Abstract
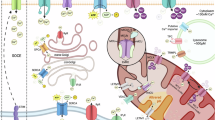
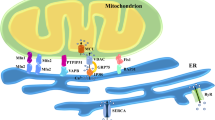
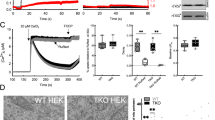
Activation of Ca2+ channels in Ca2+ stores in organelles and the plasma membrane generates cytoplasmic calcium ([Ca2+]c) signals that control almost every aspect of cell function, including metabolism, vesicle fusion and contraction. Mitochondria have a high capacity for Ca2+ uptake and chelation, alongside efficient Ca2+ release mechanisms. Still, mitochondria do not store Ca2+ in a prolonged manner under physiological conditions and lack the capacity to generate global [Ca2+]c signals. However, mitochondria take up Ca2+ at high local [Ca2+]c signals that originate from neighbouring organelles, and also during sustained global elevations of [Ca2+]c. Accumulated Ca2+ in the mitochondria stimulates oxidative metabolism and upon return to the cytoplasm, can produce spatially confined rises in [Ca2+]c to exert control over processes that are sensitive to Ca2+. Thus, the mitochondrial handling of [Ca2+]c is of physiological relevance. Furthermore, dysregulation of mitochondrial Ca2+ handling can contribute to debilitating diseases. We discuss the mechanisms and relevance of mitochondria in local and global calcium signals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 (2003).
Rizzuto, R. & Pozzan, T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006).
Spat, A., Szanda, G., Csordas, G. & Hajnoczky, G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium 44, 51–63 (2008).
Hoth, M., Fanger, C. M. & Lewis, R. S. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol. 137, 633–648 (1997).
Rizzuto, R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998).
Csordas, G., Thomas, A. P. & Hajnoczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 (1999).
Csordas, G. et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39, 121–132 (2010).
Varnai, P., Toth, B., Toth, D. J., Hunyady, L. & Balla, T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J. Biol. Chem. 282, 29678–29690 (2007).
Korzeniowski, M. K., Szanda, G., Balla, T. & Spat, A. Store-operated Ca2+ influx and subplasmalemmal mitochondria. Cell Calcium 46, 49–55 (2009).
Meschede, I. P. et al. Symmetric arrangement of mitochondria: plasma membrane contacts between adjacent photoreceptor cells regulated by Opa1. Proc. Natl Acad. Sci. USA 117, 15684–15693 (2020).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Naon, D. et al. Splice variants of mitofusin 2 shape the endoplasmic reticulum and tether it to mitochondria. Science 380, eadh9351 (2023).
De Vos, K. J. et al. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 21, 1299–1311 (2012).
Morotz, G. M. et al. The PTPIP51 coiled-coil domain is important in VAPB binding, formation of ER-mitochondria contacts and IP3 receptor delivery of Ca2+ to mitochondria. Front. Cell Dev. Biol. 10, 920947 (2022).
Yuan, Y. et al. Two-pore channel-2 and inositol trisphosphate receptors coordinate Ca2+ signals between lysosomes and the endoplasmic reticulum. Cell Rep. 43, 113628 (2024).
Ramazanov, B. R. et al. Calcium flow at ER–TGN contact sites facilitates secretory cargo export. Mol. Biol. Cell 35, ar50 (2024).
Pizzo, P., Lissandron, V., Capitanio, P. & Pozzan, T. Ca2+ signalling in the Golgi apparatus. Cell Calcium 50, 184–192 (2011).
Calvo-Rodriguez, M. et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 2146 (2020).
Pfeiffer, D. R., Gunter, T. E., Eliseev, R., Broekemeier, K. M. & Gunter, K. K. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life 52, 205–212 (2001).
Bernardi, P. et al. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 30, 1869–1885 (2023).
Vercesi, A. E. et al. Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radic. Biol. Med. 129, 1–24 (2018).
David, G., Barrett, J. N. & Barrett, E. F. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J. Physiol. 509, 59–65 (1998).
Wan, Q. F., Nixon, E. & Heidelberger, R. Regulation of presynaptic calcium in a mammalian synaptic terminal. J. Neurophysiol. 108, 3059–3067 (2012).
Divakaruni, S. S. et al. Long-term potentiation requires a rapid burst of dendritic mitochondrial fission during induction. Neuron 100, 860–875.e7 (2018).
Lewis, T. L. Jr, Kwon, S. K., Lee, A., Shaw, R. & Polleux, F. MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun. 9, 5008 (2018).
Deluca, H. F. & Engstrom, G. W. Calcium uptake by rat kidney mitochondria. Proc. Natl Acad. Sci. USA 47, 1744–1750 (1961).
Vasington, F. D. Calcium ion uptake by fragments of rat liver mitochondria and its dependence on electron transport. J. Biol. Chem. 238, 1841–1847 (1963).
Carafoli, E. & Lehninger, A. L. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem. J. 122, 681–690 (1971).
Denton, R. M. & McCormack, J. G. The role of calcium in the regulation of mitochondrial metabolism. Biochem. Soc. Trans. 8, 266–268 (1980).
Gunter, T. E. & Pfeiffer, D. R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 (1990).
Nicholls, D. G. & Crompton, M. Mitochondrial calcium transport. FEBS Lett. 111, 261–268 (1980).
Hunter, D. R., Haworth, R. A. & Southard, J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 251, 5069–5077 (1976).
Chien, K. R., Abrams, J., Pfau, R. G. & Farber, J. L. Prevention by chlorpromazine of ischemic liver cell death. Am. J. Pathol. 88, 539–557 (1977).
Tsien, R. Y., Pozzan, T. & Rink, T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J. Cell Biol. 94, 325–334 (1982).
Pozzan, T., Arslan, P., Tsien, R. Y. & Rink, T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J. Cell Biol. 94, 335–340 (1982).
Somlyo, A. P., Bond, M. & Somlyo, A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature 314, 622–625 (1985).
Rizzuto, R., Simpson, A. W., Brini, M. & Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–327 (1992).
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993).
Minta, A., Kao, J. P. & Tsien, R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 264, 8171–8178 (1989).
Burnier, M., Centeno, G., Burki, E. & Brunner, H. R. Confocal microscopy to analyze cytosolic and nuclear calcium in cultured vascular cells. Am. J. Physiol. 266, C1118–C1127 (1994).
Pralong, W. F., Spat, A. & Wollheim, C. B. Dynamic pacing of cell metabolism by intracellular Ca2+ transients. J. Biol. Chem. 269, 27310–27314 (1994).
Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995).
Szalai, G., Krishnamurthy, R. & Hajnoczky, G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18, 6349–6361 (1999).
Abramov, A. Y. & Duchen, M. R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim. Biophys. Acta 1777, 953–964 (2008).
Meldolesi, J. & Pozzan, T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci. 23, 10–14 (1998).
Duchen, M. R., Leyssens, A. & Crompton, M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J. Cell Biol. 142, 975–988 (1998).
Booth, D. M., Varnai, P., Joseph, S. K. & Hajnoczky, G. Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol. Cell 81, 3866–3876.e2 (2021).
Santo-Domingo, J., Giacomello, M., Poburko, D., Scorrano, L. & Demaurex, N. OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 32, 1927–1940 (2013).
Hoyt, K. R., McLaughlin, B. A., Higgins, D. S. Jr & Reynolds, I. J. Inhibition of glutamate-induced mitochondrial depolarization by tamoxifen in cultured neurons. J. Pharmacol. Exp. Ther. 293, 480–486 (2000).
Jouaville, L. S., Ichas, F., Holmuhamedov, E. L., Camacho, P. & Lechleiter, J. D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377, 438–441 (1995).
Hajnoczky, G., Hager, R. & Thomas, A. P. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+. J. Biol. Chem. 274, 14157–14162 (1999).
Gilabert, J. A. & Parekh, A. B. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J. 19, 6401–6407 (2000).
Hoth, M., Button, D. C. & Lewis, R. S. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl Acad. Sci. USA 97, 10607–10612 (2000).
Tinel, H. et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 18, 4999–5008 (1999).
Arnaudeau, S., Kelley, W. L., Walsh, J. V. Jr & Demaurex, N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276, 29430–29439 (2001).
Boitier, E., Rea, R. & Duchen, M. R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 145, 795–808 (1999).
Opuni, K. & Reeves, J. P. Feedback inhibition of sodium/calcium exchange by mitochondrial calcium accumulation. J. Biol. Chem. 275, 21549–21554 (2000).
Babcock, D. F., Herrington, J., Goodwin, P. C., Park, Y. B. & Hille, B. Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol. 136, 833–844 (1997).
Kirichok, Y., Krapivinsky, G. & Clapham, D. E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
Sancak, Y. et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013).
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010).
Plovanich, M. et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE 8, e55785 (2013).
Kamer, K. J., Grabarek, Z. & Mootha, V. K. High-affinity cooperative Ca2+ binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Rep. 18, 1397–1411 (2017).
Raffaello, A. et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 467, 2362–2376 (2013).
Palty, R. et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441 (2010).
Austin, S. et al. TMBIM5 is the Ca2+/H+ antiporter of mammalian mitochondria. EMBO Rep. 23, e54978 (2022).
Pan, X. et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–1472 (2013).
Antony, A. N. et al. MICU1 regulation of mitochondrial Ca2+ uptake dictates survival and tissue regeneration. Nat. Commun. 7, 10955 (2016).
Luongo, T. S. et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545, 93–97 (2017).
Logan, C. V. et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 46, 188–193 (2014).
Lewis-Smith, D. et al. Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Neurol. Genet. 2, e59 (2016).
Suzuki, J. et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 5, 4153 (2014).
Raffaello, A., Mammucari, C., Gherardi, G. & Rizzuto, R. Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 41, 1035–1049 (2016).
Emrich, S. M., Yoast, R. E. & Trebak, M. Physiological functions of CRAC channels. Annu. Rev. Physiol. 84, 355–379 (2022).
Qiu, R. & Lewis, R. S. Structural features of STIM and Orai underlying store-operated calcium entry. Curr. Opin. Cell Biol. 57, 90–98 (2019).
Gudlur, A. & Hogan, P. in Calcium Entry Channels in Non-Excitable Cells. Ch. 3 (eds Kozak J.A. & Putney J.W. Jr) 51–72 (Taylor & Francis, 2018).
Catterall, W. A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3, a003947 (2011).
Nanou, E. & Catterall, W. A. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 98, 466–481 (2018).
Dolphin, A. C. & Lee, A. Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 21, 213–229 (2020).
Mony, L. & Paoletti, P. Mechanisms of NMDA receptor regulation. Curr. Opin. Neurobiol. 83, 102815 (2023).
Baker, M. R., Fan, G., Arige, V., Yule, D. I. & Serysheva, I. I. Understanding IP3R channels: from structural underpinnings to ligand-dependent conformational landscape. Cell Calcium 114, 102770 (2023).
Foskett, J. K., White, C., Cheung, K. H. & Mak, D. O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658 (2007).
Prole, D. L. & Taylor, C. W. Structure and function of IP3 receptors. Cold Spring Harb. Perspect. Biol. 11, a035063 (2019).
Woll, K. A. & Van Petegem, F. Calcium-release channels: structure and function of IP3 receptors and ryanodine receptors. Physiol. Rev. 102, 209–268 (2022).
Smith, I. F., Swaminathan, D., Dickinson, G. D. & Parker, I. Single-molecule tracking of inositol trisphosphate receptors reveals different motilities and distributions. Biophys. J. 107, 834–845 (2014).
Thillaiappan, N. B., Chavda, A. P., Tovey, S. C., Prole, D. L. & Taylor, C. W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 8, 1505 (2017).
Thillaiappan, N. B., Smith, H. A., Atakpa-Adaji, P. & Taylor, C. W. KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals. Nat. Commun. 12, 4514 (2021).
Eisner, D., Neher, E., Taschenberger, H. & Smith, G. Physiology of intracellular calcium buffering. Physiol. Rev. 103, 2767–2845 (2023).
Schwaller, B. Cytosolic Ca2+ buffers are inherently Ca2+ signal modulators. Cold Spring Harb. Perspect. Biol. 12, a035543 (2020).
Woehler, A., Lin, K. H. & Neher, E. Calcium-buffering effects of gluconate and nucleotides, as determined by a novel fluorimetric titration method. J. Physiol. 592, 4863–4875 (2014).
Baylor, S. M. & Hollingworth, S. Model of sarcomeric Ca2+ movements, including ATP Ca2+ binding and diffusion, during activation of frog skeletal muscle. J. Gen. Physiol. 112, 297–316 (1998).
Chen, G. et al. Deficiency in parvalbumin, but not in calbindin D-28k upregulates mitochondrial volume and decreases smooth endoplasmic reticulum surface selectively in a peripheral, subplasmalemmal region in the soma of Purkinje cells. Neuroscience 142, 97–105 (2006).
Chen, G. et al. Deficiency in parvalbumin increases fatigue resistance in fast-twitch muscle and upregulates mitochondria. Am. J. Physiol. Cell Physiol. 281, C114–C122 (2001).
Tan, W. & Colombini, M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta 1768, 2510–2515 (2007).
Bathori, G., Csordas, G., Garcia-Perez, C., Davies, E. & Hajnoczky, G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 281, 17347–17358 (2006).
Rosencrans, W. M., Rajendran, M., Bezrukov, S. M. & Rostovtseva, T. K. VDAC regulation of mitochondrial calcium flux: from channel biophysics to disease. Cell Calcium 94, 102356 (2021).
Sander, P., Gudermann, T. & Schredelseker, J. A calcium guard in the outer membrane: is VDAC a regulated gatekeeper of mitochondrial calcium uptake? Int. J. Mol. Sci. 22, 946 (2021).
Filadi, R. et al. TOM70 sustains cell bioenergetics by promoting IP3R3-mediated ER to mitochondria Ca2+ transfer. Curr. Biol. 28, 369–382.e6 (2018).
Fan, M. et al. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 582, 129–133 (2020).
Rodriguez-Prados, M. et al. MICU1 occludes the mitochondrial calcium uniporter in divalent-free conditions. Proc. Natl Acad. Sci. USA 120, e2218999120 (2023).
Tsai, C. W. et al. Evidence supporting the MICU1 occlusion mechanism and against the potentiation model in the mitochondrial calcium uniporter complex. Proc. Natl Acad. Sci. USA 120, e2217665120 (2023).
Kamer, K. J., Jiang, W., Kaushik, V. K., Mootha, V. K. & Grabarek, Z. Crystal structure of MICU2 and comparison with MICU1 reveal insights into the uniporter gating mechanism. Proc. Natl Acad. Sci. USA 116, 3546–3555 (2019).
Kamer, K. J. & Mootha, V. K. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 15, 299–307 (2014).
Patron, M., Granatiero, V., Espino, J., Rizzuto, R. & De Stefani, D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 26, 179–195 (2019).
Csordas, G. et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 17, 976–987 (2013).
Jiang, L. et al. A quantitative proteome map of the human body. Cell 183, 269–283.e19 (2020).
Paillard, M. et al. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep. 18, 2291–2300 (2017).
Tsai, C. W. et al. Mechanisms and significance of tissue-specific MICU regulation of the mitochondrial calcium uniporter complex. Mol. Cell 82, 3661–3676.e8 (2022).
Hasan, P. et al. MICU1 and MICU2 control mitochondrial calcium signaling in the mammalian heart. Proc. Natl Acad. Sci. USA 121, e2402491121 (2024).
Liu, J. C. et al. MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell Rep. 16, 1561–1573 (2016).
Singh, R. et al. Uncontrolled mitochondrial calcium uptake underlies the pathogenesis of neurodegeneration in MICU1-deficient mice and patients. Sci. Adv. 8, eabj4716 (2022).
Delgado de la Herran, H. et al. Systematic mapping of mitochondrial calcium uniporter channel (MCUC)-mediated calcium signaling networks. EMBO J. 43, 5288–5326 (2024).
Hamilton, J., Brustovetsky, T. & Brustovetsky, N. The effect of mitochondrial calcium uniporter and cyclophilin D knockout on resistance of brain mitochondria to Ca2+-induced damage. J. Biol. Chem. 296, 100669 (2021).
Petersen, C. E. et al. Increased mitochondrial free Ca2+ during ischemia is suppressed, but not eliminated by, germline deletion of the mitochondrial Ca2+ uniporter. Cell Rep. 42, 112735 (2023).
Bround, M. J. et al. MCU-independent Ca2+ uptake mediates mitochondrial Ca2+ overload and necrotic cell death in a mouse model of Duchenne muscular dystrophy. Sci. Rep. 14, 6751 (2024).
Garbincius, J. F. et al. TMEM65 regulates NCLX-dependent mitochondrial calcium efflux. Preprint at bioRxiv https://doi.org/10.1101/2023.10.06.561062 (2023).
Vetralla, M. et al. TMEM65-dependent Ca2+ extrusion safeguards mitochondrial homeostasis. Preprint at bioRxiv https://doi.org/10.1101/2023.10.10.561661 (2023).
Zhang, Y. et al. Loss of TMEM65 causes mitochondrial disease mediated by mitochondrial calcium. Preprint at bioRxiv https://doi.org/10.1101/2022.08.02.502535 (2023).
Patron, M. et al. Regulation of mitochondrial proteostasis by the proton gradient. EMBO J. 41, e110476 (2022).
Zhang, L. et al. TMBIM5 loss of function alters mitochondrial matrix ion homeostasis and causes a skeletal myopathy. Life Sci. Alliance 5, e202201478 (2022).
Gunter, T. E., Chace, J. H., Puskin, J. S. & Gunter, K. K. Mechanism of sodium independent calcium efflux from rat liver mitochondria. Biochemistry 22, 6341–6351 (1983).
Tsai, M. F., Jiang, D., Zhao, L., Clapham, D. & Miller, C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J. Gen. Physiol. 143, 67–73 (2014).
Austin, S. et al. LETM1-mediated K+ and Na+ homeostasis regulates mitochondrial Ca2+ efflux. Front. Physiol. 8, 839 (2017).
De Marchi, U. et al. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J. Biol. Chem. 289, 20377–20385 (2014).
Natarajan, G. K. et al. Total matrix Ca2+ modulates Ca2+ efflux via the Ca2+/H+ exchanger in cardiac mitochondria. Front. Physiol. 11, 510600 (2020).
Lu, X., Kwong, J. Q., Molkentin, J. D. & Bers, D. M. Individual cardiac mitochondria undergo rare transient permeability transition pore openings. Circ. Res. 118, 834–841 (2016).
De Marchi, E., Bonora, M., Giorgi, C. & Pinton, P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium 56, 1–13 (2014).
Bround, M. J., Bers, D. M. & Molkentin, J. D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell Cardiol. 144, A3–A13 (2020).
Smith, R. M. & Martell, A. E. in Critical Stability Constants (Plenum, 1989).
Sokolove, P. M., Brenza, J. M. & Shamoo, A. E. Ca2+-cardiolipin interaction in a model system. Selectivity and apparent high affinity. Biochim. Biophys. Acta 732, 41–47 (1983).
Nicholls, D. G. & Chalmers, S. The integration of mitochondrial calcium transport and storage. J. Bioenerg. Biomembr. 36, 277–281 (2004).
Greenawalt, J. W., Rossi, C. S. & Lehninger, A. L. Effect of active accumulation of calcium and phosphate ions on the structure of rat liver mitochondria. J. Cell Biol. 23, 21–38 (1964).
Seifert, E. L., Ligeti, E., Mayr, J. A., Sondheimer, N. & Hajnoczky, G. The mitochondrial phosphate carrier: role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 464, 369–375 (2015).
Brenza, J. M., Neagle, C. E. & Sokolove, P. M. Interaction of Ca2+ with cardiolipin-containing liposomes and its inhibition by adriamycin. Biochem. Pharmacol. 34, 4291–4298 (1985).
Golla, V. K., Boyd, K. J. & May, E. R. Curvature sensing lipid dynamics in a mitochondrial inner membrane model. Commun. Biol. 7, 29 (2024).
Ghosh, S. et al. MCU-complex-mediated mitochondrial calcium signaling is impaired in Barth syndrome. Hum. Mol. Genet. 31, 376–385 (2022).
Csordas, G. & Hajnoczky, G. Plasticity of mitochondrial calcium signaling. J. Biol. Chem. 278, 42273–42282 (2003).
Basso, E., Rigotto, G., Zucchetti, A. E. & Pozzan, T. Slow activation of fast mitochondrial Ca2+ uptake by cytosolic Ca(2). J. Biol. Chem. 293, 17081–17094 (2018).
Debattisti, V. et al. Dysregulation of mitochondrial Ca2+ uptake and sarcolemma repair underlie muscle weakness and wasting in patients and mice lacking MICU1. Cell Rep. 29, 1274–1286 (2019).
Rodriguez-Prados, M. et al. MICU1 controls the sensitivity of the mitochondrial Ca2+ uniporter to activators and inhibitors. Cell Chem. Biol. 30, 606–617 (2023).
Santulli, G., Xie, W., Reiken, S. R. & Marks, A. R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl Acad. Sci. USA 112, 11389–11394 (2015).
Thangaratnarajah, C., Ruprecht, J. J. & Kunji, E. R. S. Calcium-induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers. Nat. Commun. 5, 5491 (2014).
Perez-Liebana, I. et al. A Ca2+-dependent mechanism boosting glycolysis and OXPHOS by activating aralar-malate-aspartate shuttle, upon neuronal stimulation. J. Neurosci. 42, 3879–3895 (2022).
Koshenov, Z. et al. Citrin mediated metabolic rewiring in response to altered basal subcellular Ca2+ homeostasis. Commun. Biol. 5, 76 (2022).
Harborne, S. P., King, M. S., Crichton, P. G. & Kunji, E. R. Calcium regulation of the human mitochondrial ATP-Mg/Pi carrier SLC25A24 uses a locking pin mechanism. Sci. Rep. 7, 45383 (2017).
Denton, R. M. & McCormack, J. G. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium 7, 377–386 (1986).
Schuster, S. M. & Olson, M. S. The regulation of pyruvate dehydrogenase in isolated beef heart mitochondria. The role of calcium, magnesium, and permeant anions. J. Biol. Chem. 249, 7159–7165 (1974).
Denton, R. M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 1787, 1309–1316 (2009).
Rutter, G. A. & Denton, R. M. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem. J. 252, 181–189 (1988).
Rusinol, A. E., Cui, Z., Chen, M. H. & Vance, J. E. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 269, 27494–27502 (1994).
Csordas, G. et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 (2006).
Lock, J. T., Smith, I. F. & Parker, I. Spatial-temporal patterning of Ca2+ signals by the subcellular distribution of IP3 and IP3 receptors. Semin. Cell Dev. Biol. 94, 3–10 (2019).
Parker, I., Choi, J. & Yao, Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium 20, 105–121 (1996).
Pacher, P., Thomas, A. P. & Hajnoczky, G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc. Natl Acad. Sci. USA 99, 2380–2385 (2002).
Thomas, A. P., Bird, G. S., Hajnoczky, G., Robb-Gaspers, L. D. & Putney, J. W. Jr. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 10, 1505–1517 (1996).
Luik, R. M., Wu, M. M., Buchanan, J. & Lewis, R. S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 (2006).
Franzini-Armstrong, C. ER-mitochondria communication. how privileged? Physiology 22, 261–268 (2007).
Giacomello, M. et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 38, 280–290 (2010).
Booth, D. M., Enyedi, B., Geiszt, M., Varnai, P. & Hajnoczky, G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol. Cell 63, 240–248 (2016).
Malli, R., Frieden, M., Trenker, M. & Graier, W. F. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J. Biol. Chem. 280, 12114–12122 (2005).
Gutierrez, T. et al. The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci. Signal. 13, eaax6660 (2020).
Krols, M., Bultynck, G. & Janssens, S. ER-Mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J. Cell Biol. 214, 367–370 (2016).
Wheeler, D. G. et al. CaV1 and CaV2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149, 1112–1124 (2012).
Malli, R., Frieden, M., Osibow, K. & Graier, W. F. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 278, 10807–10815 (2003).
Zajac, M. et al. A mechanism of lysosomal calcium entry. Sci. Adv. 10, eadk2317 (2024).
Giamogante, F. et al. A SPLICS reporter reveals α-synuclein regulation of lysosome-mitochondria contacts which affects TFEB nuclear translocation. Nat. Commun. 15, 1516 (2024).
Peng, W., Wong, Y. C. & Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl Acad. Sci. USA 117, 19266–19275 (2020).
Van Baelen, K. et al. The contribution of the SPCA1 Ca2+ pump to the Ca2+ accumulation in the Golgi apparatus of HeLa cells assessed via RNA-mediated interference. Biochem. Biophys. Res. Commun. 306, 430–436 (2003).
Voeltz, G. K., Sawyer, E. M., Hajnoczky, G. & Prinz, W. A. Making the connection: how membrane contact sites have changed our view of organelle biology. Cell 187, 257–270 (2024).
Scorrano, L. et al. Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019).
Csordas, G., Weaver, D. & Hajnoczky, G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523–540 (2018).
Szalai, G., Csordas, G., Hantash, B. M., Thomas, A. P. & Hajnoczky, G. Calcium signal transmission between ryanodine receptors and mitochondria. J. Biol. Chem. 275, 15305–15313 (2000).
Ramesh, V., Sharma, V. K., Sheu, S. S. & Franzini-Armstrong, C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann. NY Acad. Sci. 853, 341–344 (1998).
Szabadkai, G. et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911 (2006).
Mendes, C. C. et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J. Biol. Chem. 280, 40892–40900 (2005).
Hayashi, T. & Su, T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610 (2007).
Liu, Y. et al. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc. Natl Acad. Sci. USA 116, 25322–25328 (2019).
Carreras-Sureda, A. et al. Non-canonical function of IRE1α determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat. Cell Biol. 21, 755–767 (2019).
Erustes, A. G. et al. Overexpression of alpha-synuclein inhibits mitochondrial Ca2+ trafficking between the endoplasmic reticulum and mitochondria through MAMs by altering the GRP75-IP3R interaction. J. Neurosci. Res. 99, 2932–2947 (2021).
D’Eletto, M. et al. Transglutaminase type 2 regulates ER-mitochondria contact sites by interacting with GRP75. Cell Rep. 25, 3573–3581.e4 (2018).
Wu, S. et al. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation 136, 2248–2266 (2017).
Berridge, M. J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 96, 1261–1296 (2016).
Prole, D. L. & Taylor, C. W. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J. Physiol. 594, 2849–2866 (2016).
Joseph, S. K., Booth, D. M., Young, M. P. & Hajnoczky, G. Redox regulation of ER and mitochondrial Ca2+ signaling in cell survival and death. Cell Calcium 79, 89–97 (2019).
Ivanova, H. et al. Bcl-2-Protein Family as Modulators of IP3 Receptors and Other Organellar Ca2+ Channels. Cold Spring Harb. Perspect. Biol. 12, a035089 (2020).
Lock, J. T., Alzayady, K. J., Yule, D. I. & Parker, I. All three IP3 receptor isoforms generate Ca2+ puffs that display similar characteristics. Sci. Signal. 11, eaau0344 (2018).
Bartok, A. et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 10, 3726 (2019).
Katona, M. et al. Capture at the ER-mitochondrial contacts licenses IP3 receptors to stimulate local Ca2+ transfer and oxidative metabolism. Nat. Commun. 13, 6779 (2022).
Naghdi, S. & Hajnoczky, G. VDAC2-specific cellular functions and the underlying structure. Biochim. Biophys. Acta 1863, 2503–2514 (2016).
Eisner, V., Csordas, G. & Hajnoczky, G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle — pivotal roles in Ca2+ and reactive oxygen species signaling. J. Cell Sci. 126, 2965–2978 (2013).
Hall, A. R. et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 7, e2238 (2016).
Inagaki, S. et al. Mitofusin 2 positively regulates Ca2+ signaling by tethering the sarcoplasmic reticulum and mitochondria in rat aortic smooth muscle cells. Am. J. Physiol. Cell Physiol. 323, C295–C305 (2022).
Filadi, R. et al. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl Acad. Sci. USA 112, E2174–E2181 (2015).
Gomez-Suaga, P. et al. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr. Biol. 27, 371–385 (2017).
Yeo, H. K. et al. Phospholipid transfer function of PTPIP51 at mitochondria-associated ER membranes. EMBO Rep. 22, e51323 (2021).
Kornmann, B. et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481 (2009).
Hirabayashi, Y. et al. ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 358, 623–630 (2017).
Lee, K. S. et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc. Natl Acad. Sci. USA 115, E8844–E8853 (2018).
Covill-Cooke, C. et al. Shared structural features of Miro binding control mitochondrial homeostasis. EMBO J. 43, 595–614 (2024).
Guillen-Samander, A. et al. VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J. Cell Biol. 220, e202010004 (2021).
Fernandez-Busnadiego, R., Saheki, Y. & De Camilli, P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc. Natl Acad. Sci. USA 112, E2004–E2013 (2015).
Janer, A. et al. ESYT1 tethers the ER to mitochondria and is required for mitochondrial lipid and calcium homeostasis. Life Sci. Alliance 7, e202302335 (2024).
Carpio, M. A. et al. BOK controls apoptosis by Ca2+ transfer through ER-mitochondrial contact sites. Cell Rep. 34, 108827 (2021).
Lucendo, E. et al. Mcl-1 and Bok transmembrane domains: unexpected players in the modulation of apoptosis. Proc. Natl Acad. Sci. USA 117, 27980–27988 (2020).
Doghman-Bouguerra, M. et al. FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep. 17, 1264–1280 (2016).
Schober, R. et al. STIM1 and Orai1 regulate Ca2+ microdomains for activation of transcription. Biochim. Biophys. Acta Mol. Cell Res. 1866, 1079–1091 (2019).
Varadi, A., Cirulli, V. & Rutter, G. A. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium 36, 499–508 (2004).
Ben-Kasus Nissim, T. et al. Mitochondria control store-operated Ca2+ entry through Na+ and redox signals. EMBO J. 36, 797–815 (2017).
Yoast, R. E. et al. The mitochondrial Ca2+ uniporter is a central regulator of interorganellar Ca2+ transfer and NFAT activation. J. Biol. Chem. 297, 101174 (2021).
Frieden, M., Arnaudeau, S., Castelbou, C. & Demaurex, N. Subplasmalemmal mitochondria modulate the activity of plasma membrane Ca2+-ATPases. J. Biol. Chem. 280, 43198–43208 (2005).
Poburko, D., Liao, C. H., van Breemen, C. & Demaurex, N. Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells. Circ. Res. 104, 104–112 (2009).
Malli, R. et al. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 278, 44769–44779 (2003).
Quintana, A. et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 30, 3895–3912 (2011).
Schwindling, C., Quintana, A., Krause, E. & Hoth, M. Mitochondria positioning controls local calcium influx in T cells. J. Immunol. 184, 184–190 (2010).
Glancy, B. et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 19, 487–496 (2017).
Billups, B. & Forsythe, I. D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 22, 5840–5847 (2002).
Kwon, S. K. et al. LKB1 regulates mitochondria-dependent presynaptic calcium clearance and neurotransmitter release properties at excitatory synapses along cortical axons. PLoS Biol. 14, e1002516 (2016).
Vaccaro, V., Devine, M. J., Higgs, N. F. & Kittler, J. T. Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO Rep. 18, 231–240 (2017).
Devine, M. J. et al. Mitochondrial Ca2+ uniporter haploinsufficiency enhances long-term potentiation at hippocampal mossy fibre synapses. J. Cell Sci. 135, jcs259823 (2022).
Morgan, A. J., Davis, L. C. & Galione, A. Choreographing endo-lysosomal Ca2+ throughout the life of a phagosome. Biochim. Biophys. Acta Mol. Cell Res. 1868, 119040 (2021).
Cantarero, L., Garcia-Vargas, G., Hoenicka, J. & Palau, F. Differential effects of Mendelian GDAP1 clinical variants on mitochondria-lysosome membrane contacts sites. Biol. Open 12, bio059707 (2023).
Dolman, N. J. et al. Stable Golgi-mitochondria complexes and formation of Golgi Ca2+ gradients in pancreatic acinar cells. J. Biol. Chem. 280, 15794–15799 (2005).
Loncke, J. et al. Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. 31, 598–612 (2021).
Garbincius, J. F. & Elrod, J. W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 102, 893–992 (2022).
Young, M. P. et al. Metabolic adaptation to the chronic loss of Ca2+ signaling induced by KO of IP3 receptors or the mitochondrial Ca2+ uniporter. J. Biol. Chem. 298, 101436 (2022).
Groten, C. J. & MacVicar, B. A. Mitochondrial Ca2+ uptake by the MCU facilitates pyramidal neuron excitability and metabolism during action potential firing. Commun. Biol. 5, 900 (2022).
Musa, S. et al. A middle eastern founder mutation expands the genotypic and phenotypic spectrum of mitochondrial MICU1 deficiency: a report of 13 patients. JIMD Rep. 43, 79–83 (2019).
Kohlschmidt, N. et al. Molecular pathophysiology of human MICU1-deficiency. Neuropathol. Appl. Neurobiol. 47, 840–855 (2021).
Bitarafan, F. et al. Identification of a novel MICU1 nonsense variant causes myopathy with extrapyramidal signs in an Iranian consanguineous family. Mol. Cell Pediatr. 8, 6 (2021).
Mojbafan, M. et al. Reporting a rare form of myopathy, myopathy with extrapyramidal signs, in an Iranian family using next generation sequencing: a case report. BMC Med. Genet. 21, 77 (2020).
Wilton, K. M. et al. Developmental brain abnormalities and acute encephalopathy in a patient with myopathy with extrapyramidal signs secondary to pathogenic variants in MICU1. JIMD Rep. 53, 22–28 (2020).
Mallilankaraman, K. et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates Cell survival. Cell 151, 630–644 (2012).
Tomar, D. et al. MICU1 regulates mitochondrial cristae structure and function independently of the mitochondrial Ca2+ uniporter channel. Sci. Signal. 16, eabi8948 (2023).
Gottschalk, B. et al. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat. Commun. 10, 3732 (2019).
Bick, A. G. et al. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proc. Natl Acad. Sci. USA 114, E9096–E9104 (2017).
Vishnu, N. et al. Mitochondrial clearance of calcium facilitated by MICU2 controls insulin secretion. Mol. Metab. 51, 101239 (2021).
Shamseldin, H. E. et al. A null mutation in MICU2 causes abnormal mitochondrial calcium homeostasis and a severe neurodevelopmental disorder. Brain 140, 2806–2813 (2017).
Payne, R., Hoff, H., Roskowski, A. & Foskett, J. K. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1-mediated inhibition and activation of MCU. Cell Rep. 21, 3141–3154 (2017).
Puente, B. N. et al. MICU3 plays an important role in cardiovascular function. Circ. Res. 127, 1571–1573 (2020).
Liu, J. C. et al. EMRE is essential for mitochondrial calcium uniporter activity in a mouse model. JCI Insight 5, e134063 (2020).
Bulthuis, E. P. et al. SMDT1 variants impair EMRE-mediated mitochondrial calcium uptake in patients with muscle involvement. Biochim. Biophys. Acta Mol. Basis Dis. 1869, 166808 (2023).
Larrea, D. et al. MFN2 mutations in Charcot-Marie-Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum. Mol. Genet. 28, 1782–1800 (2019).
Kuo, I. Y. et al. Polycystin 2 regulates mitochondrial Ca2+ signaling, bioenergetics, and dynamics through mitofusin 2. Sci. Signal. 12, eaat7397 (2019).
Angebault, C. et al. ER-mitochondria cross-talk is regulated by the Ca2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci. Signal. 11, eaaq1380 (2018).
Patergnani, S. et al. The Wolfram-like variant WFS1E864K destabilizes MAM and compromises autophagy and mitophagy in human and mice. Autophagy 20, 2055–2066 (2024).
Paillusson, S. et al. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci. 39, 146–157 (2016).
Area-Gomez, E., Guardia-Laguarta, C., Schon, E. A. & Przedborski, S. Mitochondria, OxPhos, and neurodegeneration: cells are not just running out of gas. J. Clin. Invest. 129, 34–45 (2019).
Stutzmann, G. E., Caccamo, A., LaFerla, F. M. & Parker, I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 24, 508–513 (2004).
Stutzmann, G. E. et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 26, 5180–5189 (2006).
Chan, S. L., Mayne, M., Holden, C. P., Geiger, J. D. & Mattson, M. P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 275, 18195–18200 (2000).
Jensen, L. E. et al. Alzheimer’s disease-associated peptide Abeta42 mobilizes ER Ca2+ via InsP3R-dependent and -independent mechanisms. Front. Mol. Neurosci. 6, 36 (2013).
Smith, I. F., Hitt, B., Green, K. N., Oddo, S. & LaFerla, F. M. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neurochem. 94, 1711–1718 (2005).
Zampese, E. et al. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl Acad. Sci. USA 108, 2777–2782 (2011).
Calvo-Rodriguez, M., Hernando-Perez, E., Nunez, L. & Villalobos, C. Amyloid beta oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling. Front. Cell Neurosci. 13, 22 (2019).
Jadiya, P. et al. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 10, 3885 (2019).
Martino Adami, P. V. et al. Perturbed mitochondria-ER contacts in live neurons that model the amyloid pathology of Alzheimer’s disease. J. Cell Sci. 132, jcs229906 (2019).
Area-Gomez, E. et al. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 9, 335 (2018).
Perreault, S., Bousquet, O., Lauzon, M., Paiement, J. & Leclerc, N. Increased association between rough endoplasmic reticulum membranes and mitochondria in transgenic mice that express P301L tau. J. Neuropathol. Exp. Neurol. 68, 503–514 (2009).
Paillusson, S. et al. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 134, 129–149 (2017).
Jouaville, L. S., Pinton, P., Bastianutto, C., Rutter, G. A. & Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl Acad. Sci. USA 96, 13807–13812 (1999).
Arruda, A. P. et al. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 20, 1427–1435 (2014).
Beaulant, A. et al. Endoplasmic reticulum-mitochondria miscommunication is an early and causal trigger of hepatic insulin resistance and steatosis. J. Hepatol. 77, 710–722 (2022).
Feriod, C. N. et al. Hepatic inositol 1,4,5 trisphosphate receptor type 1 mediates fatty liver. Hepatol. Commun. 1, 23–35 (2017).
Hernandez-Alvarez, M. I. et al. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell 177, 881–895.e7 (2019).
Jin, C., Kumar, P., Gracia-Sancho, J. & Dufour, J. F. Calcium transfer between endoplasmic reticulum and mitochondria in liver diseases. FEBS Lett. 595, 1411–1421 (2021).
Danese, A. et al. Calcium regulates cell death in cancer: roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim. Biophys. Acta Bioenerg. 1858, 615–627 (2017).
Tosatto, A. et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol. Med. 8, 569–585 (2016).
Rao, G. et al. MicroRNA-195 controls MICU1 expression and tumor growth in ovarian cancer. EMBO Rep. 21, e48483 (2020).
Fernandez Garcia, E. et al. The mitochondrial Ca2+ channel MCU is critical for tumor growth by supporting cell cycle progression and proliferation. Front. Cell Dev. Biol. 11, 1082213 (2023).
Ziegler, D. V., Martin, N. & Bernard, D. Cellular senescence links mitochondria-ER contacts and aging. Commun. Biol. 4, 1323 (2021).
Wiel, C. et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 5, 3792 (2014).
Ziegler, D. V. et al. Calcium channel ITPR2 and mitochondria-ER contacts promote cellular senescence and aging. Nat. Commun. 12, 720 (2021).
van der Linden, F. H. et al. A turquoise fluorescence lifetime-based biosensor for quantitative imaging of intracellular calcium. Nat. Commun. 12, 7159 (2021).
Simmen, T. et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 24, 717–729 (2005).
Tsien, R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19, 2396–2404 (1980).
Grynkiewicz, G., Poenie, M. & Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985).
Inouye, S. et al. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc. Natl Acad. Sci. USA 82, 3154–3158 (1985).
Prasher, D., McCann, R. O. & Cormier, M. J. Cloning and expression of the cDNA coding for aequorin, a bioluminescent calcium-binding protein. Biochemical biophysical Res. Commun. 126, 1259–1268 (1985).
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 (2001).
Miyawaki, A., Griesbeck, O., Heim, R. & Tsien, R. Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl Acad. Sci. USA 96, 2135–2140 (1999).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Yellen, G. & Mongeon, R. Quantitative two-photon imaging of fluorescent biosensors. Curr. Opin. Chem. Biol. 27, 24–30 (2015).
Waldeck-Weiermair, M. et al. Development and application of sub-mitochondrial targeted Ca2+ biosensors. Front. Cell Neurosci. 13, 449 (2019).
Kosmach, A. et al. Monitoring mitochondrial calcium and metabolism in the beating MCU-KO heart. Cell Rep. 37, 109846 (2021).
Eisner, V., Picard, M. & Hajnoczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755–765 (2018).
Tabara, L. C., Segawa, M. & Prudent, J. Molecular mechanisms of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-024-00785-1 (2024).
Cereghetti, G. M. et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl Acad. Sci. USA 105, 15803–15808 (2008).
Yi, M., Weaver, D. & Hajnoczky, G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661–672 (2004).
Saotome, M. et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl Acad. Sci. USA 105, 20728–20733 (2008).
Wang, X. & Schwarz, T. L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009).
Macaskill, A. F. et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 (2009).
Agarwal, A. et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605(2017).
Eisner, V. et al. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc. Natl Acad. Sci. USA 114, E859–E868 (2017).
Eisner, V., Lenaers, G. & Hajnoczky, G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J. Cell Biol. 205, 179–195 (2014).
Willingham, T. B., Ajayi, P. T. & Glancy, B. Subcellular specialization of mitochondrial form and function in skeletal muscle cells. Front. Cell Dev. Biol. 9, 757305 (2021).
Sharma, V. K., Ramesh, V., Franzini-Armstrong, C. & Sheu, S. S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 32, 97–104 (2000).
De La Fuente, S. et al. Spatial separation of mitochondrial calcium uptake and extrusion for energy-efficient mitochondrial calcium signaling in the heart. Cell Rep. 24, 3099–3107.e4 (2018).
Pacher, P. & Hajnoczky, G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 20, 4107–4121 (2001).
Cao, C., Wang, S., Cui, T., Su, X. C. & Chou, J. J. Ion and inhibitor binding of the double-ring ion selectivity filter of the mitochondrial calcium uniporter. Proc. Natl Acad. Sci. USA 114, E2846–E2851 (2017).
Huang, Z., Spivey, J. A., MacMillan, S. N. & Wilson, J. J. A ferrocene-containing analogue of the MCU inhibitor Ru265 with increased cell permeability. Inorg. Chem. Front. 10, 591–599 (2023).
Arduino, D. M. et al. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol. Cell 67, 711–723.e7 (2017).
De Mario, A. et al. Identification and functional validation of FDA-approved positive and negative modulators of the mitochondrial calcium uniporter. Cell Rep. 35, 109275 (2021).
Paillard, M. et al. MICU1 interacts with the D-ring of the MCU pore to control its Ca2+ flux and sensitivity to Ru360. Mol. Cell 72, 778–785.e3 (2018).
Di Marco, G. et al. A high-throughput screening identifies MICU1 targeting compounds. Cell Rep. 30, 2321–2331.e6 (2020).
Marta, K., Hasan, P., Rodriguez-Prados, M., Paillard, M. & Hajnoczky, G. Pharmacological inhibition of the mitochondrial Ca2+ uniporter: relevance for pathophysiology and human therapy. J. Mol. Cell Cardiol. 151, 135–144 (2021).
Montero, M., Lobaton, C. D., Moreno, A. & Alvarez, J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 16, 1955–1957 (2002).
Acknowledgements
The authors thank E. L. Seifert and D. Weaver for pre-reviewing the manuscript. This project was funded by NIH grants (RO1 DK125897, GM151536, NS132056 and RO3 TR004644).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Amalia Dolga, Geert Bultynck and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- EF hand Ca2+-binding proteins
-
A family of proteins that contain EF hand motifs, which is the structural unit that binds Ca2+.
- Electron probe X-ray microanalysis
-
A technique that uses a focused beam of electrons to identify the chemical composition of solid materials.
- Fluorescence lifetime imaging microscopy
-
(FLIM). An advanced imaging technique used to measure fluorescence decay rate (lifetime) instead of intensity.
- Genetically-encoded calcium indicators
-
(GECI). Proteins used as reporters for cellular Ca2+ measurements, made possible by fusing Ca2+-binding motifs with fluorescent properties.
- PiC transporter
-
This phosphate carrier is an inner mitochondrial membrane protein involved in phosphate transport and mitochondrial ATP production, which has implications on mitochondrial Ca2+ transport and matrix Ca2+.
- Steatosis
-
The process in where the body accumulates lipids, affecting multiple organs and especially the liver in where this process is referred to as fatty liver disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cartes-Saavedra, B., Ghosh, A. & Hajnóczky, G. The roles of mitochondria in global and local intracellular calcium signalling. Nat Rev Mol Cell Biol 26, 456–475 (2025). https://doi.org/10.1038/s41580-024-00820-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-024-00820-1
This article is cited by
-
MitoCommun: a database for decoding mitochondrial communication networks
BMC Genomics (2026)
-
Mitochondrial dynamics and neuronal death in neurodegenerative diseases
Neurophysiology (2026)
-
Calcium dynamics unplugged: NCLX in disease and therapeutic frontiers
Molecular Biology Reports (2026)
-
Emerging roles of the ciliary-mitochondrial axis in cellular homeostasis and neuroprotection
Molecular Neurodegeneration Advances (2025)
-
CISD2 ensures adequate ER-mitochondrial coupling, critically supporting mitochondrial function in neurons
Acta Neuropathologica Communications (2025)