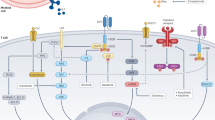
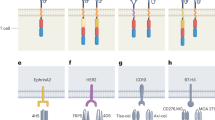
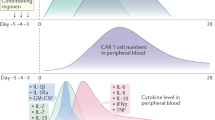
Abstract
Genetically engineered chimeric antigen receptor (CAR) T cells have emerged as a powerful treatment option in patients with B cell malignancies, but neurological adverse effects are common and hamper the success of such therapies. Immune effector cell-associated neurotoxicity syndrome encompasses a wide range of acute neurological adverse effects, including encephalopathy with alterations in cognition and behaviour, language, motor function and coordination. In patients treated with CAR T cells for CNS malignancies, a more localized on-tumour, on-target neurotoxicity syndrome termed tumour inflammation-associated neurotoxicity can develop acutely, resulting in localized oedema with mass effect or in electrophysiological dysfunction with neurological symptoms. Following B cell maturation antigen-targeting CAR T cell therapies, delayed neurological complications, including cranial nerve palsies and a unique delayed-onset parkinsonism syndrome, are increasingly recognized. Management of neurological complications includes symptomatic treatments such as antiepileptic drugs or cerebrospinal fluid diversion, temporary immunosuppression with corticosteroids, various cytokine-targeting agents, and other distinct approaches depending on the nature of the toxicity. As our understanding of the mechanisms that contribute to the various neurological adverse effects of CAR T cell and other T cell-engaging therapies increases, novel treatment strategies to alleviate symptoms, as well as innovative CAR designs, promise to improve the safety and neurotoxicity of these powerful immunotherapies.
Key points
-
Chimeric antigen receptor (CAR) T cell therapies for cancers are associated with a wide range of both acute and delayed neurological adverse events, and the underlying mechanisms of such diverse complications remain incompletely understood.
-
Immune effector cell-associated neurotoxicity syndrome, classically presenting with encephalopathy, is the most common acute neurological complication but is usually fully reversible.
-
Tumour inflammation-associated neurotoxicity is a distinct form of localized neurotoxicity syndrome that is uniquely seen in patients treated for CNS malignancies.
-
Delayed-onset neurotoxicities, such as cranial nerve palsies, peripheral neuropathies or parkinsonism, occur particularly with B cell maturation antigen-targeting CAR T cell therapies, can be irreversible and are the most challenging in clinical practice.
-
Current management strategies mostly rely on supportive care, corticosteroids and cytokine inhibitors, although their effectiveness varies depending on the type and timing of the underlying neurotoxicity syndrome.
-
Future directions to limit the most severe forms of neurotoxicity could include novel CAR designs with safety switches, and controlled trials testing neuroprotective interventions will allow us to better understand and potentially prevent these conditions and improve patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T Cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease — a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Rejeski, K. et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 10, e004475 (2022).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Karschnia, P. et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 133, 2212–2221 (2019).
Gudera, J. A., Baehring, J. M. & Karschnia, P. Parkinsonism following chimeric antigen receptor T cell therapy. JAMA Neurol. 81, 1223–1224 (2024).
Karschnia, P. et al. Neurotoxicity and management of primary and secondary central nervous system lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T-cells. Neuro Oncol. 25, 2239–2249 (2023).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
Graham, C. E. et al. Non-ICANS neurological complications after CAR T-cell therapies: recommendations from the EBMT Practice Harmonisation and Guidelines Committee. Lancet Oncol. 26, e203–e213 (2025).
Karschnia, P. et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 142, 1243–1248 (2023).
Bagley, S. J. et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat. Med. 30, 1320–1329 (2024).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Bachy, E. et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 28, 2145–2154 (2022).
Furqan, F. et al. Outpatient administration of CAR T-cell therapies using a strategy of no remote monitoring and early CRS intervention. Blood Adv. 8, 4320–4329 (2024).
Grant, S. J. et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transpl. Cell Ther. 28, 294–302 (2022).
First-ever CAR T-cell therapy approved in U.S. Cancer Discov. 7, OF1 (2017).
Gust, J., Ponce, R., Liles, W. C., Garden, G. A. & Turtle, C. J. Cytokines in CAR T cell-associated neurotoxicity. Front. Immunol. 11, 577027 (2020).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Karschnia, P. et al. Clinicopathologic findings in fatal neurotoxicity after adoptive immunotherapy with CD19-directed CAR T-cells. Hemasphere 5, e533 (2021).
Berger, S. C. et al. Molecular monitoring of T-cell kinetics and migration in severe neurotoxicity after real-world CD19-specific chimeric antigen receptor T cell therapy. Haematologica 108, 444–456 (2023).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Vinnakota, J. M. et al. Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome. Nat. Cancer 5, 1227–1249 (2024).
Schoeberl, F. et al. Neurofilament light chain serum levels correlate with the severity of neurotoxicity after CAR T-cell treatment. Blood Adv. 6, 3022–3026 (2022).
Butt, O. H. et al. Assessment of pretreatment and posttreatment evolution of neurofilament light chain levels in patients who develop immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. 8, 1652–1657 (2022).
Ursu, R. et al. Long-term neurological safety in B-cell lymphoma patients treated with anti-CD19 CAR T-cell therapy. Neurology 99, 511–515 (2022).
Beuchat, I. et al. EEG findings in CAR T-cell-associated neurotoxicity: clinical and radiological correlations. Neuro Oncol. 24, 313–325 (2022).
Stoecklein, S. et al. Functional connectivity MRI provides an imaging correlate for chimeric antigen receptor T-cell-associated neurotoxicity. Neurooncol. Adv. 5, vdad135 (2023).
Gust, J. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023).
Salvador, A. F., de Lima, K. A. & Kipnis, J. Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol. 21, 526–541 (2021).
Lin, F. Y. et al. Phase I trial of GD2.CART cells augmented with constitutive interleukin-7 receptor for treatment of high-grade pediatric CNS tumors. J. Clin. Oncol. 42, 2769–2779 (2024).
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 637, 708–715 (2024).
Abramson, J. S. et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N. Engl. J. Med. 377, 783–784 (2017).
Winkler, F. et al. Cancer neuroscience: state of the field, emerging directions. Cell 186, 1689–1707 (2023).
Zhang, B. et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Martin, T. et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 41, 1265–1274 (2023).
Cohen, A. D. et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 12, 32 (2022).
Marella, M. et al. Comprehensive BCMA expression profiling in adult normal human brain suggests a low risk of on-target neurotoxicity in BCMA-targeting multiple myeloma therapy. J. Histochem. Cytochem. 70, 273–287 (2022).
Graham, C. E. et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel-associated movement and neurocognitive toxicity. Blood 142, 1248–1252 (2023).
Alarcón, F., Zijlmans, J. C., Dueñas, G. & Cevallos, N. Post-stroke movement disorders: report of 56 patients. J. Neurol. Neurosurg. Psychiatry 75, 1568–1574 (2004).
Gust, J. BCMA-CAR T-cell treatment-associated parkinsonism. Blood 142, 1181–1183 (2023).
Van De Donk, N. W. C. J. et al. Clinical experience with cranial nerve impairment in the CARTITUDE-1, CARTITUDE-2 cohorts A, B, and C, and CARTITUDE-4 studies of ciltacabtagene autoleucel (cilta-cel). Blood 142, 3501 (2023).
Patrick, N., Bahlis, N. & Peters, S. Chimeric antigen receptor-T cell mediated bilateral facial nerve palsy: a case report. Neurohospitalist 13, 308–311 (2023).
Kathari, Y. K. et al. Immune-mediated facial nerve paralysis in a myeloma patient post B-cell maturation antigen-targeted chimeric antigen receptor T cells. Haematologica 109, 682–688 (2024).
Koch, C. et al. Diabetes insipidus and Guillain-Barré-like syndrome following CAR-T cell therapy: a case report. J. Immunother. Cancer 11, e006059 (2023).
Nair, R. et al. Acute leucoencephalomyelopathy and quadriparesis after CAR T-cell therapy. Haematologica 106, 1504–1506 (2021).
Sheikh, S. et al. Transverse myelitis after anti-CD19 directed CAR T cell therapy for relapsed large B cell lymphoma. eJHaem 3, 223–227 (2022).
Deschênes-Simard, X., Santomasso, B. D. & Dahi, P. B. Clinical features, pathophysiology, and management of acute myelopathy following CAR T-cell therapy. Blood 144, 2083–2094 (2024).
Santomasso, B. D., Gust, J. & Perna, F. How I treat unique and difficult-to-manage cases of CAR T-cell therapy-associated neurotoxicity. Blood 141, 2443–2451 (2023).
Kuboki, M. et al. Severe motor weakness due to disturbance in peripheral nerves following tisagenlecleucel treatment. In Vivo 35, 3407–3411 (2021).
Kaulen, L. D. et al. Toxicities and outcome after CD19-directed chimeric antigen receptor T-cell therapy for secondary neurolymphomatosis. Am. J. Hematol. 99, 2411–2415 (2024).
Kress, J. P. & Hall, J. B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 370, 1626–1635 (2014).
Amidi, Y. et al. Forecasting immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. J. Immunother. Cancer 10, e005459 (2022).
Rubin, D. B. et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 77, 1536–1542 (2020).
Zandaki, D. et al. EASIX and m-EASIX predict CRS and ICANS in pediatric and AYA patients after CD19-CAR T-cell therapy. Blood Adv. 9, 270–279 (2025).
Pennisi, M. et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 5, 3397–3406 (2021).
Zhao, Y. et al. Modified EASIX scores predict severe CRS/ICANS in patients with acute myeloid leukemia following CLL1 CAR-T cell therapy. Ann. Hematol. 103, 969–980 (2024).
Roddie, C. et al. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 7, 2872–2883 (2023).
Ahmed, N. et al. Optimizing the post-CAR T monitoring period in recipients of axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel. Blood Adv. 8, 5346–5354 (2024).
Winter, S. F., Martinez-Lage, M., Clement, N. F., Hochberg, E. P. & Dietrich, J. Fatal neurotoxicity after chimeric antigen receptor T-cell therapy: an unexpected case of fludarabine-associated progressive leukoencephalopathy. Eur. J. Cancer 144, 178–181 (2021).
Tanner, C. M. & Ostrem, J. L. Parkinson’s disease. N. Engl. J. Med. 391, 442–452 (2024).
England, J. D. & Asbury, A. K. Peripheral neuropathy. Lancet 363, 2151–2161 (2004).
Santomasso, B. D. et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 39, 3978–3992 (2021).
Gust, J., Taraseviciute, A. & Turtle, C. J. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 32, 1091–1101 (2018).
Locke, F. L. et al. Tocilizumab prophylaxis following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Transpl. Cell Ther. 30, 1065–1079 (2024).
Jain, M. D., Smith, M. & Shah, N. N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 141, 2430–2442 (2023).
Strati, P. et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020).
Wehrli, M. et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 10, e003847 (2022).
Gazeau, N. et al. Anakinra for refractory cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T cell therapy. Transpl. Cell Ther. 29, 430–437 (2023).
Zurko, J. C. et al. Use of early intrathecal therapy to manage high-grade immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. 8, 773–775 (2022).
Shah, N. N., Johnson, B. D., Fenske, T. S., Raj, R. V. & Hari, P. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell-associated neurotoxicity syndrome. Blood Adv. 4, 2119–2122 (2020).
Bailey, S. R. et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov. 3, 136–153 (2022).
Bajwa, A. et al. Siltuximab for chimeric antigen receptor T-cell therapy related CRS and ICANS — a multicenter retrospective analysis. Blood Adv. 9, 170–175 (2024).
Chung, M. et al. Immune checkpoint inhibitor induced anti-glutamic acid decarboxylase 65 (anti-GAD 65) limbic encephalitis responsive to intravenous immunoglobulin and plasma exchange. J. Neurol. 267, 1023–1025 (2020).
Wang, M. et al. Management of a patient with mantle cell lymphoma who developed severe neurotoxicity after chimeric antigen receptor T-cell therapy in ZUMA-2. J. Immunother. Cancer 8, e001114 (2020).
Weber, E. W. et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 3, 711–717 (2019).
Pan, J. et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J. Cell Mol. Med. 25, 1089–1099 (2021).
Sumransub, N. et al. Simvastatin with intrathecal dexamethasone reduces neurotoxicity in adults receiving chimeric antigen receptor (CAR) T-cells treatment. Blood 142, 3493 (2023).
Blumenberg, V. et al. Cyclophosphamide mitigates non-ICANS neurotoxicities after ciltacabtagene autoleucel treatment. Blood 145, 2788–2793 (2025).
Frigault, M. J. et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood 139, 2306–2315 (2022).
Park, S. H. et al. Pathological classification of the intramedullary spinal cord tumors according to 2021 World Health Organization classification of central nervous system tumors, a single-institute experience. Neurospine 19, 780–791 (2022).
Park, J. H. et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat. Med. 29, 1710–1717 (2023).
Strati, P. et al. A phase 1 study of prophylactic anakinra to mitigate ICANS in patients with large B-cell lymphoma. Blood Adv. 7, 6785–6789 (2023).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Philip, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124, 1277–1287 (2014).
Ying, Z. et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 25, 947–953 (2019).
Sahillioglu, A. C. & Schumacher, T. N. Safety switches for adoptive cell therapy. Curr. Opin. Immunol. 74, 190–198 (2022).
Perna, F. et al. CAR T-cell toxicities: from bedside to bench, how novel toxicities inform laboratory investigations. Blood Adv. 8, 4348–4358 (2024).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
P.K. has served as a consultant for the American Society of Clinical Oncology. J.D. has served as a consultant and on advisory boards for Amgen, Novartis, Janssen and Johnson & Johnson. He has received research support from Ono Pharmaceuticals and royalties from Wolters Kluwer.
Peer review
Peer review information
Nature Reviews Neurology thanks B. Santomasso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karschnia, P., Dietrich, J. Neurological complications of CAR T cell therapy for cancers. Nat Rev Neurol 21, 422–431 (2025). https://doi.org/10.1038/s41582-025-01112-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01112-8