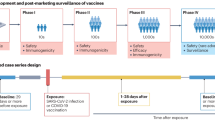
Abstract
Vaccination is widely considered to be the pre-eminent public health achievement of modern history, but declining coverage resulting from vaccine hesitancy and from interruptions in immunization campaigns in geopolitically unstable regions threatens progress against vaccine-preventable diseases. The global burden of vaccine-preventable neurological diseases is substantial, resulting in acute and chronic complications as well as high case fatality rates. In recent years, outbreaks of dengue, poliomyelitis, measles, pertussis, meningococcal disease and Japanese encephalitis virus have been linked to lack of access to vaccines, overwhelmed health-care systems, misinformation and disinformation regarding vaccine safety, and gaps in vaccination coverage caused by environmental factors and geopolitical conflicts. Coordinated global strategies, including addressing barriers to vaccination and ensuring equitable access; targeted health education about vaccine benefits and risks; integration with other public services; and advances in next-generation vaccine technologies to tackle antimicrobial resistance and non-vaccine serotype replacement, will be crucial to prevent a resurgence of vaccine-preventable neurological diseases, especially in vulnerable populations, to maintain global health security.
Key points
-
A global resurgence of vaccine-preventable neurological diseases, including recent outbreaks of measles and poliomyelitis, is being driven by declining vaccination coverage worldwide.
-
Neurological involvement in vaccine-preventable diseases leads to severe acute and chronic morbidity, including cognitive impairment, encephalitis and paralysis — conditions that are avoidable with readily available and effective vaccines.
-
Reduced immunization rates following the COVID-19 pandemic have been compounded by disruptions to targeted immunization campaigns in regions vulnerable to climate crises and geopolitical instability, as well as vaccine hesitancy fuelled by misinformation, disinformation and pervasive distrust in public health agencies.
-
Inequitable access to vaccines for low-income and middle-income countries is disproportionately high owing to inadequate public health infrastructure, economic insecurity and geographical or conflict-associated disruptions in resource-limited settings.
-
Tackling vaccine hesitancy necessitates multimodal and transparent communication strategies to repair public trust in public health officials and to increase health literacy.
-
The mRNA vaccine platforms implemented during the COVID-19 pandemic, along with nanoparticle-encapsulated delivery systems, might address gaps in vaccine availability through modular, rapid and scalable production pipelines for thermostable vaccine formulations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
18 December 2025
In the version of the article initially published, Julia Granerod’s affiliation was incorrect and has now been amended to “Dr JGW Consulting AS, Sandefjord, Norway” in the HTML and PDF versions of the article.
References
Immunization, vaccines and biologicals. WHO. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases (2025).
Carter, A. et al. Modeling the impact of vaccination for the immunization agenda 2030: deaths averted due to vaccination against 14 pathogens in 194 countries from 2021 to 2030. Vaccine 42 (Suppl. 1), S28–S37 (2024).
GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 23, 344–381 (2024)
Leibovitch, E. C. & Jacobson, S. Vaccinations for neuroinfectious disease: a global health priority. Neurother. J. Am. Soc. Exp. Neurother. 13, 562–570 (2016).
Sah, R. et al. Dengue virus and its recent outbreaks: current scenario and counteracting strategies. Int. J. Surg. Lond. Engl. 109, 2841–2845 (2023).
Namageyo-Funa, A. et al. Update on vaccine-derived poliovirus outbreaks — worldwide, January 2023–June 2024. MMWR Morb. Mortal. Wkly Rep. 73, 909–916 (2024).
Bednarczyk, R. A. & Sundaram, M. E. The continued risk of measles outbreaks in the United States resulting from suboptimal vaccination coverage. Public Health Rep. https://doi.org/10.1177/00333549241306608 (2025).
Monath, T. P. Japanese encephalitis: risk of emergence in the United States and the resulting impact. Viruses 16, 54 (2023).
Risk of vaccine-preventable disease outbreaks at 30-year high, PAHO Director says. PAHO. https://www.paho.org/en/news/20-4-2023-risk-vaccine-preventable-disease-outbreaks-30-year-high-paho-director-says (2023).
Parums, D. V. A review of the resurgence of measles, a vaccine-preventable disease, as current concerns contrast with past hopes for measles elimination. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 30, e944436 (2024).
Frenkel, L. D. The global burden of vaccine-preventable infectious diseases in children less than 5 years of age: implications for COVID-19 vaccination. How can we do better? Allergy Asthma Proc. 42, 378–385 (2021).
Lubanga, A. F. et al. Addressing the re-emergence and resurgence of vaccine-preventable diseases in Africa: a health equity perspective. Hum. Vaccines Immunother. 20, 2375081 (2024).
Granerod, J. et al. Encephalitis: an in-depth review and gap analysis of key variables affecting global disease burden. Encephalitis International https://www.encephalitis.info/wp-content/uploads/2024/02/Global-Impact-Report-Feb-2024.pdf (2024).
Blutinger, E. et al. Measles: contemporary considerations for the emergency physician. J. Am. Coll. Emerg. Physicians Open 4, e13032 (2023).
Campbell, H., Andrews, N., Brown, K. E. & Miller, E. Review of the effect of measles vaccination on the epidemiology of SSPE. Int. J. Epidemiol. 36, 1334–1348 (2007).
Lebon, P., Gelot, A., Zhang, S. Y., Casanova, J. L. & Hauw, J. J. Measles sclerosing subacute panencephalitis (SSPE), an intriguing and ever-present disease: data, assumptions and new perspectives. Rev. Neurol. 177, 1059–1068 (2021).
Shattock, A. J. et al. Contribution of vaccination to improved survival and health: modelling 50 years of the expanded programme on immunization. Lancet 403, 2307–2316 (2024).
Plotkin, S. A. & Chumakov, K. Polio eradication efforts: above all, do no harm. Science 382, 778 (2023).
Greenwood, B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130433 (2014).
Baraff, L. J., Lee, S. I. & Schriger, D. L. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr. Infect. Dis. J. 12, 389–394 (1993).
[No authors listed]. Defeating meningitis by 2030: a global road map. WHO https://www.who.int/publications/i/item/9789240026407 (2021).
Soumahoro, L., Abitbol, V., Vicic, N., Bekkat-Berkani, R. & Safadi, M. A. P. Meningococcal disease outbreaks: a moving target and a case for routine preventative vaccination. Infect. Dis. Ther. 10, 1949–1988 (2021).
Frasch, C. E., Kapre, S. V., Lee, C. H. & Préaud, J. M. Technical development of a new meningococcal conjugate vaccine. Clin. Infect. Dis. 61 (Suppl. 5), S404–S409 (2015).
Trotter, C. L. et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect. Dis. 17, 867–872 (2017).
Lazarus, J. V. et al. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023. Nat. Med. 30, 1559–1563 (2024).
Parisi, A. et al. Impact of COVID-19 pandemic on vaccine hesitancy and sentiment changes: a survey of healthcare workers in 12 countries. Public Health 238, 188–196 (2025).
Montero, D. A. et al. Two centuries of vaccination: historical and conceptual approach and future perspectives. Front. Public Health 11, 1326154 (2023).
Jones, C. E. et al. Routine vaccination coverage — worldwide, 2023. MMWR Morb. Mortal. Wkly Rep. 73, 978–984 (2024).
Delamater, P. L., Street, E. J., Leslie, T. F., Yang, Y. T. & Jacobsen, K. H. Complexity of the basic reproduction number (R0). Emerg. Infect. Dis. 25, 1–4 (2019).
Measles. WHO https://www.who.int/news-room/fact-sheets/detail/measles (2024).
Johnson, R. T. Acute encephalitis. Clin. Infect. Dis. 23, 219–224 (1996).
Feigin, V. L. et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 19, 255–265 (2020).
Wendorf, K. A. et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clin. Infect. Dis. 65, 226–232 (2017).
Ferren, M., Horvat, B. & Mathieu, C. Measles encephalitis: towards new therapeutics. Viruses 11, 1017 (2019).
Griffin, D. E. Measles virus and the nervous system. Handb. Clin. Neurol. 123, 577–590 (2014).
Minta, A. A. et al. Progress toward measles elimination — worldwide, 2000–2022. MMWR Morb. Mortal. Wkly Rep. 72, 1262–1268 (2023).
Region of the Americas is declared free of measles. PAHO https://www.paho.org/en/news/27-9-2016-region-americas-declared-free-measles (2016).
Paules, C. I., Marston, H. D. & Fauci, A. S. Measles in 2019 — going backward. N. Engl. J. Med. 380, 2185–2187 (2019).
Rapid measles outbreak response critical to protect millions of vulnerable children. WHO https://www.who.int/europe/news/item/22-02-2024-rapid-measles-outbreak-response-critical-to-protect-millions-of-vulnerable-children (2024).
Wang, R., Jing, W., Liu, M. & Liu, J. Trends of the global, regional, and national incidence of measles, vaccine coverage, and risk factors in 204 countries from 1990 to 2019. Front. Med. 8, 798031 (2021).
Kuppalli, K. & Perl, T. M. Measles in Texas: waning vaccination and a stark warning for public health. Lancet Infect. Dis. 25, 485–487 (2025).
Measles Cases and Outbreaks (CDC, accessed 19 November 2025); https://www.cdc.gov/measles/data-research/index.html.
Lam, T. A recent surge of fulminant and early onset subacute sclerosing panencephalitis (SSPE) in the United Kingdom: an emergence in a time of measles. Eur. J. Paediatr. Neurol. 34, 43–49 (2021).
Mubbashir, Z. et al. Subacute sclerosing panencephalitis: impact on public health, current insights, and future perspectives. Brain Behav. 15, e70292 (2025).
Mehndiratta, M. M., Mehndiratta, P. & Pande, R. Poliomyelitis: historical facts, epidemiology, and current challenges in eradication. Neurohospitalist 4, 223–229 (2014).
Grassly, N. C. The final stages of the global eradication of poliomyelitis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120140 (2013).
Lai, Y. A., Chen, X., Kunasekaran, M., Rahman, B. & MacIntyre, C. R. Global epidemiology of vaccine-derived poliovirus 2016–2021: a descriptive analysis and retrospective case–control study. eClinicalMedicine 50, 101508 (2022).
Wolbert, J. G., Rajnik, M., Swinkels, H. M. & Higginbotham, K. Poliomyelitis. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK558944/ (2025).
Li Hi Shing, S., Lope, J., Chipika, R. H., Hardiman, O. & Bede, P. Extra-motor manifestations in post-polio syndrome (PPS): fatigue, cognitive symptoms and radiological features. Neurol. Sci. 42, 4569–4581 (2021).
Global Polio Eradication Initiative. Polio eradication strategy 2022–2026: delivering on a promise. WHO https://polioeradication.org/wp-content/uploads/2022/06/Polio-Eradication-Strategy-2022-2026-Delivering-on-a-Promise.pdf (2021).
[No authors listed]. Poliomyelitis (polio). WHO. https://www.who.int/health-topics/poliomyelitis#tab=tab_1 (2025).
Polio eradication: falling at the final hurdle? Lancet 400, 1079 (2022).
Whitehouse, E. R. et al. Wastewater surveillance for poliovirus in selected jurisdictions, United States, 2022–2023. Emerg. Infect. Dis. 30, 2279–2287 (2024).
Geiger, K. et al. Progress toward poliomyelitis eradication — worldwide, January 2022–December 2023. MMWR Morb. Mortal. Wkly Rep. 73, 441–446 (2024).
Lee, S. E. et al. Progress toward poliomyelitis eradication — worldwide, January 2021–March 2023. MMWR Morb. Mortal. Wkly Rep. 72, 517–522 (2023).
Global Polio Eradication Initiative. OPV cessation. GPEI https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/opv-cessation/ (2023).
Suares, J. E. et al. Vaccine-associated paralytic poliomyelitis in oral polio vaccine recipients: disproportionality analysis using VAERS and systematic review. Expert Opin. Drug Saf. 23, 855–867 (2024).
Martini, M. & Orsini, D. Armed conflict in the world threatens the eradication of poliomyelitis: risks of humanitarian crises. Pathog. Glob. Health 116, 267–268 (2022).
Hardy, C. M. et al. Progress toward poliomyelitis eradication — Afghanistan, January 2023–September 2024. MMWR Morb. Mortal. Wkly Rep. 73, 1129–1134 (2024).
Mbaeyi, C. et al. Progress toward poliomyelitis eradication — Pakistan, January 2023–June 2024. MMWR Morb. Mortal. Wkly Rep. 73, 788–792 (2024).
Global Polio Eradication Initiative. Where we work. GPEI https://www.archive.polioeradication.org/where-we-work/ (2025).
Burki, T. Polio vaccination campaign in Gaza. Lancet Infect. Dis. 24, e623–e624 (2024).
Second round of polio campaign in Gaza completed amid ongoing conflict and attacks: UNICEF and WHO. WHO https://www.who.int/news/item/06-11-2024-second-round-of-polio-campaign-in-gaza-completed-amid-ongoing-conflict-and-attacks--unicef-and-who (2024).
Global Polio Eradication Initiative. Polio eradication in the African region. GPEI https://polioeradication.org/wp-content/uploads/2024/08/Africa-Regional-Polio-Eradication-Action-Plan-20242025.pdf (2024).
Runde, T. J., Anjum, F. & Hafner, J. W. Bacterial meningitis. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK470351/ (2025).
Tavares, T., Pinho, L. & Bonifácio Andrade, E. Group B streptococcal neonatal meningitis. Clin. Microbiol. Rev. 35, e0007921 (2022).
van Soest, T. M., Chekrouni, N., van Sorge, N. M., Brouwer, M. C. & van de Beek, D. Community-acquired bacterial meningitis in patients of 80 years and older. J. Am. Geriatr. Soc. 70, 2060–2069 (2022).
van de Beek, D., Brouwer, M. C., Koedel, U. & Wall, E. C. Community-acquired bacterial meningitis. Lancet 398, 1171–1183 (2021).
Mbaeyi, S. A. et al. Meningococcal disease among college-aged young adults: 2014–2016. Pediatrics 143, e20182130 (2019).
Meningitis. WHO https://www.who.int/health-topics/meningitis#tab=tab_1 (2025).
van Ettekoven, C. N., Liechti, F. D., Brouwer, M. C., Bijlsma, M. W. & van de Beek, D. Global case fatality of bacterial meningitis during an 80-year period: a systematic review and meta-analysis. JAMA Netw. Open 7, e2424802 (2024).
Wunrow, H. Y. et al. Global, regional, and national burden of meningitis and its aetiologies, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 22, 685–711 (2023).
Musher, D. M., Anderson, R. & Feldman, C. The remarkable history of pneumococcal vaccination: an ongoing challenge. Pneumonia Nathan 14, 5 (2022).
Baylac-Paouly, B. Vaccine development and collaborations: lessons from the history of the meningococcal a vaccine (1969–73). Med. Hist. 63, 435–453 (2019).
Borrow, R. & Findlow, J. The important lessons lurking in the history of meningococcal epidemiology. Expert Rev. Vaccines 23, 445–462 (2024).
Smith, K. J. et al. Higher-valency pneumococcal conjugate vaccines: an exploratory cost-effectiveness analysis in U.S. seniors. Am. J. Prev. Med. 61, 28–36 (2021).
de Boer, P. T. et al. Higher-valency pneumococcal conjugate vaccines in older adults, taking into account indirect effects from childhood vaccination: a cost-effectiveness study for the Netherlands. BMC Med. 22, 69 (2024).
King, L. M. & Lewnard, J. A. Health-economic burden attributable to novel serotypes in candidate 24- and 31-valent pneumococcal conjugate vaccines. Vaccine 42, 126310 (2024).
Knuf, M. et al. Hexavalent vaccines: what can we learn from head-to-head studies? Vaccine 39, 6025–6036 (2021).
Manson, C., Laurence, S. & Macina, D. DTaP–IPV–HB–Hib vaccine (Hexaxim): an update 10 years after first licensure. Expert Rev. Vaccines 22, 1196–1213 (2023).
Koelman, D. L. H. et al. Changing epidemiology of bacterial meningitis since introduction of conjugate vaccines: 3 decades of national meningitis surveillance in the Netherlands. Clin. Infect. Dis. 73, e1099–e1107 (2021).
Meningococcal meningitis. WHO https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/meningococcal-meningitis (2025).
McNamara, L. A. & Rubis, A. B. Meningococcal disease. CDC Yellow Book 2024: Health Information for International Travel (CDC, accessed 19 November 2025); https://www.cdc.gov/yellow-book/hcp/travel-associated-infections-diseases/meningococcal-disease.html.
Pelton, S. I. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J. Adolesc. Health 59, S3–S11 (2016).
Butler, D. F. & Myers, A. L. Changing epidemiology of Haemophilus influenzae in children. Infect. Clin. North. Am. 32, 119–128 (2018).
Slack, M. P. E., Cripps, A. W., Grimwood, K., Mackenzie, G. A. & Ulanova, M. Invasive Haemophilus influenzae infections after 3 decades of Hib protein conjugate vaccine use. Clin. Microbiol. Rev. 34, e0002821 (2021).
Flasche, S. et al. The potential for reducing the number of pneumococcal conjugate vaccine doses while sustaining herd immunity in high-income countries. PLoS Med. 12, e1001839 (2015).
WHO publishes list of bacteria for which new antibiotics are urgently needed. WHO https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (2017).
WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. WHO https://www.who.int/publications/i/item/9789240093461 (2024).
Naghavi, M. et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404, 1199–1226 (2024).
Løchen, A., Croucher, N. J. & Anderson, R. M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 10, 18977 (2020).
Aernout, I. et al. Challenges and opportunities in mRNA vaccine development against bacteria. Nat. Microbiol. 10, 1816–1828 (2025).
Simon, L. V., Sandhu, D. S., Goyal, A. & Kruse, B. Japanese encephalitis. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK470423/ (2025).
Srivastava, K. S. et al. Japanese encephalitis virus: an update on the potential antivirals and vaccines. Vaccines 11, 742 (2023).
McGuinness, S. L., Lau, C. L. & Leder, K. The evolving Japanese encephalitis situation in Australia and implications for travel medicine. J. Travel. Med. 30, taad029 (2023).
Erlanger, T. E., Weiss, S., Keiser, J., Utzinger, J. & Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 15, 1–7 (2009).
Liu, B. et al. Autoimmune encephalitis after Japanese encephalitis in children: a prospective study. J. Neurol. Sci. 424, 117394 (2021).
Mohsin, F., Suleman, S., Anzar, N., Narang, J. & Wadhwa, S. A review on Japanese encephalitis virus emergence, pathogenesis and detection: from conventional diagnostics to emerging rapid detection techniques. Int. J. Biol. Macromol. 217, 435–448 (2022).
Japanese encephalitis. WHO https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (2024).
Clinical Features and Diagnosis of Japanese Encephalitis (CDC, accessed 19 November 2025); https://www.cdc.gov/japanese-encephalitis/hcp/clinical-diagnosis/index.html.
Wang, X. et al. Long-term neurological sequelae and disease burden of Japanese encephalitis in Gansu Province, China. Ann. Glob. Health 87, 103 (2021).
Letson, G. W., Marfin, A. A., Mooney, J., Minh, H. V. & Hills, S. L. Impact of vaccination against Japanese encephalitis in endemic countries. PLoS Negl. Trop. Dis. 18, e0012390 (2024).
Heffelfinger, J. D. et al. Japanese encephalitis surveillance and immunization — Asia and Western Pacific regions, 2016. MMWR Morb. Mortal. Wkly. Rep. 66, 579–583 (2017).
Adugna, T. et al. Advancements in nanoparticle-based vaccine development against Japanese encephalitis virus: a systematic review. Front. Immunol. 15, 1505612 (2024).
Vannice, K. S. et al. The future of Japanese encephalitis vaccination: expert recommendations for achieving and maintaining optimal JE control. npj Vaccines 6, 82 (2021).
Halstead, S. B. & Thomas, S. J. Japanese encephalitis: new options for active immunization. Clin. Infect. Dis. 50, 1155–1164 (2010).
Piyasirisilp, S. & Hemachudha, T. Neurological adverse events associated with vaccination. Curr. Opin. Neurol. 15, 333–338 (2002).
McAlpine, L. S. & Zubair, A. S. Neurological sequelae of vaccines. Neurol. Sci. 44, 1505–1513 (2023).
About the Vaccine Adverse Event Reporting System (VAERS) (CDC, accessed 19 November 2025); https://www.cdc.gov/vaccine-safety-systems/vaers/index.html.
Nath, A. Neurologic complications with vaccines: what we know, what we don’t, and what we should do. Neurology 101, 621–626 (2023).
Haber, P., Sejvar, J., Mikaeloff, Y. & DeStefano, F. Vaccines and Guillain–Barré syndrome. Drug Saf. 32, 309–323 (2009).
Jeong, Y. D. et al. Global burden of vaccine-associated Guillain–Barré syndrome over 170 countries from 1967 to 2023. Sci. Rep. 14, 24561 (2024).
Schonberger, L. B. et al. Guillain–Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am. J. Epidemiol. 110, 105–123 (1979).
Stratton, K. R., Howe, C. J. & Johnston, R. B. J. (eds.) Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality (National Academies Press, 1994).
Ribeiro, A. F. et al. Neurologic disease after yellow fever vaccination, São Paulo, Brazil, 2017–2018. Emerg. Infect. Dis. 27, 1577–1587 (2021).
McMahon, A. W. et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine 25, 1727–1734 (2007).
Cohen, M. et al. Case report: yellow fever vaccine-associated neurotropic disease and associated MRI, EEG, and CSF findings. Front. Neurol. 12, 779014 (2021).
Kengsakul, K., Sathirapongsasuti, K. & Punyagupta, S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J. Med. Assoc. Thail. 85, 131–134 (2002).
Costales, C. et al. Vaccine-associated measles encephalitis in immunocompromised child, California, USA. Emerg. Infect. Dis. 28, 906–908 (2022).
Bitnun, A. et al. Measles inclusion-body encephalitis caused by the vaccine strain of measles virus. Clin. Infect. Dis. 29, 855–861 (1999).
Rakusa, M. et al. COVID-19 vaccination hesitancy among people with chronic neurological disorders: a position paper. Eur. J. Neurol. 29, 2163–2172 (2022).
Schaffer DeRoo, S., Pudalov, N. J. & Fu, L. Y. Planning for a COVID-19 vaccination program. JAMA 323, 2458–2459 (2020).
Thomas, S., Durrheim, D., Islam, F., Higgins, H. & Cashman, P. Improved childhood immunization coverage using the World Health Organization’s Tailoring Immunization Programmes guide (TIP) in a regional centre in Australia. Vaccine 40, 18–20 (2022).
Rehfuess, E. A. et al. The WHO-INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective. BMJ Glob. Health 4 (Suppl. 1), e000844 (2019).
Kaur, G. et al. Routine vaccination coverage — worldwide, 2022. MMWR Morb. Mortal. Wkly Rep. 72, 1155–1161 (2023).
Piamonte, B. L. C. et al. Addressing vaccine-preventable encephalitis in vulnerable populations. Curr. Opin. Neurol. 36, 185–197 (2023).
Oyo-Ita, A. et al. Interventions for improving coverage of childhood immunisation in low- and middle-income countries. Cochrane Database Syst. Rev. 12, CD008145 (2023).
Oyo-Ita, A. E. et al. Cost-effectiveness analysis of an intervention project engaging Traditional and Religious Leaders to improve uptake of childhood immunization in southern Nigeria. PLoS ONE 16, e0257277 (2021).
Ahmed, S. T. et al. A scoping review on integrated health campaigns for immunization in low- and middle-income countries. Health Policy Plan. 38, 1198–1224 (2023).
MacDonald, N. E. & SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine 33, 4161–4164 (2015).
Larson, H. J., Jarrett, C., Eckersberger, E., Smith, D. M. D. & Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine 32, 2150–2159 (2014).
Kolakowska, A. et al. Insufficient vaccine coverage and vaccine hesitancy in people living with HIV: a prospective study in outpatient clinics in the Paris region. Vaccine 42, 3655–3663 (2024).
Goldstein, S., MacDonald, N. E. & Guirguis, S. & SAGE Working Group on Vaccine Hesitancy. Health communication and vaccine hesitancy. Vaccine 33, 4212–4214 (2015).
Chang, Y. K., Chang, H. L., Wu, H. S. & Chen, K. T. Epidemiological features of Japanese encephalitis in Taiwan from 2000 to 2014. Am. J. Trop. Med. Hyg. 96, 382–388 (2017).
Li, M. et al. Understanding mumps dynamics: epidemiological traits and breakthrough infections in the population under 15 years of age in Jiangsu Province, China, 2023. Vaccines 12, 986 (2024).
Yoo, J. W. et al. Epidemiology of mumps, mumps complications, and mumps orchitis in Korea using the National Health Insurance Service Database. Investig. Clin. Urol. 64, 412–417 (2023).
Peer, V., Schwartz, N. & Green, M. S. Consistent, excess viral meningitis incidence rates in young males: a multi-country, multi-year, meta-analysis of national data. The importance of sex as a biological variable. eClinicalMedicine 15, 62–71 (2019).
Yibrah, M. & Damtie, D. Incidence of human rabies exposure and associated factors at the Gondar Health Center, Ethiopia: a three-year retrospective study. Infect. Dis. Poverty 4, 3 (2015).
Ali, H. A. et al. Vaccine equity in low and middle income countries: a systematic review and meta-analysis. Int. J. Equity Health 21, 82 (2022).
Ebrahimi, N. et al. Human papillomavirus vaccination in low- and middle-income countries: progression, barriers, and future prospective. Front. Immunol. 14, 1150238 (2023).
Laris-González, A., Bernal-Serrano, D., Jarde, A. & Kampmann, B. Safety of administering live vaccines during pregnancy: a systematic review and meta-analysis of pregnancy outcomes. Vaccines 8, 124 (2020).
Pardi, N. & Krammer, F. mRNA vaccines for infectious diseases — advances, challenges and opportunities. Nat. Rev. Drug Discov. 23, 838–861 (2024).
Leong, K. Y., Tham, S. K. & Poh, C. L. Revolutionizing immunization: a comprehensive review of mRNA vaccine technology and applications. Virol. J. 22, 71 (2025).
Lokras, A. G., Bobak, T. R., Baghel, S. S., Sebastiani, F. & Foged, C. Advances in the design and delivery of RNA vaccines for infectious diseases. Adv. Drug Deliv. Rev. 213, 115419 (2024).
Mir, S. & Mir, M. The mRNA vaccine, a swift warhead against a moving infectious disease target. Expert Rev. Vaccines 23, 336–348 (2024).
Liu, J. et al. A bivalent COVID-19 mRNA vaccine elicited broad immune responses and protection against Omicron subvariants infection. npj Vaccines 10, 4 (2025).
Rudolph, A. E. et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against symptomatic COVID-19 among immunocompetent individuals testing at a large US retail pharmacy. J. Infect. Dis. 229, 648–659 (2024).
Min, S. E. et al. Efficacy and safety of a novel multivalent mRNA vaccine against SARS-CoV-2 in experimental animals. Sci. Rep. 15, 21831 (2025).
Priyanka et al. Nanovaccines: a game changing approach in the fight against infectious diseases. Biomed. Pharmacother. 167, 115597 (2023).
Chen, S. et al. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Primers 3, 63 (2023).
Saleh, M., El-Moghazy, A., Elgohary, A. H., Saber, W. I. A. & Helmy, Y. A. Revolutionizing nanovaccines: a new era of immunization. Vaccines 13, 126 (2025).
Measles Vaccine Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html.
Polio Vaccine Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/polio/hcp/vaccine-considerations/index.html.
Varicella Vaccine Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/chickenpox/hcp/vaccine-considerations/index.html.
Japanese Encephalitis Vaccine (CDC, accessed 19 November 2025); https://www.cdc.gov/japanese-encephalitis/prevention/japanese-encephalitis-vaccine.html.
Tick-borne Encephalitis Vaccine Information for Healthcare Providers (CDC, accessed 19 November 2025); https://www.cdc.gov/tick-borne-encephalitis/hcp/vaccine/index.html.
Rabies Post-exposure Prophylaxis Guidance (CDC, accessed 19 November 2025); https://www.cdc.gov/rabies/hcp/clinical-care/post-exposure-prophylaxis.html.
Seasonal Influenza Vaccine Dosage & Administration (CDC, accessed 19 November 2025); https://www.cdc.gov/flu/hcp/vax-summary/vaccine-dosage-admin.html.
2025–2026 COVID-19 Vaccination Guidance (CDC, accessed 19 November 2025); https://www.cdc.gov/covid/hcp/vaccine-considerations/routine-guidance.html.
Summary of Risk-based Pneumococcal Vaccination Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/risk-indications.html.
Meningococcal Vaccine Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/meningococcal/hcp/vaccine-recommendations/index.html.
Hib vaccine recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/hi-disease/hcp/vaccine-recommendations/index.html.
Tetanus Vaccine Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/tetanus/hcp/vaccine-recommendations/index.html.
Pertussis Vaccination Recommendations (CDC, accessed 19 November 2025); https://www.cdc.gov/pertussis/hcp/vaccine-recommendations/index.html.
The College of Physicians of Philadelphia. Vaccine Timeline https://historyofvaccines.org/history/vaccine-timeline/timeline (2025).
A Brief History of Vaccination. WHO https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination (2025).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote the article. F.C.C., J.G. and K.T.T. contributed substantially to discussion of the content. F.C.C., J.G., C.Y.K. and K.T.T. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Ava Easton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
World Health Organization Immunization Data: https://immunizationdata.who.int/global
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chow, F.C., Granerod, J., Kim, C.Y. et al. The global threat of vaccine-preventable neurological diseases. Nat Rev Neurol 22, 110–122 (2026). https://doi.org/10.1038/s41582-025-01172-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01172-w