Abstract
Chronic kidney disease (CKD) is a leading cause of global morbidity and mortality and is independently associated with cardiovascular disease. The mainstay of treatment for CKD is blockade of the renin–angiotensin–aldosterone system (RAAS), which reduces blood pressure and proteinuria and slows kidney function decline. Despite this treatment, many patients progress to kidney failure, which requires dialysis or kidney transplantation, and/or die as a result of cardiovascular disease. The apelin system is an endogenous physiological regulator that is emerging as a potential therapeutic target for many diseases. This system comprises the apelin receptor and its two families of endogenous ligands, apelin and elabela/toddler. Preclinical and clinical studies show that apelin receptor ligands are endothelium-dependent vasodilators and potent inotropes, and the apelin system has a reciprocal relationship with the RAAS. In preclinical studies, apelin regulates glomerular haemodynamics and acts on the tubule to promote aquaresis. In addition, apelin is protective in several kidney injury models. Although the apelin system has not yet been studied in patients with CKD, the available data suggest that apelin is a promising potential therapeutic target for kidney disease.
Key points
-
Chronic kidney disease (CKD) is common worldwide and is associated with high cardiovascular morbidity and mortality; however, treatment options are limited.
-
The apelin system is a broad regulator of physiology that opposes the renin–angiotensin–aldosterone system (RAAS) and has beneficial cardiovascular effects in health and disease.
-
Increasing evidence indicates that apelin has favourable effects on renal physiology, including roles in the regulation of fluid balance and glomerular haemodynamics.
-
Preclinical models demonstrate direct anti-inflammatory and anti-fibrotic actions of the apelin system in models of kidney injury; targeting the apelin receptor in combination with RAAS blockers might offer synergistic benefits.
-
The apelin system is an attractive potential therapeutic target for CKD, offering direct renal protection in addition to targeting the associated cardiovascular complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830 (2013).
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Gansevoort, R. T. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382, 339–352 (2013).
Keith, D. S., Nichols, G. A., Gullion, C. M., Brown, J. B. & Smith, D. H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organisation. Arch. Intern. Med. 164, 659–663 (2004).
Schiffrin, E. L., Lipman, M. L. & Mann, J. F. Chronic kidney disease: effects on the cardiovascular system. Circulation 116, 85–97 (2007).
Anavekar, N. S. et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 351, 1285–1295 (2004).
Muntner, P. et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 55, 441–451 (2010).
Cheung, A. K. et al. Effects of intensive BP control in CKD. J. Am. Soc. Nephrol. 28, 2812–2823 (2017).
Levin, A., Singer, J., Thompson, C. R., Ross, H. & Lewis, M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am. J. Kidney Dis. 27, 347–354 (1996).
Townsend, R. R. et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am. J. Hypertens. 23, 282–289 (2010).
Kim, E. D. et al. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: the Atherosclerosis Risk in Communities (ARIC) study. Am. J. Kidney Dis. 72, 682–690 (2018).
Dhaun, N., Goddard, J. & Webb, D. J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 17, 943–955 (2006).
Gerstein, H. C. et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286, 421–426 (2001).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Jafar, T. H. et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann. Intern. Med. 139, 244–252 (2003).
O’Dowd, B. F. et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136, 355–360 (1993).
Sun, X. et al. Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens. Res. 34, 701–706 (2011).
Yang, R. et al. Apelin/APJ axis improves angiotensin II-induced endothelial cell senescence through AMPK/SIRT1 signaling pathway. Arch. Med. Sci. 14, 725–734 (2018).
Ishida, J. et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J. Biol. Chem. 279, 26274–26279 (2004).
Iwanaga, Y., Kihara, Y., Takenaka, H. & Kita, T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II–angiotensin type 1 receptor system. J. Mol. Cell Cardiol. 41, 798–806 (2006).
Siddiquee, K. et al. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 29, 724–731 (2011).
Barnes, G. D. et al. Sustained cardiovascular actions of APJ agonism during renin–angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 6, 482–491 (2013).
Sato, T. et al. ELABELA–APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc. Res. 113, 760–769 (2017).
Zhang, Z. Z. et al. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension 70, 1165–1175 (2017).
Wang, W. et al. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc. Natl Acad. Sci. USA 116, 13006–13015 (2019).
Read, C. et al. International Union of Basic and Clinical Pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacol. Rev. 71, 467–502 (2019).
Tucker, B. et al. Zebrafish angiotensin II receptor-like 1a (agtrl1a) is expressed in migrating hypoblast, vasculature, and in multiple embryonic epithelia. Gene Expr. Patterns 7, 258–265 (2007).
Scott, I. C. et al. The G protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev. Cell 12, 403–413 (2007).
Kalin, R. E. et al. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev. Biol. 305, 599–614 (2007).
Tatemoto, K. et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476 (1998).
Lee, D. K. et al. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 74, 34–41 (2000).
Chng, S. C., Ho, L., Tian, J. & Reversade, B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell 27, 672–680 (2013).
Pauli, A. et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636 (2014).
Maguire, J. J., Kleinz, M. J., Pitkin, S. L. & Davenport, A. P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 54, 598–604 (2009).
Zhen, E. Y., Higgs, R. E. & Gutierrez, J. A. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal. Biochem. 442, 1–9 (2013).
Shin, K., Pandey, A., Liu, X. Q., Anini, Y. & Rainey, J. K. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open. Bio 3, 328–333 (2013).
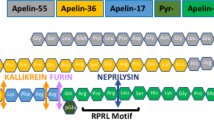
Fischer, C. et al. Plasma kallikrein cleaves and inactivates apelin-17: palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur. J. Med. Chem. 166, 119–124 (2019).
Murza, A., Belleville, K., Longpre, J. M., Sarret, P. & Marsault, E. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers 102, 297–303 (2014).
McKinnie, S. M. et al. The metalloprotease neprilysin degrades and inactivates apelin peptides. Chembiochem 17, 1495–1498 (2016).
Velazquez, E. J. et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N. Engl. J. Med. 380, 539–548 (2019).
Vickers, C. et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 277, 14838–14843 (2002).
Wang, W. et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17: physiological effects in the cardiovascular system. Hypertension 68, 365–377 (2016).
Yang, P. et al. [Pyr(1)]Apelin-13(1–12) is a biologically active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr(1)]Apelin-13. Front. Neurosci. 11, 92 (2017).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637 (2004).
Donoghue, M. et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 87, E1–E9 (2000).
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
To, K. F. & Lo, A. W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J. Pathol. 203, 740–743 (2004).
Benton, D. J. et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 588, 327–330 (2020).
Nyimanu, D. et al. Development and validation of an LC–MS/MS method for detection and quantification of in vivo derived metabolites of [Pyr(1)]apelin-13 in humans. Sci. Rep. 9, 19934 (2019).
Charo, D. N. et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol. Heart Circ. Physiol 297, H1904–H1913 (2009).
Zeng, X. X., Wilm, T. P., Sepich, D. S. & Solnica-Krezel, L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev. Cell 12, 391–402 (2007).
Freyer, L. et al. Loss of apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep. 20, 2116–2130 (2017).
Sharma, B. et al. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and APJ-deficient hearts. Dev. Cell 42, 655–666 e653 (2017).
Yang, P. et al. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 135, 1160–1173 (2017).
Hosoya, M. et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 275, 21061–21067 (2000).
O’Carroll, A. M., Selby, T. L., Palkovits, M. & Lolait, S. J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Biophys. Acta 1492, 72–80 (2000).
Medhurst, A. D. et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 84, 1162–1172 (2003).
Dray, C. et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am. J. Physiol. Endocrinol. Metab. 298, E1161–1169 (2010).
Katugampola, S. D., Maguire, J. J., Matthewson, S. R. & Davenport, A. P. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br. J. Pharmacol. 132, 1255–1260 (2001).
Kleinz, M. J., Skepper, J. N. & Davenport, A. P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 126, 233–240 (2005).
Wang, G. et al. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology 145, 1342–1348 (2004).
Kleinz, M. J. & Davenport, A. P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 118, 119–125 (2004).
Marsault, E. et al. The apelinergic system: a perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 1455, 12–33 (2019).
Boucher, J. et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 (2005).
Kawamata, Y. et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta 1538, 162–171 (2001).
Deng, C., Chen, H., Yang, N., Feng, Y. & Hsueh, A. J. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J. Biol. Chem. 290, 18261–18268 (2015).
O’Carroll, A. M. et al. Expression and functional implications of the renal apelinergic system in rodents. PLoS ONE 12, e0183094 (2017).
Perjes, A. et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic. Res. Cardiol. 111, 2 (2016).
Wang, Z. et al. Elabela–apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 5, 8170 (2015).
El Messari, S. et al. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J. Neurochem. 90, 1290–1301 (2004).
Nyimanu, D. et al. Apelin-36-[L28A] and Apelin-36-[L28C(30kDa-PEG)] peptides that improve diet induced obesity are G protein biased ligands at the apelin receptor. Peptides 121, 170139 (2019).
McKinnie, S. M. K. et al. Synthetic modification within the “RPRL” region of apelin peptides: impact on cardiovascular activity and stability to neprilysin and plasma degradation. J. Med. Chem. 60, 6408–6427 (2017).
Read, C. et al. Apelin peptides linked to anti-serum albumin domain antibodies retain affinity in vitro and are efficacious receptor agonists in vivo. Basic. Clin. Pharmacol. Toxicol. 126 (Suppl 6), 96–103 (2020).
Langelaan, D. N., Bebbington, E. M., Reddy, T. & Rainey, J. K. Structural insight into G-protein coupled receptor binding by apelin. Biochemistry 48, 537–548 (2009).
Macaluso, N. J., Pitkin, S. L., Maguire, J. J., Davenport, A. P. & Glen, R. C. Discovery of a competitive apelin receptor (APJ) antagonist. ChemMedChem 6, 1017–1023 (2011).
Murza, A. et al. Elucidation of the structure–activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem 7, 318–325 (2012).
Zhang, Y. et al. Identifying structural determinants of potency for analogs of apelin-13: integration of C-terminal truncation with structure–activity. Bioorg Med. Chem. 22, 2992–2997 (2014).
Ma, Y. et al. Structural basis for apelin control of the human apelin receptor. Structure 25, 858–866.e854 (2017).
Langelaan, D. N. et al. Structural features of the apelin receptor N-terminal tail and first transmembrane segment implicated in ligand binding and receptor trafficking. Biochim. Biophys. Acta 1828, 1471–1483 (2013).
Gerbier, R. et al. New structural insights into the apelin receptor: identification of key residues for apelin binding. FASEB J. 29, 314–322 (2015).
Couvineau, P., Llorens-Cortes, C. & Iturrioz, X. Elabela/Toddler and apelin bind differently to the apelin receptor. Faseb j. 34, 7989–8000 (2020).
Habata, Y. et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta 1452, 25–35 (1999).
Masri, B., Lahlou, H., Mazarguil, H., Knibiehler, B. & Audigier, Y. Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. Biophys. Res. Commun. 290, 539–545 (2002).
Masri, B., Morin, N., Pedebernade, L., Knibiehler, B. & Audigier, Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 281, 18317–18326 (2006).
Hashimoto, Y. et al. G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motility. Int. J. Mol. Med. 16, 787–792 (2005).
Szokodi, I. et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 91, 434–440 (2002).
Dray, C. et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 8, 437–445 (2008).
Evans, N. A. et al. Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J. Neurochem. 77, 476–485 (2001).
Lee, D. K., Ferguson, S. S., George, S. R. & O’Dowd, B. F. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem. Biophys. Res. Commun. 395, 185–189 (2010).
Zhou, N. et al. Cell–cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology 307, 22–36 (2003).
Wootten, D., Christopoulos, A., Marti-Solano, M., Babu, M. M. & Sexton, P. M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 (2018).
Scimia, M. C. et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature 488, 394–398 (2012).
Japp, A. G. et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 52, 908–913 (2008).
Japp, A. G. et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 121, 1818–1827 (2010).
Brame, A. L. et al. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension 65, 834–840 (2015).
Pope, G. R., Tilve, S., McArdle, C. A., Lolait, S. J. & O’Carroll, A. M. Agonist-induced internalization and desensitization of the apelin receptor. Mol. Cell Endocrinol. 437, 108–119 (2016).
Murza, A. et al. Discovery and structure–activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J. Med. Chem. 59, 2962–2972 (2016).
Wang, W. et al. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia–reperfusion injury: therapeutic potential of synthetic Apelin analogues. J. Am. Heart Assoc. 2, e000249 (2013).
Read, C. et al. Cardiac action of the first G protein biased small molecule apelin agonist. Biochem. Pharmacol. 116, 63–72 (2016).
Ason, B. et al. Cardiovascular response to small molecule APJ activation. JCI Insight 5, e132898 (2020).
Salcedo, A. et al. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul. Pept. 144, 50–55 (2007).
Lee, D. K. et al. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 146, 231–236 (2005).
Tatemoto, K. et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99, 87–92 (2001).
Wang, C., Du, J. F., Wu, F. & Wang, H. C. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol 294, H2540–H2546 (2008).
Perjes, A. et al. Apelin increases cardiac contractility via protein kinase Cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PLoS ONE 9, e93473 (2014).
Kuba, K. et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 101, e32–42 (2007).
Farkasfalvi, K. et al. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem. Biophys. Res. Commun. 357, 889–895 (2007).
Ashley, E. A. et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 65, 73–82 (2005).
Sato, T. et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 123, 5203–5211 (2013).
Sato, T. et al. Loss of apelin augments angiotensin II-induced cardiac dysfunction and pathological remodeling. Int J Mol Sci 20, 239 (2019).
Cheng, C. C. et al. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur. J. Clin. Invest. 43, 34–40 (2013).
Cox, C. M., D’Agostino, S. L., Miller, M. K., Heimark, R. L. & Krieg, P. A. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 296, 177–189 (2006).
Kidoya, H. et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 27, 522–534 (2008).
Kasai, A. et al. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 1717–1722 (2008).
Eyries, M. et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ. Res. 103, 432–440 (2008).
Ronkainen, V. P. et al. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. 21, 1821–1830 (2007).
Adam, F. et al. Apelin: an antithrombotic factor that inhibits platelet function. Blood 127, 908–920 (2016).
Zhang, G. et al. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood 118, 3670–3679 (2011).
Masoud, A. G. et al. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Invest. 130, 94–107 (2020).
Cheng, H. et al. Involvement of apelin/APJ axis in thrombogenesis in valve heart disease patients with atrial fibrillation. Int. Heart J. 60, 145–150 (2019).
Chun, H. J. et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Invest. 118, 3343–3354 (2008).
Hashimoto, T. et al. Requirement of apelin–apelin receptor system for oxidative stress-linked atherosclerosis. Am. J. Pathol. 171, 1705–1712 (2007).
Japp, A. G. & Newby, D. E. The apelin–APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol. 75, 1882–1892 (2008).
Földes, G. et al. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem. Biophys. Res. Commun. 308, 480–485 (2003).
Chong, K. S., Gardner, R. S., Morton, J. J., Ashley, E. A. & McDonagh, T. A. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur. J. Heart Fail. 8, 355–360 (2006).
Chen, M. M. et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 108, 1432–1439 (2003).
Fukushima, H., Kobayashi, N., Takeshima, H., Koguchi, W. & Ishimitsu, T. Effects of olmesartan on apelin/APJ and Akt/endothelial nitric oxide synthase pathway in dahl rats with end-stage heart failure. J. Cardiovasc. Pharmacol. 55, 83–88 (2010).
Berry, M. F. et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 110, II187–II193 (2004).
Azizi, Y., Faghihi, M., Imani, A., Roghani, M. & Nazari, A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides 46, 76–82 (2013).
Kido, M. et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 46, 2116–2124 (2005).
Abbasloo, E., Najafipour, H. & Vakili, A. Chronic treatment with apelin, losartan and their combination reduces myocardial infarct size and improves cardiac mechanical function. Clin. Exp. Pharmacol. Physiol. 47, 393–402 (2020).
Ellinor, P. T., Low, A. F. & Macrae, C. A. Reduced apelin levels in lone atrial fibrillation. Eur. Heart J. 27, 222–226 (2006).
Gurger, M. et al. The association between apelin-12 levels and paroxysmal supraventricular tachycardia. J. Cardiovasc. Med. 15, 642–646 (2014).
Falcone, C. et al. Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation. Int. J. Immunopathol. Pharmacol. 23, 917–925 (2010).
Kim, Y. M. et al. Apelin increases atrial conduction velocity, refractoriness, and prevents inducibility of atrial fibrillation. JCI Insight 5, e126525 (2020).
Watanabe, H. et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am. Heart J. 158, 629–636 (2009).
Ananthapanyasut, W. et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 173–181 (2010).
Kumar, S. et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J. Am. Coll. Cardiol. 74, 2204–2215 (2019).
Hus-Citharel, A. et al. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 74, 486–494 (2008).
Reaux-Le Goazigo, A., Morinville, A., Burlet, A., Llorens-Cortes, C. & Beaudet, A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology 145, 4392–4400 (2004).
Reaux, A. et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 77, 1085–1096 (2001).
De Mota, N. et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl Acad. Sci. USA 101, 10464–10469 (2004).
Azizi, M. et al. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J. Am. Soc. Nephrol. 19, 1015–1024 (2008).
Hus-Citharel, A. et al. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology 155, 4483–4493 (2014).
Boulkeroua, C. et al. Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in kidney collecting duct cells. Cell Physiol. Biochem. 53, 687–700 (2019).
Sawhney, S. et al. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am. J. Kidney Dis. 69, 18–28 (2017).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
See, E. J. et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 95, 160–172 (2019).
Chen, H. et al. Apelin protects against acute renal injury by inhibiting TGF-beta1. Biochim. Biophys. Acta 1852, 1278–1287 (2015).
Gholampour, F., Bagheri, A., Barati, A., Masoudi, R. & Owji, S. M. Remote ischemic perconditioning modulates apelin expression after renal ischemia–reperfusion injury. J. Surg. Res. 247, 429–437 (2020).
Sagiroglu, T. et al. Effects of apelin and leptin on renal functions following renal ischemia/reperfusion: an experimental study. Exp. Ther. Med. 3, 908–914 (2012).
Kim, J. S. et al. Protective role of apelin against cyclosporine-induced renal tubular injury in rats. Transpl. Proc. 49, 1499–1509 (2017).
Chen, H. et al. ELABELA and an ELABELA fragment protect against AKI. J. Am. Soc. Nephrol. 28, 2694–2707 (2017).
Couser, W. G., Remuzzi, G., Mendis, S. & Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 80, 1258–1270 (2011).
Muller, T. et al. Apelinergic system in the kidney: implications for diabetic kidney disease. Physiol. Rep. 6, e13939 (2018).
Guo, C. et al. Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities. J. Cell Mol. Med. 19, 2273–2285 (2015).
Day, R. T., Cavaglieri, R. C. & Feliers, D. Apelin retards the progression of diabetic nephropathy. Am. J. Physiol. Ren. Physiol 304, F788–F800 (2013).
Zhang, B. H., Wang, W., Wang, H., Yin, J. & Zeng, X. J. Promoting effects of the adipokine, apelin, on diabetic nephropathy. PLoS ONE 8, e60457 (2013).
Hu, H., He, L., Li, L. & Chen, L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol. Genet. Metab. 119, 20–27 (2016).
Chen, H. et al. Apelin inhibits the development of diabetic nephropathy by regulating histone acetylation in Akita mouse. J. Physiol. 592, 505–521 (2014).
Zhang, J., Yin, J., Wang, Y., Li, B. & Zeng, X. Apelin impairs myogenic response to induce diabetic nephropathy in mice. FASEB J. 32, 4315–4327 (2018).
Liu, Y., Zhang, J., Wang, Y. & Zeng, X. Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes. Cell Death Dis. 8, e3006 (2017).
Blanchard, A. et al. An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J. Clin. Endocrinol. Metab. 98, 2084–2089 (2013).
Urwyler, S. A. et al. Plasma apelin concentrations in patients with polyuria–polydipsia syndrome. J. Clin. Endocrinol. Metab. 101, 1917–1923 (2016).
Lacquaniti, A. et al. Apelin and copeptin: two opposite biomarkers associated with kidney function decline and cyst growth in autosomal dominant polycystic kidney disease. Peptides 49, 1–8 (2013).
Kocer, D., Karakukcu, C., Ozturk, F., Eroglu, E. & Kocyigit, I. Evaluation of fibrosis markers: apelin and transforming growth factor-beta1 in autosomal dominant polycystic kidney disease patients. Ther. Apher. Dial. 20, 517–522 (2016).
Nogueira, A., Pires, M. J. & Oliveira, P. A. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. Vivo 31, 1–22 (2017).
Wang, Y. et al. beta-Arrestin-biased AT1R stimulation promotes extracellular matrix synthesis in renal fibrosis. Am. J. Physiol. Ren. Physiol 313, F1–F8 (2017).
Maschio, G. et al. Effect of the angiotensin-converting-enzyme benazepril on the progression of chronic renal insufficiency. N. Engl. J. Med. 334, 939–945 (1996).
Xu, C. et al. ELABELA antagonizes intrarenal renin–angiotensin system to lower blood pressure and protects against renal injury. Am. J. Physiol. Ren. Physiol 318, F1122–F1135 (2020).
Wang, L. Y. et al. The regulatory peptide apelin: a novel inhibitor of renal interstitial fibrosis. Amino Acids 46, 2693–2704 (2014).
Schreiber, C. A., Holditch, S. J., Generous, A. & Ikeda, Y. Sustained ELABELA gene therapy in high-salt diet-induced hypertensive rats. Curr. Gene Ther. 16, 349–360 (2017).
Nishida, M. et al. The role of apelin on the alleviative effect of angiotensin receptor blocker in unilateral ureteral obstruction-induced renal fibrosis. Nephron Extra 2, 39–47 (2012).
Castan-Laurell, I., Masri, B. & Valet, P. The apelin/APJ system as a therapeutic target in metabolic diseases. Expert. Opin. Ther. Targets 23, 215–225 (2019).
Bertrand, C., Valet, P. & Castan-Laurell, I. Apelin and energy metabolism. Front. Physiol. 6, 115 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03956576 (2018).
Sorli, S. C., Le Gonidec, S., Knibiehler, B. & Audigier, Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene 26, 7692–7699 (2007).
Picault, F. X. et al. Tumour co-expression of apelin and its receptor is the basis of an autocrine loop involved in the growth of colon adenocarcinomas. Eur. J. Cancer 50, 663–674 (2014).
Heo, K. et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral. Oncol. 48, 500–506 (2012).
Berta, J. et al. Apelin expression in human non-small cell lung cancer. Role in angiogenesis and prognosis. J. Thorac. Oncol., 1120–1129 (2010).
Uribesalgo, I. et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol. Med. 11, e9266 (2019).
Davenport, A. P. et al. First in human study of a novel biased apelin receptor ligand, MM54, A G-alpha(i) agonist/beta-arrestin antagonist. Circ. Res. 123, e69–e81 (2018).
Harford-Wright, E. et al. Pharmacological targeting of apelin impairs glioblastoma growth. Brain 140, 2939–2954 (2017).
Mastrella, G. et al. Targeting APLN/APLNR improves antiangiogenic efficiency and blunts proinvasive side effects of VEGFA/VEGFR2 blockade in glioblastoma. Cancer Res. 79, 2298–2313 (2019).
Soulet, F. et al. ELA/APELA precursor cleaved by furin displays tumor suppressor function in renal cell carcinoma through mTORC1 activation. JCI Insight 5, 2298 (2020).
Patel, S. J. et al. Identification of essential genes for cancer immunotherapy. Nature 548, 537–542 (2017).
Tolkach, Y. et al. Apelin and apelin receptor expression in renal cell carcinoma. Br. J. Cancer 120, 633–639 (2019).
Yau, J. W. Y. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564 (2012).
Lu, Q., Feng, J. & Jiang, Y. R. The role of apelin in the retina of diabetic rats. PLoS ONE 8, e69703 (2013).
Qin, D., Zheng, X.-X. & Jiang, Y.-R. Apelin-13 induces proliferation, migration, and collagen I mRNA in human RPE cells via PI3K/Akt and MEK/Erk signalling pathways. Mol. Vis. 2013, 2227–2236 (2013).
Tao, Y. et al. Apelin in plasma and vitreous and in fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 51, 4237–4242 (2010).
Du, J. H. et al. Elevation of serum apelin-13 associated with proliferative diabetic retinopathy in type 2 diabetic patients. Int. J. Ophthalmol. 7, 968–973 (2014).
Kasai, A. et al. Inhibition of apelin expression switches endothelial cells from proliferative to mature state in pathological retinal angiogenesis. Angiogenesis 16, 723–734 (2013).
Acknowledgements
F.A.C. is supported by a Kidney Research UK Training Fellowship (TF_006_20171124). D.N., J.J.M. and A.P.D. are supported in whole or part by the Wellcome Trust (WT203814/Z/16/A for D.N.; WT107715/Z/15/Z for A.P.D. and J.J.M.). D.E.N. is supported by the British Heart Foundation (FS/06/064, FS/09/019, CH09/002, RG/16/10/32375, RE/18/5/34216) and Wellcome Trust (WT103782AIA). N.D. is supported by a Senior Clinical Research Fellowship from the Chief Scientist Office (SCAF/19/02).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the data, discussing the content, writing the text and reviewing or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks Mirjam Christ-Crain, who co-reviewed with Sophie Monnerat, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- ‘Message–address’ concept of peptide binding
-
The concept that agonists contain two distinct parts: one determines receptor efficacy and activation (the ‘message’); the other influences receptor selectivity (the ‘address’).
- Inotrope
-
A substance that alters the force of contraction of a muscle. This term is normally used to refer to effects on the myocardium and, unless otherwise specified, implies increased contractility.
Rights and permissions
About this article
Cite this article
Chapman, F.A., Nyimanu, D., Maguire, J.J. et al. The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol 17, 840–853 (2021). https://doi.org/10.1038/s41581-021-00461-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-021-00461-z
This article is cited by
-
Crosstalk between perivascular adipose tissue and adipocyte-derived peptide in the pathogenesis of diabetic cardiomyopathy
Cardiovascular Diabetology (2025)
-
Microenvironmental determinants of endothelial cell heterogeneity
Nature Reviews Molecular Cell Biology (2025)
-
Cxcl9 modulates aging associated microvascular metabolic and angiogenic dysfunctions in subcutaneous adipose tissue
Angiogenesis (2025)
-
Bidirectional associations between kidney function decline and carotid plaque progression: a longitudinal cohort study in the context of predictive, preventive, and personalised medicine
EPMA Journal (2025)
-
Circulating levels of visfatin and apelin as biomarkers in chronic kidney disease: a systematic review and meta-analysis
International Urology and Nephrology (2025)