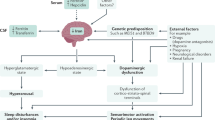
Abstract
Restless legs syndrome (RLS) and periodic limb movements (PLMs) are increasingly recognized as risk factors for cerebrovascular and cardiovascular diseases, particularly stroke. Conversely, stroke can precipitate or exacerbate the symptoms of RLS and PLMs. This Review explores the shared pathophysiological mechanisms linking RLS and PLMs to cerebrovascular pathology, highlighting a bidirectional relationship. We discuss mechanisms including neurotransmitter dysregulation, autonomic dysfunction, inflammation, oxidative stress, hypoxia and genomic and proteomic factors. Furthermore, we summarize emerging evidence and provide new insights on the potential clinical relevance of RLS in cerebrovascular risk assessment and management.
Key points
-
Restless legs syndrome (RLS) and periodic limb movements (PLMs) are linked to increased risks of stroke and cardiovascular disease. Stroke might trigger or worsen RLS and/or PLM symptoms, suggesting a bidirectional relationship.
-
Shared pathophysiological mechanisms among RLS, PLMs and stroke include neurotransmitter imbalance, autonomic dysfunction, inflammation, oxidative stress, hypoxia and overlapping proteomic and genetic pathways.
-
RLS and PLMs are linked to nocturnal blood pressure surges and heightened sympathetic activity, which can contribute to long-term vascular injury and elevated stroke risk.
-
A large study found that untreated RLS significantly increases the risk of vascular events, whereas effective RLS treatment might mitigate stroke and cardiovascular complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Manconi, M. et al. Restless legs syndrome. Nat. Rev. Dis. Prim. 7, 80 (2021).
Allen, R. P. et al. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria — history, rationale, description, and significance. Sleep Med. 15, 860–873 (2014).
Hening, W. et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 5, 237–246 (2004).
Allen, R. P., Stillman, P. & Myers, A. J. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 11, 31–37 (2010).
Bjorvatn, B. et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 6, 307–312 (2005).
Cho, Y. W., Kim, D. H., Allen, R. P. & Earley, C. J. Assessing health-related quality of life in patients with restless legs syndrome in Korea: comparison with other chronic medical diseases. Sleep Med. 13, 1158–1163 (2012).
Chenini, S. et al. Depressive symptoms and suicidal thoughts in restless legs syndrome. Mov. Disord. 37, 812–825 (2022).
Trenkwalder, C., Allen, R., Högl, B., Paulus, W. & Winkelmann, J. Restless legs syndrome associated with major diseases. Neurology 86, 1336–1343 (2016).
Patatanian, E. & Claborn, M. K. Drug-induced restless legs syndrome. Ann. Pharmacother. 52, 662–672 (2018).
Montplaisir, J. et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov. Disord. 12, 61–65 (1997).
Hoxha, O. et al. Association of periodic limb movements with medication classes. Neurology 98, e1585–e1595 (2022).
Gottlieb, D. J., Somers, V. K., Punjabi, N. M. & Winkelman, J. W. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med. 31, 10–17 (2017).
Tinh, D. X. et al. Stroke-related restless leg syndrome in hemorrhagic and ischemic stroke patients. SAGE Open Med. 13, 20503121251336900 (2025).
Wang, X.-X. et al. Risk factors and prognosis of acute ischemic stroke related restless legs syndrome. Sleep Med. 129, 75–81 (2025).
Gupta, A., Shukla, G., Mohammed, A., Goyal, V. & Behari, M. Restless legs syndrome, a predictor of subcortical stroke: a prospective study in 346 stroke patients. Sleep Med. 29, 61–67 (2017).
Gao, X. et al. Treating restless legs syndrome was associated with low risk of cardiovascular disease: a cohort study with 3.4 years of follow-up. J. Am. Heart Assoc. 10, e018674 (2021).
Koh, S. Y., Kim, M. S., Lee, S. M., Hong, J. M. & Yoon, J. H. Impaired vascular endothelial function in patients with restless legs syndrome: a new aspect of the vascular pathophysiology. J. Neurol. Sci. 359, 207–210 (2015).
Winkelman, J. W., Shahar, E., Sharief, I. & Gottlieb, D. J. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology 70, 35–42 (2008).
Wang, X.-X., Feng, Y., Tan, E.-K., Ondo, W. G. & Wu, Y.-C. Stroke-related restless legs syndrome: epidemiology, clinical characteristics, and pathophysiology. Sleep Med. 90, 238–248 (2022).
Coelho, F. M. S., Georgsson, H., Narayansingh, M., Swartz, R. H. & Murray, B. J. Higher prevalence of periodic limb movements of sleep in patients with history of stroke. J. Clin. Sleep Med. 6, 428–430 (2010).
Molnar, M. Z., Lu, J. L., Kalantar-Zadeh, K. & Kovesdy, C. P. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J. Sleep Res. 25, 47–56 (2016).
Dauvilliers, Y., Chenini, S., Barateau, L. & Somers, V. K. Restless legs syndrome, periodic leg movements, hypertension and cardiovascular diseases. Circ. Res. 137, 746–763 (2025).
Shen, Y. et al. Association between restless legs syndrome and hypertension: a meta-analysis of nine population-based studies. Neurol. Sci. 39, 235–242 (2018).
Liu, Y., Liu, G., Li, L., Yang, J. & Ma, S. Evaluation of cardiovascular risk factors and restless legs syndrome in women and men: a preliminary population-based study in China. J. Clin. Sleep Med. 14, 445–450 (2018).
Katsanos, A. H. et al. Restless legs syndrome and cerebrovascular/cardiovascular events: systematic review and meta-analysis. Acta Neurol. Scand. 137, 142–148 (2018).
Innes, K. E., Selfe, T. K. & Agarwal, P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med. Rev. 16, 309–339 (2012).
Hwang, I. C., Na, K.-S., Lee, Y. J. & Kang, S.-G. Higher prevalence of hypertension among individuals with restless legs syndrome: a meta-analysis. Psychiatry Investig. 15, 701–709 (2018).
Gupta, A. et al. Restless legs syndrome/Willis–Ekbom disease among patients with resistant hypertension versus stroke patients — a prospective study. Sleep Breath. 26, 1245–1251 (2022).
Sabic, A., Sinanovic, O., Sabic, D. & Galic, G. Restless legs syndrome in patients with hypertension and diabetes mellitus. Med. Arch. 70, 116 (2016).
Batool-Anwar, S. et al. Restless legs syndrome and hypertension in middle-aged women. Hypertension 58, 791–796 (2011).
Catzín-Kuhlmann, A. et al. Restless legs syndrome and hypertension in Mexican women. Mov. Disord. Clin. Pract. 2, 274–279 (2015).
Innes, K. E., Kandati, S., Flack, K. L., Agarwal, P. & Selfe, T. K. The relationship of restless legs syndrome to history of pregnancy-induced hypertension. J. Women’s Health 25, 397–408 (2016).
Ma, S. et al. Restless legs syndrome and hypertension in Chinese pregnant women. Neurol. Sci. 36, 877–881 (2015).
Pennestri, M. H., Montplaisir, J., Colombo, R., Lavigne, G. & Lanfranchi, P. A. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology 68, 1213–1218 (2007).
Sieminski, M., Pyrzowski, J. & Partinen, M. Periodic limb movements in sleep are followed by increases in EEG activity, blood pressure, and heart rate during sleep. Sleep Breath. 21, 497–503 (2017).
Dean, D. A. et al. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 38, 587–596 (2015).
Shin, D.-S. et al. Periodic limb movements during sleep are not associated with hypertension in a clinical cohort of Korean adults. Am. J. Hypertens. 31, 1228–1233 (2018).
Winter, A. C. et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open 2, e000866 (2012).
Schlesinger, I., Erikh, I., Nassar, M. & Sprecher, E. Restless legs syndrome in stroke patients. Sleep Med. 16, 1006–1010 (2015).
Winter, A. C. et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am. J. Med. 126, 228–235 (2013).
Shiina, T., Suzuki, K., Okamura, M., Matsubara, T. & Hirata, K. Restless legs syndrome and its variants in acute ischemic stroke. Acta Neurol. Scand. 139, 260–268 (2019).
Kendzerska, T., Kamra, M., Murray, B. J. & Boulos, M. I. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep 40, zsx013 (2017).
Boulos, M. I. et al. Restless legs syndrome after high-risk TIA and minor stroke: association with reduced quality of life. Sleep Med. 37, 135–140 (2017).
Lee, S. et al. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov. Disord. 24, 77–84 (2009).
An, T. et al. The association between vitamin D and restless legs syndrome following acute ischemic stroke. Sci. Rep. 15, 14867 (2025).
Moreau, A. et al. Complete resolution of restless legs syndrome following ischemic stroke of the right middle cerebral artery. Sleep Biol. Rhythm. 22, 531–534 (2024).
Cogez, J., Ribeiro, E., de La Sayette, V. & Viader, F. Complete recovery of restless legs syndrome after unilateral thalamic and tegmental infarction: a case report. J. Clin. Neurosci. 44, 229–230 (2017).
Zinchuk, A. et al. Association of periodic limb movements and obstructive sleep apnea with risk of cardiovascular disease and mortality. J. Am. Heart Assoc. 13, e031630 (2024).
Kendzerska, T., Gershon, A. S., Hawker, G., Leung, R. S. & Tomlinson, G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 11, e1001599 (2014).
Calabresi, P., Picconi, B., Tozzi, A. & Di Filippo, M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 30, 211–219 (2007).
Connor, J. R. et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 132, 2403–2412 (2009).
Kocar, T. D., Müller, H.-P. & Kassubek, J. Differential functional connectivity in thalamic and dopaminergic pathways in restless legs syndrome: a meta-analysis. Ther. Adv. Neurol. Disord. 13, 1756286420941670 (2020).
Walters, A. S. Restless legs syndrome, neuroleptic-induced akathisia, and the iron opioid dopamine link. Sleep 47, zsae008 (2024).
Tang, M. et al. Circadian rhythm in restless legs syndrome. Front. Neurol. 14, 1105463 (2023).
Provini, F. & Chiaro, G. Neuroimaging in restless legs syndrome. Sleep Med. Clin. 10, 215–226 (2015).
Rizzo, G., Li, X., Galantucci, S., Filippi, M. & Cho, Y. W. Brain imaging and networks in restless legs syndrome. Sleep Med. 31, 39–48 (2017).
Mizuno, S., Mihara, T., Miyaoka, T., Inagaki, T. & Horiguchi, J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J. Sleep Res. 14, 43–47 (2005).
Cochen De Cock, V. Therapies for restless legs in Parkinson’s disease. Curr. Treat. Options Neurol. 21, 56 (2019).
Allen, R. P. Restless leg syndrome/Willis–Ekbom disease pathophysiology. Sleep Med. Clin. 10, 207–214 (2015).
Trenkwalder, C. et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 17, 994–1005 (2018).
Garcia-Borreguero, D. et al. Guidelines for the first-line treatment of restless legs syndrome/Willis–Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 21, 1–11 (2016).
Winkelman, J. W. et al. Treatment of restless legs syndrome and periodic limb movement disorder: an American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 21, 137–152 (2025).
Zeng, P., Wang, T., Zhang, L. & Guo, F. Exploring the causes of augmentation in restless legs syndrome. Front. Neurol. 14, 1160112 (2023).
Ruppert, E. et al. Hyperdopaminergism in lenticulostriate stroke-related restless legs syndrome: an imaging study. Sleep. Med. 30, 136–138 (2017).
Sechi, G. & Sechi, E. Restless legs syndrome and cerebrovascular disease. Lancet Neurol. 12, 734 (2013).
Sechi, G. et al. Restless legs syndrome and periodic limb movements after ischemic stroke in the right lenticulostriate region. Parkinsonism Relat. Disord. 14, 157–160 (2008).
Guo, J., Tuo, Q. & Lei, P. Iron, ferroptosis, and ischemic stroke. J. Neurochem. 165, 487–520 (2023).
Walters, A. S., Ondo, W. G., Zhu, W. & Le, W. Does the endogenous opiate system play a role in the restless legs syndrome?: a pilot post-mortem study. J. Neurol. Sci. 279, 62–65 (2009).
Walters, A. S. et al. Review of the role of the endogenous opioid and melanocortin systems in the restless legs syndrome. Brain 147, 26–38 (2024).
Boutin, H., Catherine, A., MacKenzie, E. T., Jauzac, P. & Dauphin, F. Long-term alterations in μ, δ and κ opioidergic receptors following middle cerebral artery occlusion in mice. Acta Neuropathol. 114, 491–500 (2007).
Cumming, P., Marton, J., Lilius, T. O., Olberg, D. E. & Rominger, A. A survey of molecular imaging of opioid receptors. Molecules 24, 4190 (2019).
Willoch, F. et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain 108, 213–220 (2004).
Liampas, A. et al. Prevalence and management challenges in central post-stroke neuropathic pain: a systematic review and meta-analysis. Adv. Ther. 37, 3278–3291 (2020).
Allen, R. P., Barker, P. B., Horská, A. & Earley, C. J. Thalamic glutamate/glutamine in restless legs syndrome. Neurology 80, 2028–2034 (2013).
Garcia-Borreguero, D., Cano, I. & Granizo, J. J. Treatment of restless legs syndrome with the selective AMPA receptor antagonist perampanel. Sleep Med. 34, 105–108 (2017).
Cachope, R. & Cheer, J. F. Local control of striatal dopamine release. Front. Behav. Neurosci. 8, 188 (2014).
Avshalumov, M. V., Chen, B. T., Marshall, S. P., Peña, D. M. & Rice, M. E. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J. Neurosci. 23, 2744–2750 (2003).
Antelmi, E. et al. Sensory aspects of restless legs syndrome: clinical, neurophysiological and neuroimaging prospectives. Sleep Med. Rev. 76, 101949 (2024).
Dávalos, A., Shuaib, A. & Wahlgren, N. G. Neurotransmitters and pathophysiology of stroke: evidence for the release of glutamate and other transmitters/mediators in animals and humans. J. Stroke Cerebrovasc. Dis. 9, 2–8 (2000).
Doyle, K. P., Simon, R. P. & Stenzel-Poore, M. P. Mechanisms of ischemic brain damage. Neuropharmacology 55, 310–318 (2008).
Cao, Z., Harvey, S. S., Bliss, T. M., Cheng, M. Y. & Steinberg, G. K. Inflammatory responses in the secondary thalamic injury after cortical ischemic stroke. Front. Neurol. 11, 236 (2020).
Khan, S. et al. Exploring NMDAR pathways in ischemic stroke: implications for neurotoxic and neuroprotective mechanisms and therapeutic strategies. Naunyn Schmiedebergs Arch. Pharmacol. 398, 15173–15208 (2025).
Ku, J. et al. Diurnal variation of default mode network in patients with restless legs syndrome. Sleep Med. 41, 1–8 (2018).
Stefani, A. et al. Multimodal magnetic resonance imaging reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep 42, zsz171 (2019).
Tuovinen, N. et al. Functional connectivity and topology in patients with restless legs syndrome: a case–control resting-state functional magnetic resonance imaging study. Eur. J. Neurol. 28, 448–458 (2021).
Koch, P. J. et al. Neurotransmitter-informed connectivity maps and their application for outcome inference after stroke. Brain 148, 3935–3945 (2025).
Jiang, L., Xu, H. & Yu, C. Brain connectivity plasticity in the motor network after ischemic stroke. Neural Plast. 2013, 1–11 (2013).
Ruppert, E. et al. Stroke-related restless legs syndrome: clinical and anatomo-functional characterization of an emerging entity. Eur. J. Neurol. 29, 1011–1016 (2022).
Larivière, S., Ward, N. S. & Boudrias, M.-H. Disrupted functional network integrity and flexibility after stroke: relation to motor impairments. NeuroImage Clin. 19, 883–891 (2018).
Tuladhar, A. M. et al. Default mode network connectivity in stroke patients. PLoS ONE 8, e66556 (2013).
Dacosta-Aguayo, R. et al. Impairment of functional integration of the default mode network correlates with cognitive outcome at three months after stroke. Hum. Brain Mapp. 36, 577–590 (2015).
Ismail, U. N., Yahya, N. & Manan, H. A. Investigating functional connectivity related to stroke recovery: a systematic review. Brain Res. 1840, 149023 (2024).
Jin, B., Wang, A., Earley, C. & Allen, R. Moderate to severe but not mild RLS is associated with greater sleep-related sympathetic autonomic activation than healthy adults without RLS. Sleep Med. 68, 89–95 (2020).
Siddiqui, F. et al. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin. Neurophysiol. 118, 1923–1930 (2007).
Krzyzaniak, K., Partinen, E., Partinen, M. & Sieminski, M. Patients with a higher number of periodic limb movements have higher nocturnal blood pressure. J. Clin. Med. 11, 2829 (2022).
Sayk, F. et al. To dip or not to dip. Hypertension 49, 1070–1076 (2007).
Chenini, S. et al. Blood pressure profile and endothelial function in restless legs syndrome. Sci. Rep. 9, 15933 (2019).
Erden, E. C. et al. Incremental effects of restless legs syndrome on nocturnal blood pressure in hypertensive patients and normotensive individuals. Blood Press. Monit. 17, 231–234 (2012).
Ulu, S. M. et al. The relationship between dipping-non-dipping arterial blood pressure pattern and frequency of restless leg syndrome with related factors. Anatol. J. Cardiol. 15, 284–288 (2015).
Shin, J.-W. et al. Reduced sympatho-vagal responses to orthostatic stress in drug-naive idiopathic restless legs syndrome. J. Clin. Sleep Med. 17, 957–963 (2021).
Rassu, A. L. et al. Increased blood pressure during the suggested immobilization test in restless legs syndrome. Sleep 43, zsz263 (2020).
Izzi, F. et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 15, 1392–1397 (2014).
Shneyder, N. et al. Autonomic complaints in patients with restless legs syndrome. Sleep Med. 14, 1413–1416 (2013).
Walters, A. S. & Rye, D. B. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 32, 589–597 (2009).
Salminen, A. V., Rimpilä, V. & Polo, O. Peripheral hypoxia in restless legs syndrome (Willis–Ekbom disease). Neurology 82, 1856–1861 (2014).
Kim, M. S., Park, D. G. & Yoon, J. H. Impaired endothelial function may predict treatment response in restless legs syndrome. J. Neural Transm. 126, 1051–1059 (2019).
Manconi, M. et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 12, 47–55 (2011).
Patton, S. M., Ponnuru, P., Snyder, A. M., Podskalny, G. D. & Connor, J. R. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur. J. Neurol. 18, 1329–1335 (2011).
Araujo, J. A., Zhang, M. & Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 3, 119 (2012).
García-Martín, E. et al. Heme oxygenase-1 and 2 common genetic variants and risk for restless legs syndrome. Medicine 94, e1448 (2015).
Vizcarra-Escobar, D. et al. Is restless legs syndrome associated with chronic mountain sickness? Sleep Med. 16, 976–980 (2015).
Gupta, R., Ulfberg, J., Allen, R. P. & Goel, D. High prevalence of restless legs syndrome/Willis–Ekbom disease (RLS/WED) among people living at high altitude in the Indian Himalaya. Sleep Med. 35, 7–11 (2017).
Mitroshina, E. V., Savyuk, M. O., Ponimaskin, E. & Vedunova, M. V. Hypoxia-inducible factor (HIF) in ischemic stroke and neurodegenerative disease. Front. Cell Dev. Biol. 9, 703084 (2021).
Vatte, S. & Ugale, R. HIF-1, an important regulator in potential new therapeutic approaches to ischemic stroke. Neurochem. Int. 170, 105605 (2023).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 8416763 (2017).
Cikrikcioglu, M. A. et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur. J. Clin. Invest. 41, 734–742 (2011).
Baskol, G. et al. Assessment of nitric oxide, advanced oxidation protein products, malondialdehyde, and thiol levels in patients with restless legs syndrome. Sleep Med. 13, 414–418 (2012).
Mondello, S. et al. Searching for novel candidate biomarkers of RLS in blood by proteomic analysis. Nat. Sci. Sleep 13, 873–883 (2021).
Kucuksayan, E. et al. Plasma thiol/disulphide homeostasis changes in patients with restless legs syndrome. Clin. Chem. Lab. Med. 59, 1257–1265 (2021).
Lyu, S. et al. Further studies on the role of BTBD9 in the cerebellum, sleep-like behaviors and the restless legs syndrome. Neuroscience 505, 78–90 (2022).
Chen, P. et al. BTBD9 attenuates manganese-induced oxidative stress and neurotoxicity by regulating insulin growth factor signaling pathway. Hum. Mol. Genet. 31, 2207–2222 (2022).
Chamorro, Á., Dirnagl, U., Urra, X. & Planas, A. M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 15, 869–881 (2016).
Manzanero, S., Santro, T. & Arumugam, T. V. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem. Int. 62, 712–718 (2013).
Pawluk, H. et al. The influence of oxidative stress markers in patients with ischemic stroke. Biomolecules 14, 1130 (2024).
Menon, B., Ramalingam, K. & Kumar, R. Evaluating the role of oxidative stress in acute ischemic stroke. J. Neurosci. Rural Pract. 11, 156 (2020).
Ivanov, A. V. et al. Plasma low-molecular-weight thiol/disulphide homeostasis as an early indicator of global and focal cerebral ischaemia. Redox Rep. 22, 460–466 (2017).
Leist, M., Fava, E., Montecucco, C. & Nicotera, P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur. J. Neurosci. 9, 1488–1498 (1997).
Rashid, P. A., Whitehurst, A., Lawson, N. & Bath, P. M. W. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J. Stroke Cerebrovasc. Dis. 12, 82–87 (2003).
Castillo, J., Rama, R. & Dávalos, A. Nitric oxide-related brain damage in acute ischemic stroke. Stroke 31, 852–857 (2000).
Higuchi, T. et al. Association of restless legs syndrome with oxidative stress and inflammation in patients undergoing hemodialysis. Sleep Med. 16, 941–948 (2015).
Weinstock, L. B., Walters, A. S. & Paueksakon, P. Restless legs syndrome — theoretical roles of inflammatory and immune mechanisms. Sleep Med. Rev. 16, 341–354 (2012).
Kumar, N. et al. SARS-CoV-2 infection is associated with increased odds of insomnia, RLS and dream enactment behavior. Indian J. Psychiatry 64, 354–363 (2022).
Matsuo, M., Tsuchiya, K., Hamasaki, Y. & Singer, H. S. Restless legs syndrome. Pediatr. Neurol. 31, 119–121 (2004).
Weinstock, L. B. et al. Restless legs syndrome is associated with mast cell activation syndrome. J. Clin. Sleep Med. 16, 401–408 (2020).
Hornyak, M. et al. Low-dose hydrocortisone in the evening modulates symptom severity in restless legs syndrome. Neurology 70, 1620–1622 (2008).
Partinen, E. et al. Restless legs symptoms increased during COVID-19 pandemic. International ICOSS-Survey. Sleep Med. 119, 389–398 (2024).
Weinstock, L. B. et al. Restless legs syndrome is associated with long-COVID in women. J. Clin. Sleep Med. 18, 1413–1418 (2022).
Wipper, B., Romero-Gutierrez, C. & Winkelman, J. W. Restless legs syndrome severity in the National RLS Opioid Registry during the COVID-19 pandemic. Sleep Med. 90, 96–101 (2022).
Bellei, E. et al. Discovery of restless legs syndrome plasmatic biomarkers by proteomic analysis. Brain Behav. 8, e01062 (2018).
Dowsett, J. et al. Chronic inflammation markers and cytokine-specific autoantibodies in Danish blood donors with restless legs syndrome. Sci. Rep. 12, 1672 (2022).
Varim, C. et al. Association between the neutrophil-to-lymphocyte ratio, a new marker of systemic inflammation, and restless legs syndrome. Singap. Med. J. 57, 514–516 (2016).
Trotti, L. M. et al. Elevated C-reactive protein is associated with severe periodic leg movements of sleep in patients with restless legs syndrome. Brain Behav. Immun. 26, 1239–1243 (2012).
Chen, G. Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
McCabe, J. J. et al. Interleukin-6, C-reactive protein, and recurrence after stroke: a time-course analysis of individual-participant data. Stroke 55, 2825–2834 (2024).
Shin, J.-W. et al. Bioinformatic analysis of proteomic data for iron, inflammation, and hypoxic pathways in restless legs syndrome. Sleep Med. 75, 448–455 (2020).
Cederberg, K. L. J. et al. Proteomic insights into the pathophysiology of periodic limb movements and restless legs syndrome. Sleep Health 10, S161–S169 (2024).
Patton, S. M. et al. Proteomic analysis of the cerebrospinal fluid of patients with restless legs syndrome/Willis–Ekbom disease. Fluids Barriers CNS 10, 20 (2013).
Chen, G. et al. High-density lipoprotein associated factors apoA-I and serum amyloid A in Chinese non-diabetic patients with coronary heart disease. Chin. Med. J. 123, 658–663 (2010).
Barceló, A. et al. Prostaglandin D synthase (β trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 8, 509–511 (2007).
Bogan, R. K. Effects of restless legs syndrome (RLS) on sleep. Neuropsychiatr. Dis. Treat. 2, 513–519 (2006).
Codrich, M. et al. Neuronal hemoglobin affects dopaminergic cells’ response to stress. Cell Death Dis. 8, e2538 (2017).
Pertile, R. A. N. et al. Vitamin D: a potent regulator of dopaminergic neuron differentiation and function. J. Neurochem. 166, 779–789 (2023).
Haba-Rubio, J. et al. Prevalence and determinants of periodic limb movements in the general population. Ann. Neurol. 79, 464–474 (2016).
Edelson, J. L. et al. The genetic etiology of periodic limb movement in sleep. Sleep 46, zsac121 (2023).
Winkelmann, J. et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat. Genet. 39, 1000–1006 (2007).
Thireau, J. et al. MEIS1 variant as a determinant of autonomic imbalance in restless legs syndrome. Sci. Rep. 7, 46620 (2017).
Korostovtseva, L. Ischemic stroke and sleep: the linking genetic factors. Cardiol. Ther. 10, 349–375 (2021).
Janes, F. et al. Cerebrovascular risk in restless legs syndrome: intima–media thickness and cerebral vasomotor reactivity: a case–control study. Nat. Sci. Sleep 13, 967–975 (2021).
Han, S.-H., Park, K.-Y., Kim, J.-M., Youn, Y. C. & Shin, H.-W. Restless legs syndrome is associated with arterial stiffness and clinical outcome in stroke patients. Sleep Med. 60, 219–223 (2019).
Duering, M. et al. Neuroimaging standards for research into small vessel disease — advances since 2013. Lancet Neurol. 22, 602–618 (2023).
Ferri, R. et al. Silent cerebral small vessel disease in restless legs syndrome. Sleep 39, 1371–1377 (2016).
Rist, P. M. et al. Structural brain lesions and restless legs syndrome: a cross-sectional population-based study. BMJ Open 4, e005938 (2014).
Manconi, M., Bassetti, C. L. & Ferri, R. Periodic limb movements during sleep and white matter MRI hyperintensity in minor stroke or TIA. Sleep 40, zsx031 (2017).
Del Brutto, O. H., Mera, R. M., Del Brutto, V. J. & Castillo, P. R. Lack of association between periodic limb movements during sleep and neuroimaging signatures of cerebral small vessel disease in stroke-free community-dwelling older adults. The Atahualpa Project. J. Stroke Cerebrovasc. Dis. 29, 104497 (2020).
Veitch, M. R. et al. Association between cerebral small vessel disease and periodic limb movements of sleep in patients with stroke/TIA. Sleep 48, zsaf027 (2025).
Park, K. M., Kim, K. T., Lee, D. A. & Cho, Y. W. Small vessel disease in patients with restless legs syndrome evidenced by elevated peak width of skeletonized mean diffusivity. J. Neurol. Sci. 467, 123310 (2024).
Ouyang, F. et al. Association between periodic limb movements during sleep and neuroimaging features of cerebral small vessel disease: a preliminary cross-sectional study. J. Sleep Res. 31, e13573 (2022).
Kang, M. K., Koo, D. L., Shin, J. H., Kwon, H.-M. & Nam, H. Association between periodic limb movements during sleep and cerebral small vessel disease. Sleep Med. 51, 47–52 (2018).
Walters, A. S. et al. Restless legs syndrome shows increased silent postmortem cerebral microvascular disease with gliosis. J. Am. Heart Assoc. 10, e019627 (2021).
Tuo, H. et al. Clinical and radiological characteristics of restless legs syndrome following acute lacunar infarction. Sleep Med. 53, 81–87 (2019).
Wu, X., Xu, J. & Lu, B. Acute post-stroke restless legs syndrome: the body of caudate nucleus considerations. Sleep Med. 70, 66–70 (2020).
Kalampokini, S. et al. Restless legs syndrome due to brainstem stroke: a systematic review. Acta Neurol. Scand. 146, 440–447 (2022).
Benbir, G. & Karadeniz, D. Periodic leg movements in sleep in patients with supratentorial cerebral infarction. Acta Neurol. Belg. 112, 27–32 (2012).
Kang, S. Y., Sohn, Y. H., Lee, I. K. & Kim, J.-S. Unilateral periodic limb movement in sleep after supratentorial cerebral infarction. Parkinsonism Relat. Disord. 10, 429–431 (2004).
Buratti, L. et al. Restless legs syndrome and periodic limb movements after lacunar stroke. Sleep Med. 34, 251–252 (2017).
Woo, H. G., Lee, D., Hwang, K. J. & Ahn, T.-B. Post-stroke restless leg syndrome and periodic limb movements in sleep. Acta Neurol. Scand. 135, 204–210 (2017).
Mishra, A. et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature 611, 115–123 (2022).
Schade, R., Andersohn, F., Suissa, S., Haverkamp, W. & Garbe, E. Dopamine agonists and the risk of cardiac-valve regurgitation. N. Engl. J. Med. 356, 29–38 (2007).
Chaudhuri, K. R., Dhawan, V., Basu, S., Jackson, G. & Odin, P. Valvular heart disease and fibrotic reactions may be related to ergot dopamine agonists, but non-ergot agonists may also not be spared. Mov. Disord. 19, 1522–1523 (2004).
Bauer, A. et al. Rotigotine’s effect on PLM-associated blood pressure elevations in restless legs syndrome. Neurology 86, 1785–1793 (2016).
Trenkwalder, C. Restless legs syndrome: overdiagnosed or underdiagnosed? Nat. Clin. Pract. Neurol. 3, 474–475 (2007).
Elwood, P. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J. Epidemiol. Community Health 60, 69–73 (2006).
Li, Y. et al. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation 126, 1689–1694 (2012).
Szentkirályi, A., Völzke, H., Hoffmann, W., Happe, S. & Berger, K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J. Sleep Res. 22, 434–442 (2013).
Van Den Eeden, S. K. et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep 38, 1009–1015 (2015).
Koo, B. B. et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men. Circulation 124, 1223–1231 (2011).
Boulos, M. I. et al. Periodic limb movements and white matter hyperintensities in first-ever minor stroke or high-risk transient ischemic attack. Sleep 40, zsw080 (2017).
Zorgor, G., Kabeloglu, V. & Soysal, A. Restless legs syndrome after acute ıschemic stroke and its relation to lesion location. Sleep Biol. Rhythm 20, 551–560 (2022).
Tuo, H. et al. Restless legs syndrome secondary to pontine infarction: clinical analysis of five cases. Chronic Dis. Transl. Med. 3, 186–190 (2017).
Ruppert, E. et al. Brainstem stroke-related restless legs syndrome: frequency and anatomical considerations. Eur. Neurol. 73, 113–118 (2015).
Ruppert, E. et al. Restless legs syndrome as a first manifestation of a cerebral infarct. J. Clin. Sleep Med. 10, 1037–1038 (2014).
Han, S.-H., Park, K.-Y., Youn, Y. C. & Shin, H.-W. Restless legs syndrome and akathisia as manifestations of acute pontine infarction. J. Clin. Neurosci. 21, 354–355 (2014).
Unrath, A. & Kassubek, J. Symptomatic restless leg syndrome after lacunar stroke: a lesion study. Mov. Disord. 21, 2027–2028 (2006).
Plomaritis, P. et al. Periodic limb movements during sleep in acute stroke: prevalence, severity and impact on post-stroke recovery. J. Clin. Med. 12, 5881 (2023).
Coletti Moja, M., Cravero, E., Logozzo, I., Mairano, C. & Labate, C. Unilateral poststroke periodic limb movements: a case series. Case Rep. Neurol. 14, 162–166 (2022).
Lee, J. S., Lee, P. H. & Huh, K. Periodic limb movements in sleep after a small deep subcortical infarct. Mov. Disord. 20, 260–261 (2005).
Kim, J. et al. Periodic limb movement during sleep developed after pontine lesion. Mov. Disord. 18, 1403–1405 (2003).
Acknowledgements
No funding was received for the preparation of this Review. A.S.W. reports receiving a ‘Sleep Research in Neurology’ grant from Vanderbilt University Medical Center. K.S. reports funding from the European Union under Grant Agreement No. 101129822 (EOSC TITAN project). M.I.B. reports no conflicts of interest specific to this manuscript. Outside the submitted work, M.I.B. reports funding from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, CanStroke Recovery Trials Network, Restless Legs Syndrome Foundation, Alternative Funding Plan from the Academic Health Sciences Centres of Ontario, the Temerty Centre for AI Research and Education in Medicine (T-CAIREM), the McLaughlin Centre for Molecular Medicine and the Toronto Dementia Research Alliance. He also reports consulting fees and honoraria from Jazz Pharmaceuticals, Paladin Labs, Eisai, Precision AQ and the OntarioMD Peer Leader Program; travel support from McGill University; and receipt of sleep equipment or research support from Braebon Medical Corporation, CGX, The Mahaffy Family Research Fund and Green Mountain. M.I.B. also reports unrestricted educational grants from Jazz Pharmaceuticals and Paladin Labs.
Author information
Authors and Affiliations
Contributions
Y.S.C. prepared the primary draft of the manuscript under the supervision of M.I.B. N.F. expanded and substantially revised the paper, prepared the figures and created several of the tables. M.R. prepared the remaining tables. K.S., A.S.W. and M.I.B. provided critical input and expert guidance as senior researchers, shaping the intellectual framework and direction of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests related to this work.
Peer review
Peer review information
Nature Reviews Neurology thanks Elisabeth Ruppert, Ambra Stefani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farhani, N., Costa, Y.S., Rozik, M. et al. Restless legs syndrome and periodic limb movements of sleep — the relationship with stroke and other cerebrovascular disease. Nat Rev Neurol 22, 37–53 (2026). https://doi.org/10.1038/s41582-025-01161-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01161-z