensen, S. T. & Pedersen, L. B. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 24, 421–441 (2023).
Iomini, C., Babaev-Khaimov, V., Sassaroli, M. & Piperno, G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153, 13–24 (2001).
Pedersen, L. B., Geimer, S. & Rosenbaum, J. L. Dissecting the molecular mechanisms of intraflagellar transport in Chlamydomonas. Curr. Biol. 16, 450–459 (2006).
Mitra, A., Loseva, E. & Peterman, E. J. G. IFT cargo and motors associate sequentially with IFT trains to enter cilia of C. elegans. Nat. Commun. 15, 3456 (2024).
Buisson, J. et al. Intraflagellar transport proteins cycle between the flagellum and its base. J. Cell Sci. 126, 327–338 (2013).
Ou, G., Blacque, O. E., Snow, J. J., Leroux, M. R. & Scholey, J. M. Functional coordination of intraflagellar transport motors. Nature 436, 583–587 (2005).
King, S. M. Axonemal dynein arms. Cold Spring Harb. Persp. Biol. 8, a028100 (2016).
Gui, M. et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 28, 29–37 (2021).
Pigino, G. & Ishikawa, T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2, 50–58 (2012).
Ishikawa, T. Axoneme structure from motile cilia. Cold Spring Harb. Perspect. Biol. 9, a028076 (2016).
Wallmeier, J. et al. Motile ciliopathies. Nat. Rev. Dis. Prim. 6, 77 (2020).
Fassad, M. R. et al. Defective airway intraflagellar transport underlies a combined motile and primary ciliopathy syndrome caused by IFT74 mutations. Hum. Mol. Genet. 32, 3090–3104 (2023).
Nachury, M. V. How do cilia organize signalling cascades? Phil. Trans. R. Soc. B 369, 20130465 (2014).
Yue, Y., Engelke, M. F., Blasius, T. L. & Verhey, K. J. Hedgehog-induced ciliary trafficking of kinesin-4 motor KIF7 requires intraflagellar transport but not KIF7’s microtubule binding. Mol. Biol. Cell 33, br1 (2022).
Zhang, Y. & Beachy, P. A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 24, 668–687 (2023).
Kiesel, P. et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 27, 1115–1124 (2020).
Klena, N. & Pigino, G. Structural biology of cilia and intraflagellar transport. Annu. Rev. Cell Dev. Biol. 38, 103–123 (2022).
Sun, S., Fisher, R. L., Bowser, S. S., Pentecost, B. T. & Sui, H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl Acad. Sci. 116, 9370–9379 (2019).
Sorokin, S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377 (1962).
Benmerah, A. The ciliary pocket. Curr. Opin. Cell Biol. 25, 78–84 (2013).
Bujakowska, K. M., Liu, Q. & Pierce, E. A. Photoreceptor cilia and retinal ciliopathies. Cold Spring Harb. Persp. Biol. 9, a028274 (2017).
Reiter, J. F. & Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 (2017).
Taschner, M. & Lorentzen, E. The intraflagellar transport machinery. Cold Spring Harb. Persp. Biol. 8, a028092 (2016).
Jékely, G. & Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays 28, 191–198 (2006).
van Dam, T. J. P. et al. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl Acad. Sci. 110, 6943–6948 (2013).
Meleppattu, S., Zhou, H., Dai, J., Gui, M. & Brown, A. Mechanism of IFT-A polymerization into trains for ciliary transport. Cell 185, 4986–4998.e12 (2022).
Wu, D. et al. Ciliogenesis requires sphingolipid-dependent membrane and axoneme interaction. Proc. Natl Acad. Sci. USA 119, e2201096119 (2022).
Quidwai, T. et al. A WDR35-dependent coat protein complex transports ciliary membrane cargo vesicles to cilia. eLife 10, e69786 (2021).
Zhu, B. et al. Functional exploration of the IFT-A complex in intraflagellar transport and ciliogenesis. PLOS Genet. 13, e1006627 (2017).
Jiang, M. et al. Human IFT-A complex structures provide molecular insights into ciliary transport. Cell Res. 33, 288–298 (2023).
Behal, R. H. et al. Subunit interactions and organization of the Chlamydomonas reinhardtii intraflagellar transport complex A proteins. J. Biol. Chem. 287, 11689–11703 (2012).
Hesketh, S. J., Mukhopadhyay, A. G., Nakamura, D., Toropova, K. & Roberts, A. J. IFT-A structure reveals carriages for membrane protein transport into cilia. Cell 185, 4971–4985.e16 (2022).
Hirano, T., Katoh, Y. & Nakayama, K. Intraflagellar transport-A complex mediates ciliary entry and retrograde trafficking of ciliary G protein-coupled receptors. Mol. Biol. Cell 28, 429–439 (2017).
Ma, Y. et al. Structural insight into the intraflagellar transport complex IFT-A and its assembly in the anterograde IFT train. Nat. Commun. 14, 1506 (2023).
Lacey, S. E., Graziadei, A. & Pigino, G. Extensive structural rearrangement of intraflagellar transport trains underpins bidirectional cargo transport. Cell 187, 4621–4636.e18 (2024).
Bhogaraju, S. et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009–1012 (2013).
Taschner, M. et al. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 35, 773–790 (2016).
Katoh, Y. et al. Overall architecture of the intraflagellar transport (IFT)-B complex containing cluap1/IFT38 as an essential component of the IFT-B peripheral subcomplex. J. Biol. Chem. 291, 10962–10975 (2016).
Taschner, M., Kotsis, F., Braeuer, P., Kuehn, E. W. & Lorentzen, E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J. Cell Biol. 207, 269–282 (2014).
Wachter, S. et al. Binding of IFT22 to the intraflagellar transport complex is essential for flagellum assembly. EMBO J. 38, e101251 (2019).
Bhogaraju, S., Taschner, M., Morawetz, M., Basquin, C. & Lorentzen, E. Crystal structure of the intraflagellar transport complex 25/27. EMBO J. 30, 1907–1918 (2011).
Wang, Z., Fan, Z.-C., Williamson, S. M. & Qin, H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PLOS ONE 4, e5384 (2009).
Howard, P. W., Jue, S. F. & Maurer, R. A. Interaction of mouse TTC30/DYF-1 with multiple intraflagellar transport complex B proteins and KIF17. Exp. Cell Res. 319, 2275–2281 (2013).
Takei, R., Katoh, Y. & Nakayama, K. Robust interaction of IFT70 with IFT52–IFT88 in the IFT-B complex is required for ciliogenesis. Biol. Open 7, bio033241 (2018).
Lacey, S. E., Foster, H. E. & Pigino, G. The molecular structure of IFT-A and IFT-B in anterograde intraflagellar transport trains. Nat. Struct. Mol. Biol. 30, 584–593 (2023).
Petriman, N. A. et al. Biochemically validated structural model of the 15-subunit intraflagellar transport complex IFT-B. EMBO J. 41, e112440 (2022).
Beatson, S. & Ponting, C. P. GIFT domains: linking eukaryotic intraflagellar transport and glycosylation to bacterial gliding. Trends Biochem. Sci. 29, 396–399 (2004).
Delaval, B., Bright, A., Lawson, N. D. & Doxsey, S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 13, 461–468 (2011).
Taulet, N. et al. IFT proteins spatially control the geometry of cleavage furrow ingression and lumen positioning. Nat. Commun. 8, 1928 (2017).
Vitre, B. et al. IFT proteins interact with HSET to promote supernumerary centrosome clustering in mitosis. EMBO Rep. 21, e49234 (2020).
Follit, J. A., Tuft, R. A., Fogarty, K. E. & Pazour, G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781–3792 (2006).
Crouse, J. A. et al. Distinct functions for IFT140 and IFT20 in opsin transport. Cytoskeleton 71, 302–310 (2014).
Follit, J. A. et al. The golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLOS Genet. 4, e1000315 (2008).
Monis, W. J., Faundez, V. & Pazour, G. J. BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J. Cell Biol. 216, 2131–2150 (2017).
Follit, J. A., Xu, F., Keady, B. T. & Pazour, G. J. Characterization of mouse IFT complex B. Cell Motil. 66, 457–468 (2009).
Wang, Q. et al. Membrane association and remodeling by intraflagellar transport protein IFT172. Nat. Commun. 9, 4684 (2018).
Wedaman, K. P., Meyer, D. W., Rashid, D. J., Cole, D. G. & Scholey, J. M. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J. Cell Biol. 132, 371–380 (1996).
Yamazaki, H., Nakata, T., Okada, Y. & Hirokawa, N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J. Cell Biol. 130, 1387–1399 (1995).
Mueller, J., Perrone, C. A., Bower, R., Cole, D. G. & Porter, M. E. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell 16, 1341–1354 (2005).
Snow, J. J. et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1113 (2004).
Engelke, M. F. et al. Acute inhibition of heterotrimeric kinesin-2 function reveals mechanisms of intraflagellar transport in mammalian cilia. Curr. Biol. 29, 1137–1148.e4 (2019).
Funabashi, T. et al. Ciliary entry of KIF17 is dependent on its binding to the IFT-B complex via IFT46–IFT56 as well as on its nuclear localization signal. Mol. Biol. Cell 28, 624–633 (2017).
Waas, B. et al. Dual and opposing roles for the kinesin-2 motor, KIF17, in Hedgehog-dependent cerebellar development. Sci. Adv. 10, eade1650 (2024).
Pazour, G. J., Dickert, B. L. & Witman, G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 (1999).
Asante, D., Stevenson, N. L. & Stephens, D. J. Subunit composition of the human cytoplasmic dynein-2 complex. J. Cell Sci. 127, 4774–4787 (2014).
Toropova, K. et al. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 26, 823–829 (2019).
Stepanek, L. & Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352, 721–724 (2016).
Chhatre, A. et al. Tubulin tyrosination/detyrosination regulates the sorting of intraflagellar transport trains on axonemal microtubule doublets. Preprint at bioRxiv https://doi.org/10.1101/2024.05.03.592312 (2024).
Williams, C. L. et al. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat. Commun. 5, 5813 (2014).
Tian, X., Zhao, H. & Zhou, J. Organization, functions, and mechanisms of the BBSome in development, ciliopathies, and beyond. eLife 12, e87623 (2023).
Singh, S. K., Gui, M., Koh, F., Yip, M. C. & Brown, A. Structure and activation mechanism of the BBSome membrane protein trafficking complex. eLife 9, e53322 (2020).
Yang, S. et al. Near-atomic structures of the BBSome reveal the basis for BBSome activation and binding to GPCR cargoes. eLife 9, e55954 (2020).
Jin, H. et al. The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010).
Nachury, M. V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Klink, B. U., Gatsogiannis, C., Hofnagel, O., Wittinghofer, A. & Raunser, S. Structure of the human BBSome core complex. eLife 9, e53910 (2020).
Hernandez-Hernandez, V. et al. Bardet–Biedl syndrome proteins control the cilia length through regulation of actin polymerization. Hum. Mol. Genet. 22, 3858–3868 (2013).
Hibbard, J. V. K., Vazquez, N., Satija, R. & Wallingford, J. B. Protein turnover dynamics suggest a diffusion-to-capture mechanism for peri-basal body recruitment and retention of intraflagellar transport proteins. Mol. Biol. Cell 32, 1171–1180 (2021).
Mitra, A., Gioukakis, E., Mul, W. & Peterman, E. J. G. Sorting at ciliary base and ciliary entry of BBSome, IFT-B and IFT-A. Preprint at bioRxiv https://doi.org/10.1101/2024.03.05.583485 (2024).
Toriyama, M. et al. The ciliopathy-associated CPLANE proteins direct basal body recruitment of intraflagellar transport machinery. Nat. Genet. 48, 648–656 (2016).
Dewees, S. I. et al. Phylogenetic profiling and cellular analyses of ARL16 reveal roles in traffic of IFT140 and INPP5E. Mol. Biol. Cell 33, ar33 (2022).
Langousis, G. et al. Structure of the ciliogenesis-associated CPLANE complex. Sci. Adv. 8, eabn0832 (2022).
Brown, J. M., Cochran, D. A., Craige, B., Kubo, T. & Witman, G. B. Assembly of IFT trains at the ciliary base depends on IFT74. Curr. Biol. 25, 1583–1593 (2015).
Xin, D., Christopher, K. J., Zeng, L., Kong, Y. & Weatherbee, S. D. IFT56 regulates vertebrate developmental patterning by maintaining IFTB complex integrity and ciliary microtubule architecture. Development 144, 1544–1553 (2017).
Yang, T. T. et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 9, 2023 (2018).
Kanie, T. et al. The CEP19–RABL2 GTPase complex binds IFT-B to initiate intraflagellar transport at the ciliary base. Dev. Cell 42, 22–36.e12 (2017).
Nishijima, Y. et al. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol. Biol. Cell 28, 1652–1666 (2017).
Zhang, R.-K., Sun, W.-Y., Liu, Y.-X., Zhang, E. Y. & Fan, Z.-C. RABL2 promotes the outward transition zone passage of signaling proteins in cilia via ARL3. Proc. Natl Acad. Sci. USA 120, e2302603120 (2023).
Zhou, Z., Katoh, Y. & Nakayama, K. CEP19–RABL2–IFT-B axis controls BBSome-mediated ciliary GPCR export. Mol. Biol. Cell 33, ar126 (2022).
Klink, B. U. et al. A recombinant BBSome core complex and how it interacts with ciliary cargo. eLife 6, e27434 (2017).
Seo, S. et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc. Natl Acad. Sci. USA 107, 1488–1493 (2010).
Zhang, Q., Yu, D., Seo, S., Stone, E. M. & Sheffield, V. C. Intrinsic protein–protein interaction-mediated and chaperonin-assisted sequential assembly of stable Bardet–Biedl syndrome protein complex, the BBSome. J. Biol. Chem. 287, 20625–20635 (2012).
Prasai, A. et al. The BBSome assembly is spatially controlled by BBS1 and BBS4 in human cells. J. Biol. Chem. 295, 14279–14290 (2020).
Xue, B. et al. Intraflagellar transport protein RABL5/IFT22 recruits the BBSome to the basal body through the GTPase ARL6/BBS3. Proc. Natl Acad. Sci. 117, 2496–2505 (2020).
Guo, D.-F. et al. The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes 68, 1591–1603 (2019).
Guo, D.-F. et al. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLOS Genet. 12, e1005890 (2016).
Starks, R. D. et al. Regulation of insulin receptor trafficking by bardet biedl syndrome proteins. PLOS Genet. 11, e1005311 (2015).
Berbari, N. F., Lewis, J. S., Bishop, G. A., Askwith, C. C. & Mykytyn, K. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl Acad. Sci. USA 105, 4242–4246 (2008).
Loktev, A. V. & Jackson, P. K. Neuropeptide Y family receptors traffic via the bardet-biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep. 5, 1316–1329 (2013).
Stubbs, T., Bingman, J. I., Besse, J. & Mykytyn, K. Ciliary signaling proteins are mislocalized in the brains of Bardet–Biedl syndrome 1-null mice. Front. Cell Dev. Biol. 10, 1092161 (2023).
Su, X. et al. Bardet–Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum. Mol. Genet. 23, 5441–5451 (2014).
Nager, A. R. et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252–263.e14 (2017).
Nguyen, A. & Goetz, S. C. TTBK2 controls cilium stability by regulating distinct modules of centrosomal proteins. Mol. Biol. Cell 34, ar8 (2023).
Loukil, A., Barrington, C. & Goetz, S. C. A complex of distal appendage-associated kinases linked to human disease regulates ciliary trafficking and stability. Proc. Natl Acad. Sci. USA 118, e2018740118 (2021).
van den Hoek, H. et al. In situ architecture of the ciliary base reveals the stepwise assembly of intraflagellar transport trains. Science 377, 543–548 (2022).
Wingfield, J. L. et al. IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife 6, e26609 (2017).
Richey, E. A. & Qin, H. Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PLOS ONE 7, e43118 (2012).
Kobayashi, T., Ishida, Y., Hirano, T., Katoh, Y. & Nakayama, K. Cooperation of the IFT-A complex with the IFT-B complex is required for ciliary retrograde protein trafficking and GPCR import. Mol. Biol. Cell 32, 45–56 (2021).
Jordan, M. A., Diener, D. R., Stepanek, L. & Pigino, G. The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 20, 1250 (2018).
Zhu, X., Wang, J., Li, S., Lechtreck, K. & Pan, J. IFT54 directly interacts with kinesin-II and IFT dynein to regulate anterograde intraflagellar transport. EMBO J. 40, e105781 (2021).
Mukhopadhyay, A. G. et al. Structure and tethering mechanism of dynein-2 intermediate chains in intraflagellar transport. EMBO J. 43, 1257–1272 (2024).
Funabashi, T., Katoh, Y., Okazaki, M., Sugawa, M. & Nakayama, K. Interaction of heterotrimeric kinesin-II with IFT-B-connecting tetramer is crucial for ciliogenesis. J. Cell Biol. 217, 2867–2876 (2018).
Engel, B. D., Ludington, W. B. & Marshall, W. F. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J. Cell Biol. 187, 81–89 (2009).
Sun, Y., Chen, Z., Jin, M., Xie, H. & Zhao, C. Ciliary length regulation by intraflagellar transport in zebrafish. eLife 13, RP93168 (2024).
Ludington, W. B., Wemmer, K. A., Lechtreck, K. F., Witman, G. B. & Marshall, W. F. Avalanche-like behavior in ciliary import. Proc. Natl Acad. Sci. USA 110, 3925–3930 (2013).
Dentler, W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 170, 649–659 (2005).
Liang, Y. et al. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev. Cell 30, 585–597 (2014).
Liang, Y., Zhu, X., Wu, Q. & Pan, J. Ciliary length sensing regulates IFT entry via changes in FLA8/KIF3B phosphorylation to control ciliary assembly. Curr. Biol. 28, 2429–2435.e3 (2018).
Boegholm, N. et al. The IFT81–IFT74 complex acts as an unconventional RabL2 GTPase-activating protein during intraflagellar transport. EMBO J. 42, e111807 (2023).
Nachury, M. V. & Mick, D. U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 20, 389–405 (2019).
Pan, X. et al. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J. Cell Biol. 174, 1035–1045 (2006).
Bhogaraju, S., Weber, K., Engel, B. D., Lechtreck, K.-F. & Lorentzen, E. Getting tubulin to the tip of the cilium: one IFT train, many different tubulin cargo-binding sites? Bioessays 36, 463–467 (2014).
Craft, J. M., Harris, J. A., Hyman, S., Kner, P. & Lechtreck, K. F. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J. Cell Biol. 208, 223–237 (2015).
Hao, L. et al. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 13, 790–798 (2011).
Craft Van De Weghe, J., Harris, J. A., Kubo, T., Witman, G. B. & Lechtreck, K. F. Diffusion rather than intraflagellar transport likely provides most of the tubulin required for axonemal assembly in Chlamydomonas. J. Cell Sci. 133, jcs249805 (2020).
Ling, L. & Goeddel, D. V. MIP-T3, a novel protein linking tumor necrosis factor receptor-associated factor 3 to the microtubule network. J. Biol. Chem. 275, 23852–23860 (2000).
Bizet, A. A. et al. Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat. Commun. 6, 8666 (2015).
Zhu, X., Liang, Y., Gao, F. & Pan, J. IFT54 regulates IFT20 stability but is not essential for tubulin transport during ciliogenesis. Cell. Mol. Life Sci. 74, 3425–3437 (2017).
Kubo, T. et al. Together, the IFT81 and IFT74 N-termini form the main module for intraflagellar transport of tubulin. J. Cell Sci. 129, 2106–2119 (2016).
Jiang, X. et al. DYF-5/MAK–dependent phosphorylation promotes ciliary tubulin unloading. Proc. Natl Acad. Sci. USA 119, e2207134119 (2022).
Yamamoto, R., Hwang, J., Ishikawa, T., Kon, T. & Sale, W. S. Composition and function of ciliary inner-dynein-arm subunits studied in Chlamydomonas reinhardtii. Cytoskeleton 78, 77–96 (2021).
Mali, G. R. et al. Shulin packages axonemal outer dynein arms for ciliary targeting. Science 371, 910–916 (2021).
Ahmed, N. T., Gao, C., Lucker, B. F., Cole, D. G. & Mitchell, D. R. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 183, 313–322 (2008).
Hou, Y. & Witman, G. B. The N-terminus of IFT46 mediates intraflagellar transport of outer arm dynein and its cargo-adaptor ODA16. Mol. Biol. Cell 28, 2420–2433 (2017).
Taschner, M., Mourão, A., Awasthi, M., Basquin, J. & Lorentzen, E. Structural basis of outer dynein arm intraflagellar transport by the transport adaptor protein ODA16 and the intraflagellar transport protein IFT46. J. Biol. Chem. 292, 7462–7473 (2017).
Solomon, G. M. et al. Assessment of ciliary phenotype in primary ciliary dyskinesia by micro-optical coherence tomography. JCI Insight 2, e91702 (2017).
Bearce, E. A. et al. Daw1 regulates the timely onset of cilia motility during development. Development 149, dev200017 (2022).
Wang, J. et al. Purification and crystal structure of human ODA16: implications for ciliary import of outer dynein arms by the intraflagellar transport machinery. Protein Sci. 29, 1502–1510 (2020).
Hunter, E. L. et al. The IDA3 adapter, required for intraflagellar transport of I1 dynein, is regulated by ciliary length. Mol. Biol. Cell 29, 886–896 (2018).
Lechtreck, K. F. et al. Chlamydomonas ARMC2/PF27 is an obligate cargo adapter for intraflagellar transport of radial spokes. eLife 11, e74993 (2022).
Zhao, Q., Li, S., Shao, S., Wang, Z. & Pan, J. FLS2 is a CDK-like kinase that directly binds IFT70 and is required for proper ciliary disassembly in Chlamydomonas. PLOS Genet. 16, e1008561 (2020).
Dai, J., Barbieri, F., Mitchell, D. R. & Lechtreck, K. F. In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties. Mol. Biol. Cell 29, 2553–2565 (2018).
Wren, K. N. et al. A differential cargo-loading model of ciliary length regulation by IFT. Curr. Biol. 23, 2463–2471 (2013).
Pan, J. & Snell, W. J. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev. Cell 9, 431–438 (2005).
Haque, F. et al. Cytoskeletal regulation of a transcription factor by DNA mimicry via coiled-coil interactions. Nat. Cell Biol. 24, 1088–1098 (2022).
Ku, P. et al. Collaborative role of two distinct cilium-specific cytoskeletal systems in driving Hedgehog-responsive transcription factor trafficking. Preprint at bioRxiv https://doi.org/10.1101/2024.09.26.615198 (2024).
He, M. et al. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat. Cell Biol. 16, 663–672 (2014).
Mukhopadhyay, S. et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes. Dev. 24, 2180–2193 (2010).
Berbari, N. F., Johnson, A. D., Lewis, J. S., Askwith, C. C. & Mykytyn, K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 19, 1540–1547 (2008).
Mukhopadhyay, S. et al. The ciliary G-protein-coupled receptor gpr161 negatively regulates the sonic hedgehog pathway via cAMP signaling. Cell 152, 210–223 (2013).
Reddy Palicharla, V. & Mukhopadhyay, S. Molecular and structural perspectives on protein trafficking to the primary cilium membrane. Biochem. Soc. Trans. 52, 1473–1487 (2024).
Badgandi, H. B., Hwang, S. H., Shimada, I. S., Loriot, E. & Mukhopadhyay, S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 216, 743–760 (2017).
Hong, J. J. et al. Differential roles of Tubby family proteins in ciliary formation and trafficking. Mol. Cell 44, 591–601 (2021).
Jia, D. et al. Tulp1 deficiency causes early-onset retinal degeneration through affecting ciliogenesis and activating ferroptosis in zebrafish. Cell Death Dis. 13, 1–11 (2022).
Chávez, M. et al. Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev. Cell 34, 338–350 (2015).
Garcia-Gonzalo, F. R. et al. Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev. Cell 34, 400–409 (2015).
Hwang, S.-H. et al. Tulp3 regulates renal cystogenesis by trafficking of cystoproteins to cilia. Curr. Biol. 29, 790–802.e5 (2019).
Legué, E. & Liem, K. F. Tulp3 is a ciliary trafficking gene that regulates polycystic kidney disease. Curr. Biol. 29, 803–812.e5 (2019).
Palicharla, V. R. et al. Interactions between TULP3 tubby domain and ARL13B amphipathic helix promote lipidated protein transport to cilia. Mol. Biol. Cell 34, ar18 (2023).
Eguether, T., Cordelieres, F. P. & Pazour, G. J. Intraflagellar transport is deeply integrated in hedgehog signaling. Mol. Biol. Cell 29, 1178–1189 (2018).
Caparrós-Martín, J. A. et al. Specific variants in WDR35 cause a distinctive form of Ellis–van Creveld syndrome by disrupting the recruitment of the EvC complex and SMO into the cilium. Hum. Mol. Genet. 24, 4126–4137 (2015).
Fu, W., Wang, L., Kim, S., Li, J. & Dynlacht, B. D. Role for the IFT-A complex in selective transport to the primary cilium. Cell Rep. 17, 1505–1517 (2016).
Picariello, T. et al. A global analysis of IFT-A function reveals specialization for transport of membrane-associated proteins into cilia. J. Cell. Sci. 132, jcs220749 (2019).
Jensen, V. L. & Leroux, M. R. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 47, 83–91 (2017).
Lechtreck, K.-F. et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117–1132 (2009).
Wei, Q. et al. The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 14, 950–957 (2012).
Nozaki, S., Araya, R. F. C., Katoh, Y. & Nakayama, K. Requirement of IFT-B–BBSome complex interaction in export of GPR161 from cilia. Biol. Open 8, bio043786 (2019).
Wang, J. et al. Assembly and stability of IFT-B complex and its function in BBSome trafficking. iScience 25, 105493 (2022).
Liew, G. M. et al. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev. Cell 31, 265–278 (2014).
Dong, B. et al. Chlamydomonas IFT25 is dispensable for flagellar assembly but required to export the BBSome from flagella. Biol. Open 6, 1680–1691 (2017).
Chien, A. et al. Dynamics of the IFT machinery at the ciliary tip. eLife 6, e28606 (2017).
Mijalkovic, J., Van Krugten, J., Oswald, F., Acar, S. & Peterman, E. J. G. Single-molecule turnarounds of intraflagellar transport at the C. elegans ciliary tip. Cell Rep. 25, 1701–1707.e2 (2018).
Wingfield, J. L. et al. In vivo imaging shows continued association of several IFT-A, IFT-B and dynein complexes while IFT trains U-turn at the tip. J. Cell Sci. 134, jcs259010 (2021).
Chaya, T. et al. Ccrk-Mak/Ick signaling is a ciliary transport regulator essential for retinal photoreceptor survival. Life Sci. Alliance 7, e202402880 (2024).
Matsushime, H., Jinno, A., Takagi, N. & Shibuya, M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol. Cell. Biol. 10, 2261–2268 (1990).
Asleson, C. M. & Lefebvre, P. A. Genetic analysis of flagellar length control in Chlamydomonas reinhardtii: a new long-flagella locus and extragenic suppressor mutations. Genetics 148, 693–702 (1998).
Berman, S. A., Wilson, N. F., Haas, N. A. & Lefebvre, P. A. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 13, 1145–1149 (2003).
Broekhuis, J. R., Verhey, K. J. & Jansen, G. Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells. PLOS ONE 9, e108470 (2014).
Chaya, T., Omori, Y., Kuwahara, R. & Furukawa, T. ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J. 33, 1227–1242 (2014).
Jiang, Y.-Y. et al. LF4/MOK and a CDK-related kinase regulate the number and length of cilia in Tetrahymena. PLOS Genet. 15, e1008099 (2019).
Maurya, A. K., Rogers, T. & Sengupta, P. A CCRK and a MAK kinase modulate cilia branching and length via regulation of axonemal microtubule dynamics in Caenorhabditis elegans. Curr. Biol. 29, 1286–1300.e4 (2019).
Nakamura, K. et al. Anterograde trafficking of ciliary MAP kinase–like ICK/CILK1 by the intraflagellar transport machinery is required for intraciliary retrograde protein trafficking. J. Biol. Chem. 295, 13363–13376 (2020).
Wang, Y., Ren, Y. & Pan, J. Regulation of flagellar assembly and length in Chlamydomonas by LF4, a MAPK-related kinase. FASEB J. 33, 6431–6441 (2019).
Noguchi, T., Nakamura, K., Satoda, Y., Katoh, Y. & Nakayama, K. CCRK/CDK20 regulates ciliary retrograde protein trafficking via interacting with BROMI/TBC1D32. PLOS ONE 16, e0258497 (2021).
Gailey, C. D. et al. Phosphosite T674A mutation in kinesin family member 3A fails to reproduce tissue and ciliary defects characteristic of CILK1 loss of function. Dev. Dyn. 250, 263–273 (2021).
Nievergelt, A. P. et al. Conversion of anterograde into retrograde trains is an intrinsic property of intraflagellar transport. Curr. Biol. 32, 4071–4078.e4 (2022).
Huet, D., Blisnick, T., Perrot, S. & Bastin, P. The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport. eLife 3, e02419 (2014).
Gonçalves-Santos, F. et al. Hot-wiring dynein-2 establishes roles for IFT-A in retrograde train assembly and motility. Cell Rep. 42, 113337 (2023).
Lechtreck, K. F. et al. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J. Cell Biol. 201, 249–261 (2013).
Zhang, Q., Seo, S., Bugge, K., Stone, E. M. & Sheffield, V. C. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum. Mol. Genet. 21, 1945–1953 (2012).
Domire, J. S. et al. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet–Biedl syndrome proteins. Cell. Mol. Life Sci. 68, 2951–2960 (2011).
Eguether, T. et al. IFT27 Links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev. Cell 31, 279–290 (2014).
Ye, F., Nager, A. R. & Nachury, M. V. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 217, 1847–1868 (2018).
Liu, Y.-X., Zhang, R.-K. & Fan, Z.-C. RABL4/IFT27 in a nucleotide-independent manner promotes phospholipase D ciliary retrieval via facilitating BBSome reassembly at the ciliary tip. J. Cell. Physiol. 238, 549–565 (2023).
Dai, J. et al. Loss of ARL13 impedes BBSome-dependent cargo export from Chlamydomonas cilia. J. Cell Biol. 221, e202201050 (2022).
Liu, Y.-X., Li, W.-J., Zhang, R.-K., Sun, S.-N. & Fan, Z.-C. Unraveling the intricate cargo–BBSome coupling mechanism at the ciliary tip. Proc. Natl Acad. Sci. USA 120, e2218819120 (2023).
Green, J. A. et al. Recruitment of β-arrestin into neuronal cilia modulates somatostatin receptor subtype 3 ciliary localization. Mol. Cell Biol. 36, 223–235 (2015).
Pal, K. et al. Smoothened determines β-arrestin–mediated removal of the G protein–coupled receptor Gpr161 from the primary cilium. J. Cell Biol. 212, 861–875 (2016).
Shinde, S. R., Nager, A. R. & Nachury, M. V. Ubiquitin chains earmark GPCRs for BBSome-mediated removal from cilia. J. Cell Biol. 219, e202003020 (2020).
Shinde, S. R. et al. The ancestral ESCRT protein TOM1L2 selects ubiquitinated cargoes for retrieval from cilia. Dev. Cell 58, 677–693.e9 (2023).
Desai, P. B., Stuck, M. W., Lv, B. & Pazour, G. J. Ubiquitin links smoothened to intraflagellar transport to regulate Hedgehog signaling. J. Cell Biol. 219, e201912104 (2020).
Lv, B., Stuck, M. W., Desai, P. B., Cabrera, O. A. & Pazour, G. J. E3 ubiquitin ligase Wwp1 regulates ciliary dynamics of the Hedgehog receptor Smoothened. J. Cell Biol. 220, e202010177 (2021).
Chiuso, F. et al. Ubiquitylation of BBSome is required for ciliary assembly and signaling. EMBO Rep. 24, e55571 (2023).
Huang, K., Diener, D. R. & Rosenbaum, J. L. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J. Cell Biol. 186, 601–613 (2009).
Langousis, G. et al. Loss of the BBSome perturbs endocytic trafficking and disrupts virulence of Trypanosoma brucei. Proc. Natl Acad. Sci. USA 113, 632–637 (2016).
Liu, P. & Lechtreck, K. F. The Bardet–Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc. Natl Acad. Sci. 115, E934–E943 (2018).
Qin, H., Diener, D. R., Geimer, S., Cole, D. G. & Rosenbaum, J. L. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 255–266 (2004).
Wang, Q., Peng, Z., Long, H., Deng, X. & Huang, K. Polyubiquitylation of α-tubulin at K304 is required for flagellar disassembly in Chlamydomonas. J. Cell Sci. 132, jcs229047 (2019).
Engel, B. D. et al. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J. Cell Biol. 199, 151–167 (2012).
Mirvis, M., Siemers, K. A., Nelson, W. J. & Stearns, T. P. Primary cilium loss in mammalian cells occurs predominantly by whole-cilium shedding. PLOS Biol. 17, e3000381 (2019).
Wang, G. et al. Rab7 regulates primary cilia disassembly through cilia excision. J. Cell Biol. 218, 4030–4041 (2019).
Long, H. et al. Comparative analysis of ciliary membranes and ectosomes. Curr. Biol. 26, 3327–3335 (2016).
Datta, P. et al. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet–Biedl syndrome. Proc. Natl Acad. Sci. USA 112, E4400–E4409 (2015).
Hsu, Y. et al. BBSome function is required for both the morphogenesis and maintenance of the photoreceptor outer segment. PLOS Genet. 13, e1007057 (2017).
Kwon, Y. T. & Ciechanover, A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem. Sci. 42, 873–886 (2017).
Mercey, O., Mukherjee, S., Guichard, P. & Hamel, V. The molecular architecture of the ciliary transition zones. Curr. Opin. Cell Biol. 88, 102361 (2024).
LeGuennec, M., Klena, N., Aeschlimann, G., Hamel, V. & Guichard, P. Overview of the centriole architecture. Curr. Opin. Struct. Biol. 66, 58–65 (2021).
Breslow, D. K. & Holland, A. J. Mechanism and regulation of centriole and cilium biogenesis. Annu. Rev. Biochem. 88, 691–724 (2019).
Kee, H. L. et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431–437 (2012).
Lin, Y.-C. et al. Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nat. Chem. Biol. 9, 437–443 (2013).
Breslow, D. K., Koslover, E. F., Seydel, F., Spakowitz, A. J. & Nachury, M. V. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol. 203, 129–147 (2013).
Gilula, N. B. & Satir, P. The ciliary necklace: a ciliary membrane specialization. J. Cell Biol. 53, 494–509 (1972).
Craige, B. et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 (2010).
Hu, Q. et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010).
Oswald, F., Prevo, B., Acar, S. & Peterman, E. J. G. Interplay between ciliary ultrastructure and ift-train dynamics revealed by single-molecule super-resolution imaging. Cell Rep. 25, 224–235 (2018).
Yang, T. T., Tran, M. N. T., Chong, W. M., Huang, C.-E. & Liao, J.-C. Single-particle tracking localization microscopy reveals nonaxonemal dynamics of intraflagellar transport proteins at the base of mammalian primary cilia. Mol. Biol. Cell 30, 828–837 (2019).
Scheidel, N. & Blacque, O. E. Intraflagellar transport complex a genes differentially regulate cilium formation and transition zone gating. Curr. Biol. 28, 3279–3287.e2 (2018).
Marshall, W. F. Chlamydomonas as a model system to study cilia and flagella using genetics, biochemistry, and microscopy. Front. Cell Dev. Biol. 12, 1412641 (2024).
Awasthi, M., Ranjan, P., Sharma, K., Veetil, S. K. & Kateriya, S. The trafficking of bacterial type rhodopsins into the Chlamydomonas eyespot and flagella is IFT mediated. Sci. Rep. 6, 34646 (2016).
Fujiu, K., Nakayama, Y., Iida, H., Sokabe, M. & Yoshimura, K. Mechanoreception in motile flagella of Chlamydomonas. Nat. Cell Biol. 13, 630–632 (2011).
Fujiu, K., Nakayama, Y., Yanagisawa, A., Sokabe, M. & Yoshimura, K. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133–139 (2009).
Huang, K. et al. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501–514 (2007).
Nauli, S. M. et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 (2003).
Beneke, T. et al. IFT and BBSome proteins are required for Leishmania mexicana pathogenicity, but flagellar motility is dispensable. Preprint at bioRxiv https://doi.org/10.1101/2024.09.13.612850 (2024).
Broadhead, R. et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440, 224–227 (2006).
Inglis, P. N., Ou, G., Leroux, M. R. & Scholey, J. M. The sensory cilia of Caenorhabditis elegans. In WormBook: The Online Review of C. elegans Biology (WormBook, 2018).
Omori, Y. et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10, 437–444 (2008).
Blacque, O. E. et al. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 15, 935–941 (2005).
Nonaka, S. et al. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998).
Binó, L. et al. A protocol for generation and live-cell imaging analysis of primary cilia reporter cell lines. STAR Protoc. 3, 101199 (2022).
Ocbina, P. J. R. & Anderson, K. V. Intraflagellar transport, cilia and mammalian hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 237, 2030–2038 (2008).
Baldassi, D., Gabold, B. & Merkel, O. Air–liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges and future directions. Adv. Nanobiomed. Res. 1, 2000111 (2021).
Acknowledgements
This work is supported by the Human Technopole and the European Research Council under the EU Horizon 2020 Research and Innovation Programme (grant number 819826) to G.P.
Author information
Authors and Affiliations
Contributions
S.E.L. researched data for the article. Both authors contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Saikat Mukhopadhyay, who co-reviewed with Venkata Vivek Reddy Palicharla, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Ciliopathies
-
A group of syndromes with diverse developmental phenotypes caused by mutations to genes involved in the formation and function of cilia.
- Cryo-electron tomography
-
An electron microscopy technique capable of visualizing cellular ultrastructure in three dimensions.
- Ectosomes
-
Small vesicles released from the tip of the cilium that can contain high concentrations of receptors and signalling proteins.
- Expansion microscopy
-
A super-resolution fluorescence microscopy technique in which a sample is cross-linked to an expandable hydrogel to physically separate the resolvable features.
- G-protein-coupled receptors
-
(GPCRs). Integral membrane proteins that transduce the binding of an extracellular agonist into an intracellular response through activation of cytoplasm G proteins.
- Polycystic kidney disease
-
A set of ciliopathy diseases characterized by the aberrant formation of cysts in the kidneys.
- Steady-state cilium
-
A full-length cilium that is neither growing or shrinking.
- Tetratricopeptide repeats
-
A short structural motif made up of antiparallel α-helices that is generally repeated in tandem to form a TPR domain; these often form solenoids or long helical bundles that mediate protein–protein interactions and allow for structural flexibility.
- Tryptophan–aspartic acid (WD) domains
-
β-propeller (annular) structural domains that often mediate protein–protein interactions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lacey, S.E., Pigino, G. The intraflagellar transport cycle. Nat Rev Mol Cell Biol 26, 175–192 (2025). https://doi.org/10.1038/s41580-024-00797-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-024-00797-x
This article is cited by
-
Function of manchette and intra-manchette transport in spermatogenesis and male fertility
Cell Communication and Signaling (2025)
-
Mutations in CFAP57 disrupt the localization of MYH10 and IFT88, leading to flagellogenesis failure in humans and mice
Human Genomics (2025)