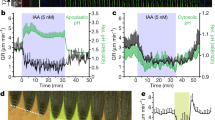
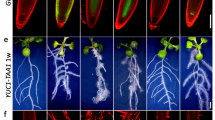
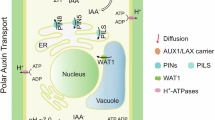
Abstract
The phytohormone auxin is a major signal coordinating growth and development in plants. The variety of its effects arises from its ability to form local auxin maxima and gradients within tissues, generated through directional cell-to-cell transport and elaborate metabolic control. These auxin distribution patterns instruct cells in a context-dependent manner to undergo predefined developmental transitions. In this Review, we discuss advances in auxin action at the level of homeostasis and signalling. We highlight key insights into the structural basis of PIN-mediated intercellular auxin transport and explore two novel non-transcriptional auxin signalling mechanisms: one involving intracellular Ca2+ transients and another involving cell-surface auxin perception that mediates global, ultrafast phosphorylation. Furthermore, we examine emerging evidence indicating the involvement of cyclic adenosine monophosphate as a second messenger in the transcriptional auxin response. Together, these recent developments in auxin research have profoundly deepened our understanding of the complex and diverse activities of auxin in plant growth and development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Darwin, C. & Darwin, F. E. The Power of Movement in Plants (D. Appleton, 1880).
Thimann, K. V. & Koepfli, J. B. Identity of the growth-promoting and root-forming substances of plants. Nature 135, 101–102 (1935).
Kögl, F., Erxleben, H. & Haagen-Smit, A. J. Über die Isolierung der Auxine a und b aus pflanzlichen Materialien. IX. Mitteilung. Physiol. Chem. 243, 209–226 (1934).
Carrillo-Carrasco, V. P., Hernandez-Garcia, J., Mutte, S. K. & Weijers, D. The birth of a giant: evolutionary insights into the origin of auxin responses in plants. EMBO J. 42, e113018 (2023).
Schmidt, V. et al. Phytohormone profiling in an evolutionary framework. Nat. Commun. 15, 3875 (2024).
Dubrovsky, J. G. et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl Acad. Sci. USA 105, 8790–8794 (2008).
Reinhardt, D. et al. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003).
Vanneste, S. & Friml, J. Auxin: a trigger for change in plant development. Cell 136, 1005–1016 (2009).
Leyser, O. Auxin signaling. Plant Physiol. 176, 465–479 (2018).
de Roij, M., Borst, J. W. & Weijers, D. Protein degradation in auxin response. Plant Cell 36, 3025–3035 (2024).
Lavy, M. & Estelle, M. Mechanisms of auxin signaling. Development 143, 3226–3229 (2016).
Dubey, S. M., Serre, N. B. C., Oulehlová, D., Vittal, P. & Fendrych, M. No time for transcription-rapid auxin responses in plants. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a039891 (2021).
Ludwig-Müller, J., Jülke, S., Bierfreund, N. M., Decker, E. L. & Reski, R. Moss (Physcomitrella patens) GH3 proteins act in auxin homeostasis. N. Phytol. 181, 323–338 (2009).
Eklund, D. M. et al. Auxin produced by the indole-3-pyruvic acid pathway regulates development and gemmae dormancy in the liverwort Marchantia polymorpha. Plant Cell 27, 1650–1669 (2015).
Kato, H. et al. Design principles of a minimal auxin response system. Nat. Plants 6, 473–482 (2020).
Tang, H. et al. Divergence of trafficking and polarization mechanisms for PIN auxin transporters during land plant evolution. Plant Commun. 5, 100669 (2024).
Fisher, T. J., Flores-Sandoval, E., Alvarez, J. P. & Bowman, J. L. PIN-FORMED is required for shoot phototropism/gravitropism and facilitates meristem formation in Marchantia polymorpha. N. Phytol. 238, 1498–1515 (2023).
Kaneko, S. et al. An evolutionarily primitive and distinct auxin metabolism in the lycophyte Selaginella moellendorffii. Plant Cell Physiol. 61, 1724–1732 (2020).
Prigge, M. J., Lavy, M., Ashton, N. W. & Estelle, M. Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr. Biol. 20, 1907–1912 (2010).
Skokan, R. et al. PIN-driven auxin transport emerged early in streptophyte evolution. Nat. Plants 5, 1114–1119 (2019).
Kuhn, A. et al. RAF-like protein kinases mediate a deeply conserved, rapid auxin response. Cell 187, 130–148 (2024).
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Mashiguchi, K. et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18512–18517 (2011).
Won, C. et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18518–18523 (2011).
Tivendale, N. D. et al. Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiol. 159, 1055–1063 (2012).
Casanova-Saez, R., Mateo-Bonmati, E. & Ljung, K. Auxin metabolism in plants. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a039867 (2021).
Sugawara, S. et al. Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol. 56, 1641–1654 (2015).
Wang, B. et al. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl Acad. Sci. USA 112, 4821–4826 (2015).
Frick, E. M. & Strader, L. C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 69, 169–177 (2018).
Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 69, 417–435 (2018).
Cheng, Y., Dai, X. & Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20, 1790–1799 (2006).
Brumos, J. et al. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 47, 306–318 (2018).
Krahmer, J. & Fankhauser, C. Environmental control of hypocotyl elongation. Annu. Rev. Plant Biol. 75, 489–519 (2024).
Ursache, R. et al. Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development 141, 1250–1259 (2014).
Sheldrake, A. R. The production of auxin by dying cells. J. Exp. Bot. 72, 2288–2300 (2021).
Dobrev, P. I. et al. Study of auxin metabolism using stable isotope labeling and LCMS; evidence for in planta auxin decarboxylation pathway. Preprint at bioRxiv https://doi.org/10.1101/2023.06.02.543384 (2023).
Mateo-Bonmatí, E., Casanova-Sáez, R., Šimura, J. & Ljung, K. Broadening the roles of UDP-glycosyltransferases in auxin homeostasis and plant development. N. Phytol. 232, 642–654 (2021).
Ishimaru, K. et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707–711 (2013).
Yang, Y. et al. Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiol. 147, 1034–1045 (2008).
Takubo, E. et al. Role of Arabidopsis INDOLE-3-ACETIC ACID CARBOXYL METHYLTRANSFERASE 1 in auxin metabolism. Biochem. Biophys. Res. Commun. 527, 1033–1038 (2020).
Abbas, M. et al. Auxin methylation is required for differential growth in Arabidopsis. Proc. Natl Acad. Sci. USA 115, 6864–6869 (2018).
Goto, T., Soyano, T., Liu, M., Mori, T. & Kawaguchi, M. Auxin methylation by IAMT1, duplicated in the legume lineage, promotes root nodule development in Lotus japonicus. Proc. Natl Acad. Sci. USA 119, e2116549119 (2022).
Zhang, F. et al. A feedback regulation between ARF7-mediated auxin signaling and auxin homeostasis involving MES17 affects plant gravitropism. J. Integr. Plant Biol. 64, 1339–1351 (2022).
Hayashi, K. I. et al. The main oxidative inactivation pathway of the plant hormone auxin. Nat. Commun. 12, 6752 (2021).
Müller, K. et al. DIOXYGENASE FOR AUXIN OXIDATION 1 catalyzes the oxidation of IAA amino acid conjugates. Plant Physiol. 187, 103–115 (2021).
Zheng, Z. et al. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat. Plants 2, 16025 (2016).
Casanova-Sáez, R. et al. Inactivation of the entire Arabidopsis group II GH3s confers tolerance to salinity and water deficit. N. Phytol. 235, 263–275 (2022).
Wang, Q. et al. GH3-mediated auxin inactivation attenuates multiple stages of lateral root development. N. Phytol. 240, 1900–1912 (2023).
Sauer, M. & Kleine-Vehn, J. PIN-FORMED and PIN-LIKES auxin transport facilitators. Development https://doi.org/10.1242/dev.168088 (2019).
Hladík, P. et al. Phenylacetic acid metabolism in land plants: novel pathways and metabolites. J. Exp. Bot. https://doi.org/10.1093/jxb/eraf092 (2025).
Ung, K. L. et al. Structures and mechanism of the plant PIN-FORMED auxin transporter. Nature 609, 605–610 (2022).
Hammes, U. Z. & Pedersen, B. P. Structure and function of auxin transporters. Annu. Rev. Plant Biol. https://doi.org/10.1146/annurev-arplant-070523-034109 (2024).
Miao, R., Russinova, E. & Rodriguez, P. L. Tripartite hormonal regulation of plasma membrane H+-ATPase activity. Trends Plant Sci. 27, 588–600 (2022).
Raven, J. A. Transport of indole acetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. N. Phytol. 74, 163–172 (1975).
Rubery, P. H. & Sheldrake, A. R. Carrier-mediated auxin transport. Planta 118, 101–121 (1974).
Yang, Y., Hammes, U. Z., Taylor, C. G., Schachtman, D. P. & Nielsen, E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16, 1123–1127 (2006).
Corratgé-Faillie, C. & Lacombe, B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 68, 3107–3113 (2017).
Watanabe, S. et al. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. Proc. Natl Acad. Sci. USA 117, 31500–31509 (2020).
Kamimoto, Y. et al. Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant Cell Physiol. 53, 2090–2100 (2012).
Kubeš, M. et al. The Arabidopsis concentration-dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. Plant J. 69, 640–654 (2012).
Yang, Z. et al. Structural insights into auxin recognition and efflux by Arabidopsis PIN1. Nature 609, 611–615 (2022).
Su, N. et al. Structures and mechanisms of the Arabidopsis auxin transporter PIN3. Nature 609, 616–621 (2022).
Wisniewska, J. et al. Polar PIN localization directs auxin flow in plants. Science 312, 883 (2006).
Luschnig, C. & Friml, J. Over 25 years of decrypting PIN-mediated plant development. Nat. Commun. 15, 9904 (2024).
Bayly-Jones, C. et al. LYCHOS is a human hybrid of a plant-like PIN transporter and a GPCR. Nature 634, 1238–1244 (2024).
Janacek, D. P. et al. Transport properties of canonical PIN-FORMED proteins from Arabidopsis and the role of the loop domain in auxin transport. Dev. Cell 59, 3259–3271 (2024).
Ding, Z. et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 3, 941 (2012).
Mravec, J. et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136–1140 (2009).
Barbez, E. et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485, 119–122 (2012).
Lanassa Bassukas, A. E., Xiao, Y. & Schwechheimer, C. Phosphorylation control of PIN auxin transporters. Curr. Opin. Plant Biol. 65, 102146 (2022).
Tan, S., Luschnig, C. & Friml, J. Pho-view of auxin: reversible protein phosphorylation in auxin biosynthesis, transport and signaling. Mol. Plant 14, 151–165 (2021).
Geisler, M. M. A retro-perspective on auxin transport. Front. Plant Sci. 12, 756968 (2021).
Růžička, K. et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl Acad. Sci. 107, 10749–10753 (2010).
Aryal, B. et al. ABCG36/PEN3/PDR8 is an exporter of the auxin precursor, indole-3-butyric acid, and involved in auxin-controlled development. Front. Plant Sci. 10, 899 (2019).
Ying, W. et al. Structure and function of the Arabidopsis ABC transporter ABCB19 in brassinosteroid export. Science 383, eadj4591 (2024).
Wei, H. et al. Structural insights into brassinosteroid export mediated by the Arabidopsis ABC transporter ABCB1. Plant Commun. https://doi.org/10.1016/j.xplc.2024.101181 (2024).
Geisler, M. M. Embracing substrate multispecificity in plant ABC transporters. Mol. Plant 17, 990–992 (2024).
Ung, K. L., Schulz, L., Kleine-Vehn, J., Pedersen, B. P. & Hammes, U. Z. Auxin transport at the endoplasmic reticulum: roles and structural similarity of PIN-FORMED and PIN-LIKES. J. Exp. Bot. 74, 6893–6903 (2023).
Sawchuk, M. G., Edgar, A. & Scarpella, E. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet. 9, e1003294 (2013).
Seifu, Y. W. et al. Mapping the membrane orientation of auxin homeostasis regulators PIN5 and PIN8 in Arabidopsis thaliana root cells reveals their divergent topology. Plant Methods 20, 84 (2024).
Ranocha, P. et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 4, 2625 (2013).
Michniewicz, M. et al. TRANSPORTER OF IBA1 links auxin and cytokinin to influence root architecture. Dev. Cell 50, 599–609 (2019).
Gao, C. et al. Directionality of plasmodesmata-mediated transport in Arabidopsis leaves supports auxin channeling. Curr. Biol. 30, 1970–1977 (2020).
Mellor, N. L. et al. Auxin fluxes through plasmodesmata modify root-tip auxin distribution. Development https://doi.org/10.1242/dev.181669 (2020).
Mehra, P. et al. Hydraulic flux-responsive hormone redistribution determines root branching. Science 378, 762–768 (2022).
Han, X. et al. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev. Cell 28, 132–146 (2014).
Ainley, W. M., Walker, J. C., Nagao, R. T. & Key, J. L. Sequence and characterization of two auxin-regulated genes from soybean. J. Biol. Chem. 263, 10658–10666 (1988).
Theologis, A., Huynh, T. V. & Davis, R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J. Mol. Biol. 183, 53–68 (1985).
dos Santos Maraschin, F., Memelink, J., Offringa, R. & Auxin-induced, S. C. F. Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J. 59, 100–109 (2009).
Gray, W. M., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001).
Ramos, J. A., Zenser, N., Leyser, O. & Callis, J. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13, 2349–2360 (2001).
Winkler, M. et al. Variation in auxin sensing guides AUX/IAA transcriptional repressor ubiquitylation and destruction. Nat. Commun. 8, 15706 (2017).
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005).
Kepinski, S. & Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 (2005).
Dindas, J. et al. AUX1-mediated root hair auxin influx governs SCF(TIR1/AFB)-type Ca2+ signaling. Nat. Commun. 9, 1174 (2018).
Fendrych, M. et al. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 4, 453–459 (2018).
Qi, L. et al. Guanylate cyclase activity of TIR1/AFB auxin receptors in rapid auxin responses. Preprint at bioRxiv https://doi.org/10.1101/2023.11.18.567481 (2024).
Qi, L. et al. Adenylate cyclase activity of TIR1/AFB auxin receptors in plants. Nature 611, 133–138 (2022).
Bascom, C. Jr. et al. Clade-D auxin response factors regulate auxin signaling and development in the moss Physcomitrium patens. PLoS Biol. 21, e3002163 (2023).
Morffy, N. et al. Identification of plant transcriptional activation domains. Nature 632, 166–173 (2024).
Mutte, S. K. et al. Origin and evolution of the nuclear auxin response system. eLife 7, e33399 (2018).
Boer, D. R. et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156, 577–589 (2014).
Freire-Rios, A. et al. Architecture of DNA elements mediating ARF transcription factor binding and auxin-responsive gene expression in Arabidopsis. Proc. Natl Acad. Sci. USA 117, 24557–24566 (2020).
Truskina, J. et al. A network of transcriptional repressors modulates auxin responses. Nature 589, 116–119 (2021).
Cavalleri, A. et al. Auxin-dependent post-translational regulation of MONOPTEROS in the Arabidopsis root. Cell Rep. 43, 115083 (2024).
Vert, G., Walcher, C. L., Chory, J. & Nemhauser, J. L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl Acad. Sci. USA 105, 9829–9834 (2008).
Cho, H. et al. A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat. Cell Biol. 16, 66–76 (2014).
Orosa-Puente, B. et al. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362, 1407–1410 (2018).
Jing, H. et al. Regulation of AUXIN RESPONSE FACTOR condensation and nucleo-cytoplasmic partitioning. Nat. Commun. 13, 4015 (2022).
Powers, S. K. et al. Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in Arabidopsis thaliana. Mol. Cell 76, 177–190 (2019).
Xuan, L. et al. MCTP controls nucleocytoplasmic partitioning of AUXIN RESPONSE FACTORs during lateral root development. Dev. Cell https://doi.org/10.1016/j.devcel.2024.09.026 (2024).
Ebstrup, E. et al. NBR1-mediated selective autophagy of ARF7 modulates root branching. EMBO Rep. 25, 2571–2591 (2024).
Das, S. et al. Quantitative imaging reveals the role of MpARF proteasomal degradation during gemma germination. Plant Commun. https://doi.org/10.1016/j.xplc.2024.101039 (2024).
Cancé, C., Martin-Arevalillo, R., Boubekeur, K. & Dumas, R. Auxin response factors are keys to the many auxin doors. N. Phytol. 235, 402–419 (2022).
Pierre-Jerome, E., Moss, B. L., Lanctot, A., Hageman, A. & Nemhauser, J. L. Functional analysis of molecular interactions in synthetic auxin response circuits. Proc. Natl Acad. Sci. USA 113, 11354–11359 (2016).
Krogan, N. T., Ckurshumova, W., Marcos, D., Caragea, A. E. & Berleth, T. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. N. Phytol. 194, 391–401 (2012).
Lau, S., De Smet, I., Kolb, M., Meinhardt, H. & Jürgens, G. Auxin triggers a genetic switch. Nat. Cell Biol. 13, 611–615 (2011).
Simonini, S. et al. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 30, 2286–2296 (2016).
Kuhn, A. et al. Direct ETTIN-auxin interaction controls chromatin states in gynoecium development. eLife https://doi.org/10.7554/eLife.51787 (2020).
Liscum, E. & Reed, J. W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49, 387–400 (2002).
Abel, S. & Theologis, A. Early genes and auxin action. Plant Physiol. 111, 9–17 (1996).
Guilfoyle, T. J. The PB1 domain in auxin response factor and Aux/IAA proteins: a versatile protein interaction module in the auxin response. Plant Cell 27, 33–43 (2015).
Korasick, D. A. et al. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc. Natl Acad. Sci. USA 111, 5427–5432 (2014).
Nanao, M. H. et al. Structural basis for oligomerization of auxin transcriptional regulators. Nat. Commun. 5, 3617 (2014).
Figueiredo, M. R. A. & Strader, L. C. Intrinsic and extrinsic regulators of Aux/IAA protein degradation dynamics. Trends Biochem. Sci. 47, 865–874 (2022).
Szemenyei, H., Hannon, M. & Long, J. A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384–1386 (2008).
Cho, H. T. et al. A dose-dependent bimodal switch by homologous Aux/IAA transcriptional repressors. Mol. Plant 17, 1407–1422 (2024).
Leydon, A. R. et al. Repression by the Arabidopsis TOPLESS corepressor requires association with the core Mediator complex. eLife 10, e66739 (2021).
Leydon, A. R. et al. A function of TPL/TBL1-type corepressors is to nucleate the assembly of the preinitiation complex. J. Cell Biol. https://doi.org/10.1083/jcb.202404103 (2024).
Kubalová, M. et al. Auxin co-receptor IAA17/AXR3 controls cell elongation in Arabidopsis thaliana root solely by modulation of nuclear auxin pathway. N. Phytol. 241, 2448–2463 (2024).
Ruegger, M. et al. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev. 12, 198–207 (1998).
Tan, X. et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645 (2007).
Lee, S. et al. Defining binding efficiency and specificity of auxins for SCF(TIR1/AFB)-Aux/IAA co-receptor complex formation. ACS Chem. Biol. 9, 673–682 (2014).
Weijers, D. & Wagner, D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 67, 539–574 (2016).
Galvan-Ampudia, C. S. et al. Temporal integration of auxin information for the regulation of patterning. eLife https://doi.org/10.7554/eLife.55832 (2020).
Mahonen, A. P. et al. PLETHORA gradient formation mechanism separates auxin responses. Nature 515, 125–129 (2014).
Caumon, H. & Vernoux, T. A matter of time: auxin signaling dynamics and the regulation of auxin responses during plant development. J. Exp. Bot. 74, 3887–3902 (2023).
Chen, H. et al. TIR1-produced cAMP as a second messenger in transcriptional auxin signalling. Nature https://doi.org/10.1038/s41586-025-08669-w (2025).
Ito, J. et al. Auxin-dependent compositional change in Mediator in ARF7- and ARF19-mediated transcription. Proc. Natl Acad. Sci. 113, 6562–6567 (2016).
Fukaki, H., Taniguchi, N. & Tasaka, M. PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J. 48, 380–389 (2006).
von Wangenheim, D. et al. Live tracking of moving samples in confocal microscopy for vertically grown roots. eLife https://doi.org/10.7554/eLife.26792 (2017).
Shih, H. W., DePew, C. L., Miller, N. D. & Monshausen, G. B. The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr. Biol. 25, 3119–3125 (2015).
Serre, N. B. C. et al. The AUX1-AFB1-CNGC14 module establishes a longitudinal root surface pH profile. eLife https://doi.org/10.7554/eLife.85193 (2023).
Dubey, S. M. et al. The AFB1 auxin receptor controls the cytoplasmic auxin response pathway in Arabidopsis thaliana. Mol. Plant 16, 1120–1130 (2023).
Chen, H., Li, L., Zou, M., Qi, L. & Friml, J. Distinct functions of TIR1 and AFB1 receptors in auxin signaling. Mol. Plant 16, 1117–1119 (2023).
Prigge, M. J. et al. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife https://doi.org/10.7554/eLife.54740 (2020).
Serre, N. B. C. et al. AFB1 controls rapid auxin signalling through membrane depolarization in Arabidopsis thaliana root. Nat. Plants 7, 1229–1238 (2021).
Lu, B. et al. FERONIA-mediated TIR1/AFB2 oxidation stimulates auxin signalling in Arabidopsis. Mol. Plant https://doi.org/10.1016/j.molp.2024.04.002 (2024).
Kulich, I. et al. Calcium-triggered apoplastic ROS bursts balance gravity and mechanical signals to navigate soil. Preprint at bioRxiv https://doi.org/10.1101/2025.01.07.631646 (2025).
Wang, J. et al. Cryo-EM structures of Arabidopsis CNGC1 and CNGC5 reveal molecular mechanisms underlying gating and calcium selectivity. Nat. Plants https://doi.org/10.1038/s41477-025-01923-z (2025).
Zhang, Y., Xiao, G., Wang, X., Zhang, X. & Friml, J. Evolution of fast root gravitropism in seed plants. Nat. Commun. 10, 3480 (2019).
Fendrych, M., Leung, J. & Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife https://doi.org/10.7554/eLife.19048 (2016).
Friml, J. et al. ABP1-TMK auxin perception for global phosphorylation and auxin canalization. Nature 609, 575–581 (2022).
Narasimhan, M. et al. Systematic analysis of specific and non-specific auxin effects on endocytosis and trafficking. Plant Physiol. https://doi.org/10.1093/plphys/kiab134 (2021).
Habets, M. E. & Offringa, R. Auxin Binding Protein 1: a red herring after all? Mol. Plant 8, 1131–1134 (2015).
Napier, R. M., David, K. M. & Perrot-Rechenmann, C. in Auxin Molecular Biology (eds C. Perrot-Rechenmann & G. Hagen) 339–348 (Springer, 2002).
Chen, J. G., Ullah, H., Young, J. C., Sussman, M. R. & Jones, A. M. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 15, 902–911 (2001).
Gao, Y. et al. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl Acad. Sci. USA 112, 2275–2280 (2015).
Grones, P. et al. Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J. Exp. Bot. 66, 5055–5065 (2015).
Ohmiya, A., Tanaka, Y., Kadowaki, K. & Hayashi, T. Cloning of genes encoding auxin-binding proteins (ABP19/20) from peach: significant peptide sequence similarity with germin-like proteins. Plant Cell Physiol. 39, 492–499 (1998).
Yu, Y. et al. ABLs and TMKs are co-receptors for extracellular auxin. Cell 186, 5457–5471.e17 (2023).
Rodriguez, L. et al. ABP1/ABL3-TMK1 cell-surface auxin signaling directly targets PIN2-mediated auxin fluxes for root gravitropism. Preprint at bioRxiv https://doi.org/10.1101/2022.11.30.518503 (2025).
Xu, T. et al. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343, 1025–1028 (2014).
Kim, M. H. et al. Identification of Arabidopsis BAK1-associating receptor-like kinase 1 (BARK1) and characterization of its gene expression and brassinosteroid-regulated root phenotypes. Plant Cell Physiol. 54, 1620–1634 (2013).
Marquès-Bueno, M. M. et al. Auxin-regulated reversible inhibition of TMK1 signaling by MAKR2 modulates the dynamics of root gravitropism. Curr. Biol. 31, 228–237 (2021).
DeFalco, T. A. & Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 81, 3449–3467 (2021).
Nolan, T. M., Vukašinović, N., Liu, D., Russinova, E. & Yin, Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32, 295–318 (2020).
Li, L. et al. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 599, 273–277 (2021).
Dai, N., Wang, W., Patterson, S. E. & Bleecker, A. B. The TMK subfamily of receptor-like kinases in Arabidopsis display an essential role in growth and a reduced sensitivity to auxin. PLoS ONE 8, e60990 (2013).
Lin, W. et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature https://doi.org/10.1038/s41586-021-03976-4 (2021).
Wang, J. et al. Self-regulation of PIN1-driven auxin transport by cell surface-based auxin signaling in Arabidopsis. Preprint at bioRxiv https://doi.org/10.1101/2022.11.30.518523 (2022).
Rodriguez, L. et al. Cell surface auxin signalling directly targets PIN-mediated auxin fluxes for adaptive plant development. Preprint at bioRxiv https://doi.org/10.1101/2022.11.30.518503 (2022).
Pan, X. et al. Auxin-induced signaling protein nanoclustering contributes to cell polarity formation. Nat. Commun. 11, 3914 (2020).
Platre, M. P. et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 364, 57–62 (2019).
Kleine-Vehn, J. et al. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 7, 540 (2011).
Wang, Y. et al. Transmembrane kinase 1-mediated auxin signal regulates membrane-associated clathrin in Arabidopsis roots. J. Integr. Plant Biol. 65, 82–99 (2023).
Robert, S. et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143, 111–121 (2010).
Han, H. et al. Rapid auxin-mediated phosphorylation of Myosin regulates trafficking and polarity in Arabidopsis. Preprint at bioRxiv https://doi.org/10.1101/2021.04.13.439603 (2021).
Thimann, K. V. Hormones and the analysis of growth. Plant Physiol. 13, 437–449 (1938).
Yang, J. et al. TMK1-based auxin signaling regulates abscisic acid responses via phosphorylating ABI1/2 in Arabidopsis. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2102544118 (2021).
Huang, R. et al. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc. Natl Acad. Sci. USA 116, 21285–21290 (2019).
Wang, Q. et al. A phosphorylation-based switch controls TAA1-mediated auxin biosynthesis in plants. Nat. Commun. 11, 679 (2020).
Gu, B. et al. Modulation of receptor-like transmembrane kinase 1 nuclear localization by DA1 peptidases in Arabidopsis. Proc. Natl Acad. Sci. USA 119, e2205757119 (2022).
Cao, M. et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243 (2019).
Wang, J. L. et al. WAV E3 ubiquitin ligases mediate degradation of IAA32/34 in the TMK1-mediated auxin signaling pathway during apical hook development. Proc. Natl Acad. Sci. USA 121, e2314353121 (2024).
Li, C. et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4, e06587 (2015).
Xu, T. et al. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143, 99–110 (2010).
Du, M., Spalding, E. P. & Gray, W. M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 71, 379–402 (2020).
Li, L., Gallei, M. & Friml, J. Bending to auxin: fast acid growth for tropisms. Trends Plant Sci. 27, 440–449 (2022).
Hager, A., Menzel, H. & Krauss, A. Experiments and hypothesis concerning the primary action of auxin in elongation growth. Planta 100, 47–75 (1971).
Takahashi, K., Hayashi, K. & Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 159, 632–641 (2012).
Svennelid, F. et al. Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11, 2379–2391 (1999).
Maudoux, O. et al. A plant plasma membrane H+-ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J. Biol. Chem. 275, 17762–17770 (2000).
Pedersen, B. P., Buch-Pedersen, M. J., Preben Morth, J., Palmgren, M. G. & Nissen, P. Crystal structure of the plasma membrane proton pump. Nature 450, 1111–1114 (2007).
Ren, H., Park, M. Y., Spartz, A. K., Wong, J. H. & Gray, W. M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 14, e1007455 (2018).
Spartz, A. K. et al. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26, 2129–2142 (2014).
Wang, J. et al. The apoplastic pH is a key determinant in the hypocotyl growth response to auxin dosage and light. Nat. Plants 11, 279–294 (2025).
Ren, H. & Gray, W. M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 8, 1153–1164 (2015).
Spartz, A. K. et al. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 70, 978–990 (2012).
Ding, Z. & Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl Acad. Sci. USA 107, 12046–12051 (2010).
Du, M. et al. Biphasic control of cell expansion by auxin coordinates etiolated seedling development. Sci. Adv. 8, eabj1570 (2022).
de Wit, M., Ljung, K. & Fankhauser, C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. N. Phytol. 208, 198–209 (2015).
Calderon Villalobos, L. I. et al. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 8, 477–485 (2012).
Rademacher, E. H. et al. Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 22, 211–222 (2012).
Vernoux, T. et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol. Syst. Biol. 7, 508 (2011).
Robert, H. S. et al. Maternal auxin supply contributes to early embryo patterning in Arabidopsis. Nat. Plants 4, 548–553 (2018).
Robert, H. S. et al. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 23, 2506–2512 (2013).
Wabnik, K., Robert, H. S., Smith, R. S. & Friml, J. Modeling framework for the establishment of the apical-basal embryonic axis in plants. Curr. Biol. 23, 2513–2518 (2013).
Blilou, I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005).
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673 (2002).
Xuan, W. et al. Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351, 384–387 (2016).
Motte, H., Vanneste, S. & Beeckman, T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 70, 465–488 (2019).
Galvan-Ampudia, C. S., Chaumeret, A. M., Godin, C. & Vernoux, T. Phyllotaxis: from patterns of organogenesis at the meristem to shoot architecture. Wiley Interdiscip. Rev. Dev. Biol. 5, 460–473 (2016).
Jönsson, H., Heisler, M. G., Shapiro, B. E., Meyerowitz, E. M. & Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1633–1638 (2006).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Ursache, R. et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 7, 353–364 (2021).
Sorefan, K. et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459, 583–586 (2009).
Barkoulas, M., Hay, A., Kougioumoutzi, E. & Tsiantis, M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40, 1136–1141 (2008).
Hu, Z. L. et al. A CUC1/auxin genetic module links cell polarity to patterned tissue growth and leaf shape diversity in crucifer plants. Proc. Natl Acad. Sci. USA 121, e2321877121 (2024).
Dong, Y. et al. HEARTBREAK controls post-translational modification of INDEHISCENT to regulate fruit morphology in Capsella. Curr. Biol. 30, 3880–3888 (2020).
Sachs, T. The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 9, 151–262 (1981).
Ravichandran, S. J., Linh, N. M. & Scarpella, E. The canalization hypothesis — challenges and alternatives. N. Phytol. 227, 1051–1059 (2020).
Aliaga Fandino, A. C. & Hardtke, C. S. Auxin transport in developing protophloem: a case study in canalization. J. Plant Physiol. 269, 153594 (2022).
Chen, Q. et al. A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat. Commun. 6, 8821 (2015).
Garay-Arroyo, A. et al. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. EMBO J. 32, 2884–2895 (2013).
Abualia, R. et al. Molecular framework integrating nitrate sensing in root and auxin-guided shoot adaptive responses. Proc. Natl Acad. Sci. USA 119, e2122460119 (2022).
Abas, L. et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8, 249–256 (2006).
Baster, P. et al. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 32, 260–274 (2013).
Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256 (2005).
Prat, T. et al. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLoS Genet. 14, e1007177 (2018).
Barbosa, I. C. R., Hammes, U. Z. & Schwechheimer, C. Activation and polarity control of PIN-FORMED auxin transporters by phosphorylation. Trends Plant Sci. 23, 523–538 (2018).
Hajný, J., Tan, S. & Friml, J. Auxin canalization: from speculative models toward molecular players. Curr. Opin. Plant Biol. 65, 102174 (2022).
Zhang, Y., Hartinger, C., Wang, X. & Friml, J. Directional auxin fluxes in plants by intramolecular domain-domain coevolution of PIN auxin transporters. N. Phytol. 227, 1406–1416 (2020).
Hajný, J. et al. Receptor kinase module targets PIN-dependent auxin transport during canalization. Science 370, 550–557 (2020).
Wabnik, K. et al. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol. Syst. Biol. 6, 447 (2010).
Kareem, A., Bhatia, N., Ohno, C. & Heisler, M. G. PIN-FORMED1 polarity in the plant shoot epidermis is insensitive to the polarity of neighboring cells. iScience 25, 105062 (2022).
Friml, J. Fourteen stations of auxin. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a039859 (2022).
Zhang, J., Nodzynski, T., Pencík, A., Rolcík, J. & Friml, J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc. Natl Acad. Sci. USA 107, 918–922 (2010).
Chen, J. et al. Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants. Cell 186, 4788–4802 (2023).
Kulich, I., Schmid, J., Teplova, A., Qi, L. & Friml, J. Rapid translocation of NGR proteins driving polarization of PIN-activating D6 protein kinase during root gravitropism. eLife https://doi.org/10.7554/eLife.91523 (2024).
Nishimura, T. et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 381, 1006–1010 (2023).
Fiedler, L. & Friml, J. Rapid auxin signaling: unknowns old and new. Curr. Opin. Plant Biol. 75, 102443 (2023).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, wrote the article and/or edited the manuscript before submission. J.F. and S.V. contributed substantially to discussion of the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Dong-Wei Di and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 14-3-3 proteins
-
A family of phospho-binding proteins that regulate the activity of many proteins involved in diverse cellular processes.
- Cupin fold
-
A small, conserved β-barrel shape that is characteristic of the GERMIN-like protein (GLP) family.
- Cytoplasmic streaming
-
The active intracellular flow of organelles and cellular components.
- EAR domain
-
A domain that often confers inhibitory activity to transcriptional regulators, such as Aux/IAA and B-type ARFs in the example of auxin signalling.
- FERONIA
-
A malectin domain-containing receptor-like kinase that is involved in cell wall integrity sensing and RAPID ALKALINIZATION FACTOR (RALF) peptide signalling.
- Mediator complex
-
A conserved large, multisubunit complex that relays regulatory signals from the transcription factors to RNA polymerase II, for example, through module exchange and large-scale structural changes.
- Pre-initiation complex
-
A large multiprotein assembly at the promotor of a gene that is essential for the initiation of transcription by RNA polymerase II. It includes general transcription factors and RNA polymerase II and is co-activated by the Mediator complex.
- Receptor-like kinases
-
A multigene family of transmembrane receptor kinases involved in sensing various extracellular signals and activating cellular responses.
- RootChip
-
An integrated microfluidics chip specifically designed to monitor in real-time root responses to a stimulus.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vanneste, S., Pei, Y. & Friml, J. Mechanisms of auxin action in plant growth and development. Nat Rev Mol Cell Biol 26, 648–666 (2025). https://doi.org/10.1038/s41580-025-00851-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-025-00851-2
This article is cited by
-
Bioimaging application and growth-promoting behavior of Auxin-derived carbon dots on cancer cell and seed performance
Journal of Biological Engineering (2025)
-
The curious origins of a high-stress training technique mainlining: its molecular, biochemical, and agronomic perspectives for the cultivation of Cannabis sativa
Journal of Cannabis Research (2025)
-
A redox–auxin connection in response to water deficit
Nature Reviews Molecular Cell Biology (2025)
-
HSP90 differentially stabilizes plant ABCB-type auxin transporters on the plasma membrane
Nature Communications (2025)
-
Auxin transport inhibition triggers pedicel expansion and alters abscission zone dynamics in tomato fruit
Brazilian Journal of Botany (2025)