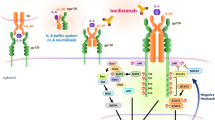
Abstract
In 1973, IL-6 was identified as a soluble factor that is secreted by T cells and is important for antibody production by B cells. Since its discovery more than 40 years ago, the IL-6 pathway has emerged as a pivotal pathway involved in immune regulation in health and dysregulation in many diseases. Targeting of the IL-6 pathway has led to innovative therapeutic approaches for various rheumatic diseases, such as rheumatoid arthritis, juvenile idiopathic arthritis, adult-onset Still’s disease, giant cell arteritis and Takayasu arteritis, as well as other conditions such as Castleman disease and cytokine release syndrome. Targeting this pathway has also identified avenues for potential expansion into several other indications, such as uveitis, neuromyelitis optica and, most recently, COVID-19 pneumonia. To mark the tenth anniversary of anti-IL-6 receptor therapy worldwide, we discuss the history of research into IL-6 biology and the development of therapies that target IL-6 signalling, including the successes and challenges and with an emphasis on rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kishimoto, T. & Ishizaka, K. Regulation of antibody response in vitro. VII. Enhancing soluble factors for IgG and IgE antibody response. J. Immunol. 111, 1194–1205 (1973).
Hirano, T. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76 (1986).
Hashizume, M. et al. Tocilizumab, a humanized anti-IL-6R antibody, as an emerging therapeutic option for rheumatoid arthritis: molecular and cellular mechanistic insights. Int. Rev. Immunol. 34, 265–279 (2015).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018).
Kang, S., Tanaka, T., Narazaki, M. & Kishimoto, T. Targeting interleukin-6 signaling in clinic. Immunity 50, 1007–1023 (2019).
Hibi, M. et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63, 1149–1157 (1990).
Yawata, H. et al. Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J. 12, 1705–1712 (1993).
Waage, A., Kaufmann, C., Espevik, T. & Husby, G. Interleukin-6 in synovial fluid from patients with arthritis. Clin. Immunol. Immunopathol. 50, 394–398 (1989).
Meyers, F. J. et al. Bladder cancer. human leukocyte antigen II, interleukin-6, and interleukin-6 receptor expression determined by the polymerase chain reaction. Cancer 67, 2087–2095 (1991).
Riechmann, L., Clark, M., Waldmann, H. & Winter, G. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
Sato, K. et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 53, 851–856 (1993).
Mihara, M. et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int. Immunopharmacol. 5, 1731–1740 (2005).
Kawano, M. et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332, 83–85 (1988).
Bataille, R. et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood 86, 685–691 (1995).
Lu, Z. Y. et al. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood 86, 3123–3231 (1995).
San-Miguel, J. et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 123, 4136–4142 (2014).
Yoshizaki, K. et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 74, 1360–1367 (1989).
Suematsu, S. et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc. Natl Acad. Sci. USA 86, 7547–7551 (1989).
Katsume, A. et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease like symptoms emerged in IL-6 transgenic mice. Cytokine 20, 304–311 (2002).
Beck, J. T. et al. Brief report: alleviation of systemic manifestations of Castleman’s disease by monoclonal anti-interleukin-6 antibody. N. Engl. J. Med. 330, 602–605 (1994).
Nishimoto, N. et al. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 95, 56–61 (2000).
Nishimoto, N. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 106, 2627–2632 (2005).
van Rhee, F. et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 15, 966–974 (2014).
Mitsuyama, K. et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut 36, 45–49 (1995).
Ito, H. et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 126, 989–996 (2004).
Monemi, S. et al. Incidence of gastrointestinal perforations in patients with rheumatoid arthritis treated with tocilizumab from clinical trial, postmarketing, and real-world data sources. Rheumatol. Ther. 3, 337–352 (2016).
Guerne, P. A., Zuraw, B. L., Vaughan, J. H., Carson, D. A. & Lotz, M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J. Clin. Invest. 83, 585–592 (1989).
Jilka, R. L. et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257, 88–91 (1992).
van de Loo, F. A., Joosten, L. A., van Lent, P. L., Arntz, O. J. & van den Berg, W. B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 38, 164–172 (1995).
Poli, V. et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 13, 1189–1196 (1994).
Takagi, N. et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 41, 2117–2121 (1998).
Fujimoto, M. et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory TH17 responses. Arthritis Rheum. 58, 3710–3719 (2008).
Ohshima, S. et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc. Natl Acad. Sci. USA 95, 8222–8226 (1998).
Wendling, D., Racadot, E. & Wijdenes, J. Treatment of severe rheumatoid arthritis by anti-interleukin 6 monoclonal antibody. J. Rheumatol. 20, 259–262 (1993).
Nishimoto, N., Kishimoto, T. & Yoshizaki, K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann. Rheum. Dis. 59 (Suppl. 1), i21–i27 (2000).
Choy, E. H. et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 46, 3143–3150 (2002).
Nishimoto, N. et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 50, 1761–1769 (2004).
Maini, R. N. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 54, 2817–2829 (2006).
Nishimoto, N. et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 66, 1162–1167 (2007).
Jones, G. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann. Rheum. Dis. 69, 88–96 (2010).
Emery, P. et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67, 1516–1523 (2008).
Genovese, M. C. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 (2008).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Kremer, J. M. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 63, 609–621 (2011).
Takeuchi, T. et al. Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients—REACTION 52-week study. Rheumatology 50, 1908–1915 (2011).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Dougados, M. et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann. Rheum. Dis. 72, 43–50 (2013).
Dougados, M. et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann. Rheum. Dis. 73, 803–809 (2014).
Kaneko, Y. et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann. Rheum. Dis. 75, 1917–1923 (2016).
Teitsma, X. M., Marijnissen, A. K., Bijlsma, J. W., Lafeber, F. P. & Jacobs, J. W. Tocilizumab as monotherapy or combination therapy for treating active rheumatoid arthritis: a meta-analysis of efficacy and safety reported in randomized controlled trials. Arthritis Res. Ther. 18, 211 (2016).
Burmester, G. R. et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann. Rheum. Dis. 75, 1081–1091 (2016).
Strand, V. et al. Impact of tocilizumab monotherapy on patient-reported outcomes in patients with rheumatoid arthritis from two randomised controlled trials. RMD Open 3, e000496 (2017).
Jones, G. et al. Five-year efficacy and safety of tocilizumab monotherapy in patients with rheumatoid arthritis who were methotrexate- and biologic-naive or free of methotrexate for 6 months: the AMBITION study. J. Rheumatol. 44, 142–146 (2017).
Burmester, G. R. et al. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann. Rheum. Dis. 76, 1279–1284 (2017).
Edwards, C. J., Ostor, A. J. K., Naisbett-Groet, B. & Kiely, P. Tapering versus steady-state methotrexate in combination with tocilizumab for rheumatoid arthritis: a randomized, double-blind trial. Rheumatology 57, 84–91 (2018).
Kaneko, Y. et al. Tocilizumab discontinuation after attaining remission in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate: results from a prospective randomised controlled study (the second year of the SURPRISE study). Ann. Rheum. Dis. 77, 1268–1275 (2018).
Kremer, J. M. et al. Sustained response following discontinuation of methotrexate in patients with rheumatoid arthritis treated with subcutaneous tocilizumab: results from a randomized, controlled trial. Arthritis Rheumatol. 70, 1200–1208 (2018).
Rubbert-Roth, A., Furst, D. E., Nebesky, J. M., Jin, A. & Berber, E. A review of recent advances using tocilizumab in the treatment of rheumatic diseases. Rheumatol. Ther. 5, 21–42 (2018).
Teitsma, X. M. et al. Inadequate response to treat-to-target methotrexate therapy in patients with new-onset rheumatoid arthritis: development and validation of clinical predictors. Ann. Rheum. Dis. 77, 1261–1267 (2018).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216655 (2020).
Dutch Association for Rheumatology. NVR Standpunt Tocilizumab Verklaring Commissie Kwaliteit. https://www.nvr.nl/wp-content/uploads/2018/09/NVR-Standpunt-Tocilizumab-verklaring-commissie-kwaliteit-nov-2009.pdf (2009).
Finzel, S. et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann. Rheum. Dis. 78, 1186–1191 (2019).
Fonseca, J. E. et al. Portuguese guidelines for the use of biological agents in rheumatoid arthritis—October 2011 update. Acta Reumatol. Port. 36, 385–358 (2011).
Swedish Rheumatological Association. Guidelines for the pharmaceutical management of rheumatoid arthritis. http://svenskreumatologi.se/wp-content/uploads/2016/08/guidelines_for_the_pharmaceutical_management_of_rheumatoid_arthritis.pdf (2011).
Gaujoux-Viala, C. et al. Recommendations of the French Society for Rheumatology for managing rheumatoid arthritis. Joint Bone Spine 81, 287–297 (2014).
Albrecht, K. et al. German guidelines for the sequential medical treatment of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. Rheumatol. Int. 34, 1–9 (2014).
National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. https://www.nice.org.uk/guidance/TA375 (NICE, 2016).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 76, 960–977 (2017).
Garcia-Vicuna, R. et al. Recommendations by the Spanish Society of Rheumatology for the management of patients diagnosed with rheumatoid arthritis who cannot be treated with methotrexate. Reumatol. Clin. 13, 127–138 (2017).
Duarte, C. et al. Portuguese recommendations for the use of biological therapies in patients with rheumatoid arthritis—2016 update. Acta Reumatol. Port. 42, 112–126 (2017).
Moreland, L. W. & Curtis, J. R. Systemic nonarticular manifestations of rheumatoid arthritis: focus on inflammatory mechanisms. Semin. Arthritis Rheum. 39, 132–143 (2009).
Davis, M. C. et al. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav. Immun. 22, 24–32 (2008).
Boyapati, A. et al. Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of MOBILITY. Arthritis Res. Ther. 18, 225 (2016).
Fleischmann, R. et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 69, 277–290 (2017).
Burmester, G. R. et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann. Rheum. Dis. 76, 840–847 (2017).
Takeuchi, T. et al. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann. Rheum. Dis. 76, 2001–2008 (2017).
Aletaha, D. et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet 389, 1206–1217 (2017).
Taylor, P. C. et al. Efficacy and safety of monotherapy with sirukumab compared with adalimumab monotherapy in biologic-naive patients with active rheumatoid arthritis (SIRROUND-H): a randomised, double-blind, parallel-group, multinational, 52-week, phase 3 study. Ann. Rheum. Dis. 77, 658–666 (2018).
Genovese, M. C. et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase IIb study. Ann. Rheum. Dis. 73, 1607–1615 (2014).
Ravelli, A. & Martini, A. Juvenile idiopathic arthritis. Lancet 369, 767–778 (2007).
De Benedetti, F. et al. Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J. Clin. Invest. 93, 2114–2119 (1994).
Cazzola, M. et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood 87, 4824–4830 (1996).
De Benedetti, F. et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J. Clin. Invest. 99, 643–650 (1997).
De Benedetti, F. et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 54, 3551–3563 (2006).
Hinze, C., Gohar, F. & Foell, D. Management of juvenile idiopathic arthritis: hitting the target. Nat. Rev. Rheumatol. 11, 290–300 (2015).
Woo, P. et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res. Ther. 7, R1281–R1288 (2005).
Yokota, S. et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 52, 818–825 (2005).
Yokota, S. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 371, 998–1006 (2008).
De Benedetti, F. et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 367, 2385–2395 (2012).
Yokota, S. et al. Long-term treatment of systemic juvenile idiopathic arthritis with tocilizumab: results of an open-label extension study in Japan. Ann. Rheum. Dis. 72, 627–628 (2013).
Yokota, S. et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J. Rheumatol. 41, 759–767 (2014).
De Benedetti, F. et al. Catch-up growth during tocilizumab therapy for systemic juvenile idiopathic arthritis: results from a phase III trial. Arthritis Rheumatol. 67, 840–848 (2015).
Yokota, S. et al. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann. Rheum. Dis. 75, 1654–1660 (2016).
Kaneko, Y. et al. Tocilizumab in patients with adult-onset Still’s disease refractory to glucocorticoid treatment: a randomised, double-blind, placebo-controlled phase III trial. Ann. Rheum. Dis. 77, 1720–1729 (2018).
Imagawa, T. et al. Safety and efficacy of tocilizumab, an anti-IL-6-receptor monoclonal antibody, in patients with polyarticular-course juvenile idiopathic arthritis. Mod. Rheumatol. 22, 109–115 (2012).
Brunner, H. I. et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann. Rheum. Dis. 74, 1110–1117 (2015).
Bharucha, K. N. et al. Growth during tocilizumab therapy for polyarticular-course juvenile idiopathic arthritis: 2-year data from a phase III clinical trial. J. Rheumatol. 45, 1173–1179 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02776735 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02991469 (2020).
Ambarus, C., Yeremenko, N., Tak, P. P. & Baeten, D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr. Opin. Rheumatol. 24, 351–358 (2012).
Ranganathan, V., Gracey, E., Brown, M. A., Inman, R. D. & Haroon, N. Pathogenesis of ankylosing spondylitis — recent advances and future directions. Nat. Rev. Rheumatol. 13, 359–367 (2017).
Gratacos, J. et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br. J. Rheumatol. 33, 927–931 (1994).
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O. & Dougados, M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100 (2014).
Sieper, J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057 (2015).
Scher, J. U., Ogdie, A., Merola, J. F. & Ritchlin, C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat. Rev. Rheumatol. 15, 153–166 (2019).
Partsch, G. et al. Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J. Rheumatol. 24, 518–523 (1997).
van Kuijk, A. W., Reinders-Blankert, P., Smeets, T. J., Dijkmans, B. A. & Tak, P. P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann. Rheum. Dis. 65, 1551–1557 (2006).
Mease, P. J. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Mihara, M. & Ohsugi, Y. Possible role of IL-6 in pathogenesis of immune complex-mediated glomerulonephritis in NZB/W F1 mice: induction of IgG class anti-DNA autoantibody production. Int. Arch. Allergy. Appl. Immunol. 93, 89–92 (1990).
Hirohata, S. & Miyamoto, T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system involvement. Arthritis Rheum. 33, 644–649 (1990).
Gordon, C. et al. Urinary IL-6: a marker for mesangial proliferative glomerulonephritis? Clin. Exp. Immunol. 86, 145–149 (1991).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
Shirota, Y. et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 72, 118–128 (2013).
Rovin, B. H. et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 68, 2174–2183 (2016).
Wallace, D. J. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 76, 534–542 (2017).
Kitaba, S. et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am. J. Pathol. 180, 165–176 (2012).
Kadono, T., Kikuchi, K., Ihn, H., Takehara, K. & Tamaki, K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J. Rheumatol. 25, 296–301 (1998).
De Lauretis, A. et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 40, 435–446 (2013).
Shima, Y. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 49, 2408–2412 (2010).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Khanna, D. et al. Efficacy and safety of tocilizumab for the treatment of systemic sclerosis: results from a phase 3 randomized controlled trial. Arthritis Rheumatol. 70 (Suppl. 10), 898 (2018).
Dejaco, C. et al. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat. Rev. Rheumatol. 13, 578–592 (2017).
Kim, E. S. H. & Beckman, J. Takayasu arteritis: challenges in diagnosis and management. Heart 104, 558–565 (2018).
Dasgupta, B. & Panayi, G. S. Interleukin-6 in serum of patients with polymyalgia rheumatica and giant cell arteritis. Br. J. Rheumatol. 29, 456–458 (1990).
Noris, M., Daina, E., Gamba, S., Bonazzola, S. & Remuzzi, G. Interleukin-6 and RANTES in Takayasu arteritis: a guide for therapeutic decisions? Circulation 100, 55–60 (1999).
Villiger, P. M. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 387, 1921–1927 (2016).
Stone, J. H. et al. Trial of tocilizumab in giant-cell arteritis. N. Engl. J. Med. 377, 317–328 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03600805 (2020).
Nakaoka, Y. et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann. Rheum. Dis. 77, 348–354 (2018).
Macchioni, P. et al. Tocilizumab for polymyalgia rheumatica: report of two cases and review of the literature. Semin. Arthritis Rheum. 43, 113–118 (2013).
Lally, L., Forbess, L., Hatzis, C. & Spiera, R. Brief report: a prospective open-label phase IIa trial of tocilizumab in the treatment of polymyalgia rheumatica. Arthritis Rheumatol. 68, 2550–2554 (2016).
Devauchelle-Pensec, V. et al. Efficacy of first-line tocilizumab therapy in early polymyalgia rheumatica: a prospective longitudinal study. Ann. Rheum. Dis. 75, 1506–1510 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03263715 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03600818 (2020).
Hay, K. A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 183, 364–374 (2018).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Chinese Clinical Trial Registry. Chictr.org.cn http://www.chictr.org.cn/showprojen.aspx?proj=49409 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04317092 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04320615 (2020).
Koike, T. et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann. Rheum. Dis. 70, 2148–2151 (2011).
Genovese, M. C. et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J. Rheumatol. 40, 768–780 (2013).
Koike, T. et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J. Rheumatol. 41, 15–23 (2014).
Yamamoto, K. et al. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J. Rheumatol. 42, 1368–1375 (2015).
Burmester, G. R. et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann. Rheum. Dis. 75, 68–74 (2016).
Kremer, J. M. et al. Clinical efficacy and safety maintained up to 5 years in patients with rheumatoid arthritis treated with tocilizumab in a randomised trial. Clin. Exp. Rheumatol. 34, 625–633 (2016).
Flaig, T. et al. Tocilizumab-induced pancreatitis: case report and review of data from the FDA adverse event reporting system. J. Clin. Pharm. Ther. 41, 718–721 (2016).
Hoeltzenbein, M. et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin. Arthritis Rheum. 46, 238–245 (2016).
Kivitz, A. et al. Long-term safety and efficacy of subcutaneously administered tocilizumab for adult rheumatoid arthritis: a multicenter phase 3b long-term extension study. Rheumatol. Ther. 3, 291–304 (2016).
Genovese, M. C. et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol. 69, 1751–1761 (2017).
Kim, S. C. et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol. 69, 1154–1164 (2017).
Papalopoulos, I. et al. Liver safety of non-tumour necrosis factor inhibitors in rheumatic patients with past hepatitis B virus infection: an observational, controlled, long-term study. Clin. Exp. Rheumatol. 36, 102–109 (2018).
Kim, S. C. et al. No difference in cardiovascular risk of tocilizumab versus abatacept for rheumatoid arthritis: a multi-database cohort study. Semin. Arthritis Rheum. 48, 399–405 (2018).
Rutherford, A. I., Subesinghe, S., Hyrich, K. L. & Galloway, J. B. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 77, 905–910 (2018).
Gron, K. L. et al. Risk of serious infections in patients with rheumatoid arthritis treated in routine care with abatacept, rituximab and tocilizumab in Denmark and Sweden. Ann. Rheum. Dis. 78, 320–327 (2019).
Curtis, J. R. et al. Tocilizumab in rheumatoid arthritis: a case study of safety evaluations of a large postmarketing data set from multiple data sources. Semin. Arthritis Rheum. 44, 381–388 (2015).
Sakai, R. et al. Head-to-head comparison of the safety of tocilizumab and tumor necrosis factor inhibitors in rheumatoid arthritis patients (RA) in clinical practice: results from the registry of Japanese RA patients on biologics for long-term safety (REAL) registry. Arthritis Res. Ther. 17, 74 (2015).
Morel, J. et al. Risk factors of serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatology 56, 1746–1754 (2017).
Choy, E. et al. Evaluation of the efficacy and safety of sarilumab combination therapy in patients with rheumatoid arthritis with inadequate response to conventional disease-modifying antirheumatic drugs or tumour necrosis factor alpha inhibitors: systematic literature review and network meta-analyses. RMD Open 5, e000798 (2019).
Emery, P. et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology 58, 849–858 (2018).
Genentech. Tocilizumab package insert in the USA. Genentech https://www.gene.com/download/pdf/actemra_prescribing.pdf (2019).
European Medicines Agency. Tocilizumab summary of product characteristics in EU. EMA http://ec.europa.eu/health/documents/community-register/2018/20181029142753/anx_142753_en.pdf (2018).
Sanofi. Sarilumab package insert in the USA. Sanofi http://products.sanofi.us/kevzara/kevzara.pdf (2018).
European Medicines Agency. Sarilumab summary of product characteristics in EU. EMA https://www.ema.europa.eu/en/documents/product-information/kevzara-epar-product-information_en.pdf (2017).
Pardeo, M. et al. Neutropenia during tocilizumab treatment is not associated with infection risk in systemic or polyarticular-course juvenile idiopathic arthritis. J. Rheumatol. 46, 1117–1126 (2019).
Nishimoto, N. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann. Rheum. Dis. 68, 1580–1584 (2009).
McInnes, I. B. et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 74, 694–702 (2015).
Gabay, C. et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1806–1812 (2016).
Fioravanti, A. et al. Tocilizumab modulates serum levels of adiponectin and chemerin in patients with rheumatoid arthritis: potential cardiovascular protective role of IL-6 inhibition. Clin. Exp. Rheumatol. 37, 293–300 (2019).
Scott, L. J. Tocilizumab: a review in rheumatoid arthritis. Drugs 77, 1865–1879 (2017).
Burmester, G. R. et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 76, 1078–1085 (2017).
FDA. FDA summary minutes of the Arthritis Advisory Committee Meeting https://www.fda.gov/media/107409/download (FDA, 2017).
Tsunenari, T. et al. New xenograft model of multiple myeloma and efficacy of a humanized antibody against human interleukin-6 receptor. Blood 90, 2437–2444 (1997).
Bataille, R., Jourdan, M., Zhang, X. G. & Klein, B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J. Clin. Invest. 84, 2008–2011 (1989).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Hosokawa, T. et al. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J. Gastroenterol. Hepatol. 14, 987–996 (1999).
Pignatti, P. et al. Abnormal regulation of interleukin 6 in systemic juvenile idiopathic arthritis. J. Rheumatol. 28, 1670–1676 (2001).
De Benedetti, F. et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 34, 1158–1163 (1991).
Opoka-Winiarska, V. et al. Long-term, interventional, open-label extension study evaluating the safety of tocilizumab treatment in patients with polyarticular-course juvenile idiopathic arthritis from Poland and Russia who completed the global, international CHERISH trial. Clin. Rheumatol. 37, 1807–1816 (2018).
Hoshino, T. et al. Elevated serum interleukin 6, interferon-gamma, and tumor necrosis factor-alpha levels in patients with adult Still’s disease. J. Rheumatol. 25, 396–398 (1998).
Tanaka, Y. et al. Production of B cell-stimulating factors by B cells in patients with systemic lupus erythematosus. J. Immunol. 141, 3043–309 (1988).
Gurram, M., Pahwa, S. & Frieri, M. Augmented interleukin-6 secretion in collagen-stimulated peripheral blood mononuclear cells from patients with systemic sclerosis. Ann. Allergy 73, 493–496 (1994).
Acknowledgements
The authors acknowledge the support of B. Sudbeck in compiling clinical trial information.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.H.C. has received research grants from Bio-Cancer, Biogen, Novartis, Pfizer, Roche, Sanofi and UCB; consultancy fees from AbbVie, Amgen, Biogen, Chugai Pharma, Eli Lilly, Janssen, Novartis, Pfizer, Regeneron, Roche, R-Pharm and Sanofi; speaker’s fees from Amgen, Bristol-Myers Squibb, Chugai Pharma, Eli Lilly, Janssen, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB. F.D.B. has received research grants from AbbVie, Novartis, Pfizer, Roche, Sanofi, Novimmune and SOBI. T.T. has received research grants from AbbVie, Asahi Kasei Pharma, Astellas Pharma, AYUMI Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Nippon Kayaku, Novartis Pharma K.K., Pfizer Japan, Takeda Pharmaceutical; and personal fees from AbbVie G.K., Astellas Pharma, AstraZeneca K.K., Bristol-Myers K.K., Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan K.K., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Nippon Kayaku, Novartis Pharma K.K., Pfizer Japan, Sanofi K.K., Teijin Pharma, Taiho Pharmaceutical, Taisho Pharmaceutical, Takeda Pharmaceutical, UCB Japan. M.H. is an employee of Chugai Pharmaceutical. M.R.J. is employed by Roche and owns shares in Roche. T.K. has a patent for tocilizumab. Work by T.K.’s group is supported in part by the Kishimoto Foundation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choy, E.H., De Benedetti, F., Takeuchi, T. et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 16, 335–345 (2020). https://doi.org/10.1038/s41584-020-0419-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-020-0419-z
This article is cited by
-
Role of Interleukin-6 in Atherothrombosis and Myocardial Infarction
Current Atherosclerosis Reports (2026)
-
FGF4 drives tumor progression in triple-negative breast cancer via IL6/STAT3-mediated macrophage M2 polarization and immune suppression
Cell Division (2025)
-
CDO1 phosphorylation is required for IL-6-induced tumor cell proliferation through governing cysteine availability
Cell Communication and Signaling (2025)
-
RBD-displaying OMV nanovaccine boosts immunity against SARS-CoV-2
Journal of Nanobiotechnology (2025)
-
Cancer-associated fibroblasts: central players in cancer hallmarks and therapeutic resistance
Cell Communication and Signaling (2025)