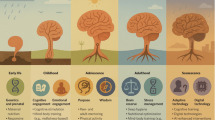
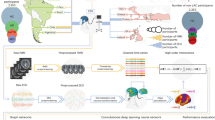
Abstract
The variability in cognitive and brain ageing trajectories may be influenced by inter-individual and community-level differences in resilience that result from differential exposures to social and structural determinants of health and be affected by an individual’s sex and gender. However, no clear guidance exists on how to best integrate these diversity-related factors (that is, sex, gender and social and structural determinants of health) into clinical and cognitive neuroscience research on resilience in ageing and dementia. The international Brain Resilience and Diversity in Aging and Dementia (BReDAD) Collaboratory was established in 2024 with the goals of synthesizing knowledge, identifying knowledge gaps and developing recommendations for conducting more inclusive research on resilience in this area. On the basis of a focused review of the literature, and discussions held and recommendations made by the Collaboratory, in this Roadmap article, we present a way forward for integrating diversity in future resilience research. This proposal comprises: (i) developing trust and meaningful long-term relationships with communities historically excluded from research; (ii) diversifying who is engaged in all aspects of the research process; (iii) adopting a life-course perspective; (iv) improving and expanding research designs and measurement tools; and (v) using sensitive computational analytics and mixed methods for testing complex, intersectional models. We conclude by recommending a transdisciplinary approach in resilience research to better reflect the complexities inherent in studying diversity and developing precision medicine outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Anstey, K. J. et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20–76 years. Sci. Rep. 11, 7710 (2021).
Ekstrom, I., Josefsson, M., Backman, L. & Laukka, E. J. Predictors of cognitive aging profiles over 15 years: a longitudinal population-based study. Psychol. Aging 39, 467–483 (2024).
McFall, G. P., McDermott, K. L. & Dixon, R. A. Modifiable risk factors discriminate memory trajectories in non-demented aging: precision factors and targets for promoting healthier brain aging and preventing dementia. J. Alzheimers Dis. 70, S101–S118 (2019).
Salthouse, T. A. Trajectories of normal cognitive aging. Psychol. Aging 34, 17–24 (2019).
LaPlume, A. A., Anderson, N. D., McKetton, L., Levine, B. & Troyer, A. K. When I’m 64: age-related variability in over 40,000 online cognitive test takers. J. Gerontol. B Psychol. Sci. Soc. Sci. 77, 104–117 (2022).
Charness, N. & Boot, W. R. A grand challenge for psychology: reducing the age-related digital divide. Curr. Dir. Psychol. Sci. 31, 187–193 (2022).
de Frias, C. M., Lövdén, M., Lindenberger, U. & Nilsson, L.-G. Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence 35, 381–392 (2007).
Drouin, S. M. et al. Data-driven analyses of longitudinal hippocampal imaging trajectories: discrimination and biomarker prediction of change classes. J. Alzheimers Dis. 88, 97–115 (2022).
Lin, K. et al. Risk factors and cognitive correlates of white matter hyperintensities in ethnically diverse populations without dementia: the COSMIC consortium. Alzheimers Dement. 16, e12567 (2024).
Wilson, R. S. et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging 17, 179–193 (2002).
Barnes, L. L. et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 85, 528–534 (2015).
Tang, M. X. et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755 (1998).
Vega, I. E., Cabrera, L. Y., Wygant, C. M., Velez-Ortiz, D. & Counts, S. E. Alzheimer’s disease in the Latino community: intersection of genetics and social determinants of health. J. Alzheimers Dis. 58, 979–992 (2017).
Mielke, M. M. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times 35, 14–17 (2018).
Arenaza-Urquijo, E. M. & Vemuri, P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology 90, 695–703 (2018).
Stern, Y. et al. A framework for concepts of reserve and resilience in aging. Neurobiol. Aging 124, 100–103 (2023).
Vemuri, P. et al. Amyloid, vascular, and resilience pathways associated with cognitive aging. Ann. Neurol. 86, 866–877 (2019).
Montine, T. J. et al. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res. Ther. 11, 22 (2019).
Katzman, R. et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 23, 138–144 (1988).
Nyberg, L., Lovden, M., Riklund, K., Lindenberger, U. & Backman, L. Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305 (2012).
Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460 (2002).
Vonk, J. M. J. et al. The role of cognitive and brain reserve in memory decline and atrophy rate in mid and late-life: the SMART-MR study. Cortex 148, 204–214 (2022).
Norling, A. M. & Lipsitz, L. A. Exercise to mitigate cerebrovascular aging: a geroscience perspective. J. Gerontol. A Biol. Sci. Med. Sci https://doi.org/10.1093/gerona/glae083 (2024).
Braveman, P., Egerter, S. & Williams, D. R. The social determinants of health: coming of age. Annu. Rev. Public Health 32, 381–398 (2011).
Liu, S., Jones, R. N. & Glymour, M. M. Implications of lifecourse epidemiology for research on determinants of adult disease. Public Health Rev. 32, 489–511 (2010).
Majoka, M. A. & Schimming, C. Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clin. Ther. 43, 922–929 (2021).
Anstey, K. J., Ee, N., Eramudugolla, R., Jagger, C. & Peters, R. A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J. Alzheimers Dis. 70, S165–S186 (2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 404, 572–628 (2024).
Calvo, N. et al. Associated risk and resilience factors of Alzheimer’s disease in women with early bilateral oophorectomy: data from the UK Biobank. J. Alzheimers Dis. 102, 119–128 (2024).
Mavrodaris, A., Lafortune, L. & Brayne, C. E. The future longevity: designing a synergistic approach for healthy ageing, sustainability, and equity. Lancet Healthy Longev. 3, e584–e586 (2022).
Subramaniapillai, S., Almey, A., Rajah, M. N. & Einstein, G. Sex and gender differences in cognitive and brain reserve: implications for Alzheimer’s disease in women. Front. Neuroendocrinol. 60, 100879 (2021).
Hilal, S. & Brayne, C. Epidemiologic trends, social determinants, and brain health: the role of life course inequalities. Stroke https://doi.org/10.1161/STROKEAHA.121.032609 (2022).
Willroth, E. C. et al. A review of the literature on wellbeing and modifiable dementia risk factors. Ageing Res. Rev. 99, 102380 (2024).
Elcombe, E. L. et al. Hippocampal volume in older adults at risk of cognitive decline: the role of sleep, vascular risk, and depression. J. Alzheimers Dis. 44, 1279–1290 (2015).
Fjell, A. M. et al. Neuroinflammation and tau interact with amyloid in predicting sleep problems in aging independently of atrophy. Cereb. Cortex 28, 2775–2785 (2018).
Montero-Odasso, M. et al. Effects of exercise alone or combined with cognitive training and vitamin D supplementation to improve cognition in adults with mild cognitive impairment: a randomized clinical trial. JAMA Netw. Open 6, e2324465 (2023).
Joseph, J., Cole, G., Head, E. & Ingram, D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 29, 12795–12801 (2009).
Sloan, R. P. et al. Insights into the role of diet and dietary flavanols in cognitive aging: results of a randomized controlled trial. Sci. Rep. 11, 3837 (2021).
Frisoni, G. B. et al. Alzheimer’s disease outlook: controversies and future directions. Lancet 406, 1424–1442 (2025).
Kivipelto, M., Mangialasche, F. & Anstey, K. J. Pivotal points in the science of dementia risk reduction. Lancet 404, 501–503 (2024).
Arenaza-Urquijo, E. M. et al. Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimers Dement. 20, 5695–5719 (2024).
Kaup, A. R. et al. Cognitive resilience to apolipoprotein E ε4: contributing factors in black and white older adults. JAMA Neurol. 72, 340–348 (2015).
McDermott, K. L., McFall, G. P., Andrews, S. J., Anstey, K. J. & Dixon, R. A. Memory resilience to Alzheimer’s genetic risk: sex effects in predictor profiles. J. Gerontol. B Psychol. Sci. Soc. Sci. 72, 937–946 (2017).
Zheng, L. et al. Gender specific factors contributing to cognitive resilience in APOE ɛ4 positive older adults in a population-based sample. Sci. Rep. 13, 8037 (2023).
Wu, Y. T. et al. The changing prevalence and incidence of dementia over time — current evidence. Nat. Rev. Neurol. 13, 327–339 (2017).
Arbel, I., Bingham, K. S. & Dawson, D. R. A scoping review of literature on sex and gender differences among dementia spousal caregivers. Gerontologist 59, e802–e815 (2019).
Wierenga, L. M. et al. Recommendations for a better understanding of sex and gender in the neuroscience of mental health. Biol. Psychiatry Glob. Open Sci. 4, 100283 (2024).
Barth, C., Crestol, A., de Lange, A. G. & Galea, L. A. M. Sex steroids and the female brain across the lifespan: insights into risk of depression and Alzheimer’s disease. Lancet Diabetes Endocrinol. 11, 926–941 (2023).
Buckley, R. F. et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 76, 542–551 (2019).
Wang, X., Sundermann, E. E., Buckley, R. F., Banks, S. J. & Team, A. S. Sex differences in the association between tau PET and cognitive performance in a non-Hispanic white cohort with preclinical AD. Alzheimers Dement. 20, 25–33 (2024).
Gong, J. et al. Sex differences in dementia risk and risk factors: individual-participant data analysis using 21 cohorts across six continents from the COSMIC consortium. Alzheimers Dement. 19, 3365–3378 (2023).
Cho, S., Crenshaw, K. & McCall, L. Toward a field of intersectionality studies: theory, applications, and praxis. Signs 38, 785–810 (2013).
Nietzel, M. T. Women continue to outpace men in college enrollment and graduation. Forbes Magazine https://www.forbes.com/sites/michaeltnietzel/2024/08/07/women-continue-to-outpace-men-in-college-enrollment-and-graduation/ (Forbes, 2024).
Livingston, G. et al. Dementia prevention, intervention, and care. Lancet 390, 2673–2734 (2017).
Nianogo, R. A. et al. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 79, 584–591 (2022).
Niu, Z., Haley, A. P., Clark, A. L. & Duarte, A. Age exacerbates the negative effect of depression on executive functioning in racial and ethnic minorities. Brain Imaging Behav. https://doi.org/10.1007/s11682-024-00898-3 (2024).
Hokett, E. & Duarte, A. Age and race-related differences in sleep discontinuity linked to associative memory performance and its neural underpinnings. Front. Hum. Neurosci. 13, 176 (2019).
Hokett, E., Mirjalili, S. & Duarte, A. Greater sleep variance related to decrements in memory performance and event-specific neural similarity: a racially/ethnically diverse lifespan sample. Neurobiol. Aging 117, 33–43 (2022).
Duarte-Guterman, P. et al. Inflammation in Alzheimer’s disease: do sex and APOE matter? J. Alzheimers Dis. 78, 627–641 (2020).
Duchesne, A., Pletzer, B., Pavlova, M. A., Lai, M. C. & Einstein, G. Editorial: bridging gaps between sex and gender in neurosciences. Front. Neurosci. 14, 561 (2020).
Nebel, R. A. et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 14, 1171–1183 (2018).
Subramaniapillai, S. et al. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. NeuroImage Clin. 30, 102620 (2021).
Lin, K. A. et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 1, 103–110 (2015).
Tifratene, K., Robert, P., Metelkina, A., Pradier, C. & Dartigues, J. F. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 85, 331–338 (2015).
Sperling, R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1097, 146–155 (2007).
LaPlume, A., Lissaman, R., Kearley, J. & Rajah, M. N. in Encyclopedia of the Human Brain Vol. 4 (ed. Grafman, J.) 95–112 (Elsevier, 2025).
Koran, M. E. I., Wagener, M., Hohman, T. J. & Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 11, 205–213 (2017).
Kearley, J., Lissaman, R., Laugier, A. & Rajah, M. N. Association between CAIDE risk score and episodic memory in middle-aged females: the impact of spontaneous menopause. Horm. Behav. 171, 105739 (2025).
Kaur, A. et al. Sex differences in the association of age at hypertension diagnosis with brain structure. Hypertension 81, 291–301 (2024).
Lissaman, R., Rajagopal, S., Kearley, J., Pasvanis, S. & Rajah, M. N. Menopause status- and sex-related differences in age associations with spatial context memory and white matter microstructure at midlife. Neurobiol. Aging 141, 151–159 (2024).
Edwards, H., Duchesne, A., Au, A. & Einstein, G. The many menopauses: searching the cognitive research literature for menopause types. Menopause 26, 45–65 (2018).
Duarte-Guterman, P., Leuner, B. & Galea, L. A. M. The long and short term effects of motherhood on the brain. Front. Neuroendocrinol. 53, 100740 (2019).
Puri, T. A., Richard, J. E. & Galea, L. A. M. Beyond sex differences: short- and long-term effects of pregnancy on the brain. Trends Neurosci. 46, 459–471 (2023).
Avila, J. F. et al. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 15, 1516–1523 (2019).
Casaletto, K. B. et al. Cognitive aging is not created equally: differentiating unique cognitive phenotypes in ‘normal’ adults. Neurobiol. Aging 77, 13–19 (2019).
Glymour, M. M. & Whitmer, R. A. Using cross-cultural studies to improve evidence on dementia prevention: lessons from the special issue sponsored by the International Research Network on Dementia Prevention (IRNDP). J. Alzheimers Dis. 70, S5–S10 (2019).
Kalaria, R. et al. The 2022 symposium on dementia and brain aging in low- and middle-income countries: highlights on research, diagnosis, care, and impact. Alzheimers Dement. 20, 4290–4314 (2024).
Avila, J. F. et al. Measurement invariance of neuropsychological measures of cognitive aging across race/ethnicity by sex/gender groups. Neuropsychology 34, 3–14 (2020).
Anstey, K. J. et al. Future directions for dementia risk reduction and prevention research: an international research network on dementia prevention consensus. J. Alzheimers Dis. 78, 3–12 (2020).
Subramaniapillai, S. et al. Sex differences in the neural correlates of spatial context memory decline in healthy aging. J. Cogn. Neurosci. 31, 1895–1916 (2019).
Gervais, N. J. et al. Cognitive markers of dementia risk in middle-aged women with bilateral salpingo-oophorectomy prior to menopause. Neurobiol. Aging 94, 1–6 (2020).
Crestol, A. et al. Menopause status and within-group differences in chronological age affect the functional neural correlates of spatial context memory in middle-aged females. J. Neurosci. 43, 8756–8768 (2023).
Calvo, N. & Einstein, G. Clarifying our language for better women’s brain health: what do we really mean when we say ‘menopause’ and ‘hormone therapy’? Br. J. Psychiatry 226, 343–345 (2025).
Einstein, G., Au, A., Klemensberg, J., Shin, E. M. & Pun, N. The gendered ovary: whole body effects of oophorectomy. Can. J. Nurs. Res. 44, 7–17 (2012).
Adedeji, D. O. et al. Longitudinal study of Alzheimer’s disease biomarkers, allostatic load, and cognition among memory clinic patients. Brain Behav. Immun. Health 28, 100592 (2023).
Juster, R. P., Rutherford, C., Keyes, K. & Hatzenbuehler, M. L. Associations between structural stigma and allostatic load among sexual minorities: results from a population-based study. Psychosom. Med. 86, 157–168 (2024).
Messiha, K. et al. Grey literature scoping review: a synthesis of the application of participatory methodologies in underrepresented groups at an elevated risk of dementia. BMC Med. Res. Methodol. 25, 122 (2025).
Moorthie, S. et al. A scoping review of approaches to improving quality of data relating to health inequalities. Int. J. Environ. Res. Public Health 19, 15874 (2022).
Badhwar, A. et al. A multiomics approach to heterogeneity in Alzheimer's disease: focused review and roadmap. Brain 143, 1315–1331 (2020).
Pickett, J. et al. A roadmap to advance dementia research in prevention, diagnosis, intervention, and care by 2025. Int. J. Geriatr. Psychiatry 33, 900–906 (2018).
Hutchison, E. D. Dimensions of Human Behavior. The Changing Life Course 3rd edn (Sage, 2008).
Christophe, N. K. & Stein, G. L. Facilitating the study of familism across racial/ethnic groups: creation of the short attitudinal familism scale. J. Fam. Psychol. 36, 534–544 (2022).
Baltes, P. B., Staudinger, U. M. & Lindenberger, U. Lifespan psychology: theory and application to intellectual functioning. Annu. Rev. Psychol. 50, 471–507 (1999).
Blazer, D. G., Yaffe, K. & Karlawish, J. Cognitive aging: a report from the Institute of Medicine. JAMA 313, 2121–2122 (2015).
Eyre, H. A. et al. Life-course brain health as a determinant of late-life mental health: American Association for Geriatric Psychiatry Expert Panel recommendations. Am. J. Geriatr. Psychiatry 31, 1017–1031 (2023).
Stein, G. L. et al. When discrimination hurts: the longitudinal impact of increases in peer discrimination on anxiety and depressive symptoms in Mexican-origin youth. J. Youth Adolesc. 48, 864–875 (2019).
Cabeza, R. et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710 (2018).
Birkenbihl, C. et al. Rethinking the residual approach: leveraging machine learning to operationalize cognitive resilience in Alzheimer’s disease. Brain Inform. 12, 3 (2025).
Kremen, W. S. et al. Cognitive reserve and related constructs: a unified framework across cognitive and brain dimensions of aging. Front. Aging Neurosci. 14, 834765 (2022).
Stern, Y. et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. https://doi.org/10.1016/j.jalz.2018.07.219 (2018).
Whitehall, L. et al. Psychometric evaluation of the Making it CLEAR questionnaire: a resilience measure for older adults. Innov. Aging 5, igab030 (2021).
Elman, J. A. et al. Issues and recommendations for the residual approach to quantifying cognitive resilience and reserve. Alzheimers Res. Ther. 14, 102 (2022).
Bocancea, D. I. et al. Measuring resilience and resistance in aging and Alzheimer disease using residual methods: a systematic review and meta-analysis. Neurology 97, 474–488 (2021).
Hou, J. et al. Polygenic resilience scores capture protective genetic effects for Alzheimer’s disease. Transl. Psychiatry 12, 296 (2022).
Krieger, N. et al. Epigenetic aging and racialized, economic, and environmental injustice: NIMHD Social Epigenomics Program. JAMA Netw. Open 7, e2421832 (2024).
Kerr, P., Kheloui, S., Rossi, M., Desilets, M. & Juster, R. P. Allostatic load and women’s brain health: a systematic review. Front. Neuroendocrinol. 59, 100858 (2020).
McEwen, B. S. & Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101 (1993).
Crimmins, E. M., Johnston, M., Hayward, M. & Seeman, T. Age differences in allostatic load: an index of physiological dysregulation. Exp. Gerontol. 38, 731–734 (2003).
D’Amico, D., Amestoy, M. E. & Fiocco, A. J. The association between allostatic load and cognitive function: a systematic and meta-analytic review. Psychoneuroendocrinology 121, 104849 (2020).
Yu, Y. L. & Juster, R. P. Spousal synchrony in allostatic load among older couples in the Health and Retirement Study. Psychosom. Med. 85, 716–726 (2023).
Geronimus, A. T. et al. Weathering in Detroit: place, race, ethnicity, and poverty as conceptually fluctuating social constructs shaping variation in allostatic load. Milbank Q. 98, 1171–1218 (2020).
Juster, R. P. et al. Sex differences and gender diversity in stress responses and allostatic load among workers and LGBT people. Curr. Psychiatry Rep. 21, 110 (2019).
Mays, V. M., Juster, R. P., Williamson, T. J., Seeman, T. E. & Cochran, S. D. Chronic physiologic effects of stress among lesbian, gay, and bisexual adults: results from the National Health and Nutrition Examination Survey. Psychosom. Med. 80, 551–563 (2018).
Kochan, N. A. et al. Reliability, validity, and user-experience of remote unsupervised computerized neuropsychological assessments in community-living 55- to 75-year-olds. J. Alzheimers Dis. 90, 1629–1645 (2022).
Jacova, C. et al. C-TOC (Cognitive Testing on Computer): investigating the usability and validity of a novel self-administered cognitive assessment tool in aging and early dementia. Alzheimer Dis. Assoc. Disord. 29, 213–221 (2015).
Wild, K., Howieson, D., Webbe, F., Seelye, A. & Kaye, J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 4, 428–437 (2008).
Li, D. & Mielke, M. M. An update on blood-based markers of Alzheimer’s disease using the SiMoA platform. Neurol. Ther. 8, 73–82 (2019).
Nesselroade, J.R. & Baltes, P.B. (Eds.) Longitudinal Research in the Study of Behavior and Development (Academic, 1979).
Iturria-Medina, Y. et al. Unified epigenomic, transcriptomic, proteomic, and metabolomic taxonomy of Alzheimer’s disease progression and heterogeneity. Sci. Adv. 8, eabo6764 (2022).
Misic, B. & Sporns, O. From regions to connections and networks: new bridges between brain and behavior. Curr. Opin. Neurobiol. 40, 1–7 (2016).
Khan, A. F. & Iturria-Medina, Y. Beyond the usual suspects: multi-factorial computational models in the search for neurodegenerative disease mechanisms. Transl. Psychiatry 14, 386 (2024).
White, J., Tannenbaum, C., Klinge, I., Schiebinger, L. & Clayton, J. The integration of sex and gender considerations into biomedical research: lessons from International Funding Agencies. J. Clin. Endocrinol. Metab. 106, 3034–3048 (2021).
Tannenbaum, C., Schwarz, J. M., Clayton, J. A., de Vries, G. J. & Sullivan, C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol. Sex Differ. 7, 13 (2016).
Colineaux, H., Soulier, A., Lepage, B. & Kelly-Irving, M. Considering sex and gender in epidemiology: a challenge beyond terminology. From conceptual analysis to methodological strategies. Biol. Sex Differ. 13, 23 (2022).
Rioux, C. et al. Sex and gender terminology: a glossary for gender-inclusive epidemiology. J. Epidemiol. Community Health https://doi.org/10.1136/jech-2022-219171 (2022).
Ackley, S. F. et al. Discordance in chromosomal and self-reported sex in the UK Biobank: implications for transgender- and intersex-inclusive data collection. Proc. Natl Acad. Sci. USA 120, e2218700120 (2023).
Acknowledgements
The authors thank all attendees at the BReDAD Collaboratory symposium. The BReDAD Collaboratory is funded by the Canadian Institutes of Health Research (CIHR) Institute of Aging Operating Grant: Brain Health and Reduction of Risk for Age-related Cognitive Impairment — Knowledge Synthesis and Mobilization Grants, reference no. 191181 awarded to M.N.R. as the principal investigator (PI), with R.A.D., G.E. and Y.S. as co-PIs. M.N.R. acknowledges support from the Canada Research Chairs Program CRC-2022-00,240, CIHR Sex & Gender Research Chair GS9-171,369, and NSERC Discovery Grant RGPIN-2018-05,761. G.E. acknowledges support from the CIHR no. WJP-150643 (the Wilfred and Joyce Posluns Chair in Women’s Brain Health and Aging), Canadian Cancer Society no. 310336, Alzheimer Society of Canada no. 72953944, CIHR no. MOP-130490, Ontario Brain Institute, CIHR no. CNA-163902 and the Jacqueline Ford Fund for Gender and Health. R.A.D. acknowledges support from the Canadian Consortium on Neurodegeneration in Aging with funding from Alberta Innovates (no. G2020000063) and CIHR (no. 163902).
Author information
Authors and Affiliations
Consortia
Contributions
M.N.R., R.A.D., G.E. and Y.S. researched data for the article, provided substantial contributions to discussion of its content, wrote the article and reviewed and edited the manuscript before submission. BReDAD Collaboratory members A.B., R.B., K.C., K.C., J.D., A.D., L.G., M.G., Y.I., I.I., R.J., J.M., B.M., M.R., M.S. and P.V. contributed content for the article and provided substantial input on the discussion of its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Carol Brayne, Eero Vuoksimaa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Brain Resilience and Diversity in Aging and Dementia (BReDAD) Collaboratory: https://resilienceandaging.com/
Collaboratory on Research Definitions for Reserve and Resilience in Cognitive Aging and Dementia: https://reserveandresilience.com
Supplementary information
Rights and permissions
About this article
Cite this article
Rajah, M.N., Dixon, R.A., Einstein, G. et al. A roadmap for conducting more inclusive research on brain resilience in ageing and dementia. Nat. Rev. Neurosci. (2026). https://doi.org/10.1038/s41583-025-01021-1
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41583-025-01021-1