Abstract
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels1 are essential for pacemaking activity and neural signalling2,3. Drugs inhibiting HCN1 are promising candidates for management of neuropathic pain4 and epileptic seizures5. The general anaesthetic propofol (2,6-di-iso-propylphenol) is a known HCN1 allosteric inhibitor6 with unknown structural basis. Here, using single-particle cryo-electron microscopy and electrophysiology, we show that propofol inhibits HCN1 by binding to a mechanistic hotspot in a groove between the S5 and S6 transmembrane helices. We found that propofol restored voltage-dependent closing in two HCN1 epilepsy-associated polymorphisms that act by destabilizing the channel closed state: M305L, located in the propofol-binding site in S5, and D401H in S6 (refs. 7,8). To understand the mechanism of propofol inhibition and restoration of voltage-gating, we tracked voltage-sensor movement in spHCN channels and found that propofol inhibition is independent of voltage-sensor conformational changes. Mutations at the homologous methionine in spHCN and an adjacent conserved phenylalanine in S6 similarly destabilize closing without disrupting voltage-sensor movements, indicating that voltage-dependent closure requires this interface intact. We propose a model for voltage-dependent gating in which propofol stabilizes coupling between the voltage sensor and pore at this conserved methionine–phenylalanine interface in HCN channels. These findings unlock potential exploitation of this site to design specific drugs targeting HCN channelopathies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The maps for HCN1 WT and M305L in nanodiscs in complex with propofol (accession codes: EMD-42116, EMD-44425) and without propofol (accession codes: EMD-42117, EMD-44426) have been deposited in the Electron Microscopy Data Bank (EMDB). Atomic coordinates for the HCN1 WT and M305L structures in nanodiscs with propofol (accession codes: 8UC7, 9BC6) and without propofol (accession codes: 8UC8, 9BC7) have been deposited in the Protein Data Bank (PDB). Figures 2–5 and Extended Data Figs. 3–5 and 7 have raw data associated with them. Raw electrophysiology and fluorescence traces are available from the corresponding authors upon request. The atomic coordinates of one replica of the free molecular dynamics simulation of DOPC:POPE:POPS lipid-solvated HCN1 channel with propofol bound at sites 1 and 2 at t = 0 and 400 ns and the topology and force field files of all system components including propofol are available at Zenodo (https://doi.org/10.5281/zenodo.11528212)84. Source data are provided with this paper.
References
Kaupp, U. B. & Seifert, R. Molecular diversity of pacemaker ion channels. Annu. Rev. Physiol. 63, 235–257 (2001).
DiFrancesco, D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 55, 455–472 (1993).
Biel, M., Wahl-Schott, C., Michalakis, S. & Zong, X. Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89, 847–885 (2009).
Tibbs, G. R. et al. An anchor-tether ‘hindered’ HCN1 inhibitor is antihyperalgesic in a rat spared nerve injury neuropathic pain model. Br. J. Anaesth. 131, 745–763 (2023).
Bleakley, L. E. et al. Cation leak underlies neuronal excitability in an HCN1 developmental and epileptic encephalopathy. Brain 144, 2060–2073 (2021).
Lyashchenko, A. K., Redd, K. J., Yang, J. & Tibbs, G. R. Propofol inhibits HCN1 pacemaker channels by selective association with the closed states of the membrane embedded channel core. J. Physiol. 583, 37–56 (2007).
Poduri, A. HCN1 gain-of-function mutations—a new cause of epileptic encephalopathy. Epilepsy Curr. 14, 348–349 (2014).
Marini, C. et al. HCN1 mutation spectrum: from neonatal epileptic encephalopathy to benign generalized epilepsy and beyond. Brain 141, 3160–3178 (2018).
Lee, C. H. & MacKinnon, R. Structures of the human HCN1 hyperpolarization-activated channel. Cell 168, 111–120 e111 (2017).
Lee, C. H. & MacKinnon, R. Voltage sensor movements during hyperpolarization in the HCN channel. Cell 179, 1582–1589 e1587 (2019).
Mannikko, R., Elinder, F. & Larsson, H. P. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature 419, 837–841 (2002).
Vemana, S., Pandey, S. & Larsson, H. P. S4 movement in a mammalian HCN channel. J. Gen. Physiol. 123, 21–32 (2004).
Wu, X., Cunningham, K. P., Ramentol, R., Perez, M. E. & Larsson, H. P. Similar voltage-sensor movement in spHCN channels can cause closing, opening, or inactivation. J. Gen. Physiol. 155, e202213170 (2023).
Mandala, V. S. & MacKinnon, R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl Acad. Sci. USA 119, e2214151119 (2022).
Whicher, J. R. & MacKinnon, R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 353, 664–669 (2016).
Kim, D. M. & Nimigean, C. M. Voltage-gated potassium channels: a structural examination of selectivity and gating. Cold Spring Harb. Perspect. Biol. 8, a029231 (2016).
Dai, G., Aman, T. K., DiMaio, F. & Zagotta, W. N. The HCN channel voltage sensor undergoes a large downward motion during hyperpolarization. Nat. Struct. Mol. Biol. 26, 686–694 (2019).
Wu, X., Ramentol, R., Perez, M. E., Noskov, S. Y. & Larsson, H. P. A second S4 movement opens hyperpolarization-activated HCN channels. Proc. Natl Acad. Sci. USA 118, e2102036118 (2021).
Lu, Z., Klem, A. M. & Ramu, Y. Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J. Gen. Physiol. 120, 663–676 (2002).
Long, S. B., Campbell, E. B. & Mackinnon, R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309, 903–908 (2005).
Lorinczi, E. et al. Voltage-dependent gating of KCNH potassium channels lacking a covalent link between voltage-sensing and pore domains. Nat. Commun. 6, 6672 (2015).
Fernandez-Marino, A. I., Harpole, T. J., Oelstrom, K., Delemotte, L. & Chanda, B. Gating interaction maps reveal a noncanonical electromechanical coupling mode in the Shaker K+ channel. Nat. Struct. Mol. Biol. 25, 320–326 (2018).
de la Pena, P., Dominguez, P. & Barros, F. Gating mechanism of Kv11.1 (hERG) K+ channels without covalent connection between voltage sensor and pore domains. Pflugers Arch. 470, 517–536 (2018).
Flynn, G. E. & Zagotta, W. N. Insights into the molecular mechanism for hyperpolarization-dependent activation of HCN channels. Proc. Natl Acad. Sci. USA 115, E8086–E8095 (2018).
Cowgill, J. & Chanda, B. Mapping electromechanical coupling pathways in voltage-gated ion channels: challenges and the way forward. J. Mol. Biol. 433, 167104 (2021).
Rivolta, I., Binda, A., Masi, A. & DiFrancesco, J. C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. 472, 931–951 (2020).
Butler, K. M., da Silva, C., Alexander, J. J., Hegde, M. & Escayg, A. Diagnostic yield from 339 epilepsy patients screened on a clinical gene panel. Pediatr. Neurol. 77, 61–66 (2017).
Bleakley, L. E. & Reid, C. A. HCN1 epilepsy: from genetics and mechanisms to precision therapies. J. Neurochem. https://doi.org/10.1111/jnc.15928 (2023).
Thollon, C. et al. Use-dependent inhibition of hHCN4 by ivabradine and relationship with reduction in pacemaker activity. Br. J. Pharmacol. 150, 37–46 (2007).
Lu, X., Smaill, J. B. & Ding, K. New promise and opportunities for allosteric kinase inhibitors. Angew. Chem. Int. Ed. Engl. 59, 13764–13776 (2020).
Kim, E. D. et al. Allosteric drug discrimination is coupled to mechanochemical changes in the kinesin-5 motor core. J. Biol. Chem. 285, 18650–18661 (2010).
Zhu, S. et al. Structural and dynamic mechanisms of GABAA receptor modulators with opposing activities. Nat. Commun. 13, 4582 (2022).
Ramirez, D., Zuniga, R., Concha, G. & Zuniga, L. HCN channels: new therapeutic targets for pain treatment. Molecules 23, 2094 (2018).
Cacheaux, L. P. et al. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J. Pharmacol. Exp. Ther. 315, 517–525 (2005).
Saponaro, A. et al. Gating movements and ion permeation in HCN4 pacemaker channels. Mol. Cell 81, 2929–2943 e2926 (2021).
Tanguay, J., Callahan, K. M. & D’Avanzo, N. Characterization of drug binding within the HCN1 channel pore. Sci. Rep. 9, 465 (2019).
Faulkner, C., Santos-Carballal, D., Plant, D. F. & de Leeuw, N. H. Atomistic molecular dynamics simulations of propofol and fentanyl in phosphatidylcholine lipid bilayers. ACS Omega 5, 14340–14353 (2020).
Joyce, R. L. et al. Alkylphenol inverse agonists of HCN1 gating: H-bond propensity, ring saturation and adduct geometry differentially determine efficacy and potency. Biochem. Pharmacol. 163, 493–508 (2019).
Shintre, C. et al. Human hyperpolarization activated cyclic nucleotide gated ion channel 4. Zenodo https://doi.org/10.5281/zenodo.1434068 (2018).
Schmidpeter, P. A. M. et al. Anionic lipids unlock the gates of select ion channels in the pacemaker family. Nat. Struct. Mol. Biol. 29, 1092–1100 (2022).
Hung, A. et al. Biophysical analysis of an HCN1 epilepsy variant suggests a critical role for S5 helix Met-305 in voltage sensor to pore domain coupling. Prog. Biophys. Mol. Biol. 166, 156–172 (2021).
Ludwig, A., Zong, X., Jeglitsch, M., Hofmann, F. & Biel, M. A family of hyperpolarization-activated mammalian cation channels. Nature 393, 587–591 (1998).
Decher, N., Chen, J. & Sanguinetti, M. C. Voltage-dependent gating of hyperpolarization-activated, cyclic nucleotide-gated pacemaker channels: molecular coupling between the S4-S5 and C-linkers. J. Biol. Chem. 279, 13859–13865 (2004).
Flynn, G. E. & Zagotta, W. N. Molecular mechanism underlying phosphatidylinositol 4,5-bisphosphate-induced inhibition of SpIH channels. J. Biol. Chem. 286, 15535–15542 (2011).
Bruening-Wright, A., Elinder, F. & Larsson, H. P. Kinetic relationship between the voltage sensor and the activation gate in spHCN channels. J. Gen. Physiol. 130, 71–81 (2007).
Ramentol, R., Perez, M. E. & Larsson, H. P. Gating mechanism of hyperpolarization-activated HCN pacemaker channels. Nat. Commun. 11, 1419 (2020).
Valley, C. C. et al. The methionine-aromatic motif plays a unique role in stabilizing protein structure. J. Biol. Chem. 287, 34979–34991 (2012).
Ryu, S. & Yellen, G. Charge movement in gating-locked HCN channels reveals weak coupling of voltage sensors and gate. J. Gen. Physiol. 140, 469–479 (2012).
Porro, A. et al. Do the functional properties of HCN1 mutants correlate with the clinical features in epileptic patients? Prog. Biophys. Mol. Biol. 166, 147–155 (2021).
Boonsimma, P. et al. Exome sequencing as first-tier genetic testing in infantile-onset pharmacoresistant epilepsy: diagnostic yield and treatment impact. Eur. J. Hum. Genet. 31, 179–187 (2023).
Kim, J. J. et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 585, 303–308 (2020).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Casanal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 29, 1069–1078 (2020).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Liu, Y. et al. CB-Dock2: improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 50, W159–W164 (2022).
Wang, S., Xie, J., Pei, J. & Lai, L. CavityPlus 2022 update: an integrated platform for comprehensive protein cavity detection and property analyses with user-friendly tools and cavity databases. J. Mol. Biol. 435, 168141 (2023).
Maglic, J. B. & Lavendomme, R. MoloVol: an easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures. J. Appl. Crystallogr. 55, 1033–1044 (2022).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Jubb, H. C. et al. Arpeggio: a web server for calculating and visualising interatomic interactions in protein structures. J. Mol. Biol. 429, 365–371 (2017).
Procter, J. B. et al. Alignment of biological sequences with Jalview. Methods Mol. Biol. 2231, 203–224 (2021).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 54, 5.6.1–5.6.37 (2016).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Kasimova, M. A. et al. Helix breaking transition in the S4 of HCN channel is critical for hyperpolarization-dependent gating. eLife 8, e53400 (2019).
Elbahnsi, A. et al. Interplay between VSD, pore, and membrane lipids in electromechanical coupling in HCN channels. eLife 12, e80303 (2023).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ɸ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Arcario, M. J., Mayne, C. G. & Tajkhorshid, E. Atomistic models of general anesthetics for use in in silico biological studies. J. Phys. Chem. B 118, 12075–12086 (2014).
Olsson, M. H., Sondergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 7, 525–537 (2011).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
Darden, T. A., York, D. M. & Pedersen, L. G. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Kim, E. D. et al. Propofol rescues voltage-dependent gating of HCN1 channel epilepsy mutants. Zenodo https://doi.org/10.5281/zenodo.11528212 (2024).
Acknowledgements
We thank M. Falzone and P. Schmidpeter for assistance with cryo-EM freezing and processing; M. Ferrer for help with two-electrode voltage clamp recordings; J. Wojcik for mutagenesis of human HCN1; L. Khajoueinejad for Xenopus oocyte preparation; M. Su for baculovirus guidance; and the members of C. Nimigean’s, P. Larsson’s and P. Riegelhaupt’s labs for their scientific input. Screening and data collection were performed by H. Kuang, B. Wang and W. Rice at New York University Langone Health’s Cryo-Electron Microscopy Laboratory (RRID:SCR_019202); by J. Mendez, C. Hernandez, K. Maruthi and E. Eng at the Simons Electron Microscopy Center at the New York Structural Biology Center; and by D. Acehan and E. C. Fluck at the Weill Cornell Medicine Cryo-EM Core Facility. Negative stain screening and confocal imaging were conducted at the Weill Cornell Medicine CLC Imaging Core Facility. The computational resources were provided by the Scientific Computing Unit at Weill Cornell Medicine. We thank R. Mackinnon for the pEG BacMam-HCN1EM and PET32a-eGFP nanobody constructs. The work presented here was sponsored in part by grant no. NIH GM124451 and grant no. NIH NS137561 to C.M.N., grant no. NIH GM139164 to H.P.L., grant no. NIH GM128420 to A.A., grant no. NIH R42NS129370 to P.A.G., grant no. NIH F32GM145091 to E.D.K. and a Hartwell Foundation Postdoctoral Fellowship to E.D.K. The New York University Langone Health Cryo-Electron Microscopy laboratory is partially supported by the Laura and Isaac Perlmutter Cancer Center Support Grant no. NIH NCI P30CA016087 and work at the Simons Electron Microscopy Center at the New York Structural Biology Center is supported by the Simons Foundation (grant no. SF349247). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
E.D.K. performed mutagenesis and TEVC for human HCN1, prepared samples for and collected the cryo-EM data, and analysed the TEVC and cryo-EM data. X.W. performed mutagenesis and VCF for spHCN channels. K.P.C. performed VCF for spHCN channels. H.P.L. performed the kinetic modelling. M.E.P. performed mutagenesis for spHCN channels. S.L. and A.A. designed, performed and analysed the molecular dynamics simulations. E.D.Z. performed transfection, confocal microscopy and colocalization analysis and mutagenesis of human HCN1. G.R.T. and P.A.G. contributed to the conception of this work, established TEVC conditions and synthesized and provided the pGHE human full-length HCN1 plasmid for TEVC. H.P.L. and C.M.N. supervised, designed and acquired funding for the research. E.D.K., X.W., H.P.L. and C.M.N. interpreted the data, prepared figures and wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
G.R.T. and P.A.G. are co-inventors on patents related to the development of novel alkylphenols for the treatment of neuropathic pain. G.R.T. and P.A.G. serve on the Scientific Advisory Board for Akelos Inc., a research-based biotechnology company that has a licensing agreement for the use of those patents.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
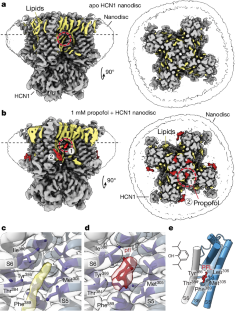
Extended Data Fig. 1 Cryo-EM data processing of HCN1 WT nanodisc in the absence and presence of 1 mM propofol.
a, SEC chromatograms and SDS-PAGE of HCN1 WT nanodisc purifications in the presence and absence of propofol. For gel source data, see Supplementary Fig. 1. The cryo-EM processing schematics are shown for b, apo HCN1 WT nanodisc and c, HCN1 WT nanodisc + pfl. Micrograph scale bar represents 50 nm. d, Backbone r.m.s.d. deviations between apo, propofol (pfl), and detergent (PDB 5U6O9) structures.
Extended Data Fig. 2 Local cryo-EM densities of propofol binding sites 1 and 2.
Shown are side and top views of the site 1 and site 2 densities from the 3D Refinement map, PostProcess map, DeepEMhancer map, and half map 1 for a, apo WT, b, WT + 1 mM propofol, c, holo M305L, and d, M305L + 1 mM propofol datasets. The HCN1 protein is in gray, tubular lipid densities in yellow, and the identified propofol densities in red. DeepEMhancer representations are used in the manuscript.
Extended Data Fig. 3 Site 2 is state-independent and does not confer propofol inhibition of HCN1 channels.
a, Overview of the propofol-HCN1 cryoEM map, from Fig. 1b, highlighting binding site 1 and site 2. b, Zoomed in view of the residues surrounding the density at site 2. c, Overlay of HCN1 + 1 mM propofol (this study, red-brown), HCN1 crosslinked (PDB 6UQF10, cyan), HCN4 open (PDB 7NMN35, light blue), and HCN1 closed (PDB 5U6O9, white). Shown are the response of HCN1 d, L218A and e, K219A in the absence (left) and presence (right) of propofol by two electrode voltage clamp. Voltage clamp ranged from +45 mV to −135 mV with tail currents measured at +50 mV. The current response at −85 mV is highlighted in red. Corresponding Boltzmann fits are shown in f, L218A (apo: V1/2 = −55.3 ± 4.0 mV, k = 6.3 ± 0.8 mV, n = 3; pfl: V1/2 = −86.2 ± 3.4 mV, k = 10.1 ± 1.2 mV, n = 4, P < 0.0001) and g, K219A (apo: V1/2 = −50.7 ± 2.8 mV, k = 6.8 ± 0.4 mV, n = 4; pfl: V1/2 = −83.0 ± 2.5 mV, k = 10.2 ± 1.6, n = 4, P < 0.0001). L218A ΔV1/2 = −31.0 ± 5.2 mV and K219A ΔV1/2 = −32.3 ± 3.7 mV, compared to that of WT ΔV1/2 = −30.9 ± 6.4 mV. P-values were determined by two-way ANOVA using a Tukey post hoc test between apo and propofol V1/2, with significance defined as P < 0.05, and n denotes biological replicates. Empty and filled symbols with error bars represent mean ± s.d. for normalized apo and propofol data, respectively.
Extended Data Fig. 4 Propofol makes hydrophobic contacts with and exhibits longer residence in site 1.
a, HCN1 was solvated in lipids (yellow-red-blue sticks) and propofol bound at site 1 and site 2 are shown in red spheres. K+ and Cl− ions are shown in green and gray spheres, respectively. Waters are not shown for simplicity. R.m.s.d. of propofol from their originating position in a MD simulation of the HCN1 WT tetramer in a b, DOPC:POPE:POPS and c, pure POPC lipid bilayer. All 12 propofols unbound from site 2 in both lipid compositions, while 11 of 12 propofols remained bound to site 1 in the DOPC:POPE:POPS bilayer. In the POPC bilayer, 10 of 12 propofols remained at site 1. Propofol (red) at site 1 adopts multiple binding poses in both the d, DOPC:POPE:POPS and e, POPC lipid bilayers. Propofols at the center of each of the three highest populated clusters which contribute to 97% of the total frames are shown in stick model and colored by orange, red-brown, and yellow respectively. Distinct HCN1 subunits are shown in light gray and slate. Amino acid residues lining the binding pocket are in purple. f, Docking65 of propofol to HCN1 identified 5 transmembrane locations, labeled 1 through 5. Site 1 identified by cryoEM is equivalent to docking position 1. However, site 2 from the cryo-EM experiment was not identified. g, Docking of propofol to HCN1 M305L found 4 transmembrane locations. The site 1 was identified, but not site 2. Positions 2 and 4 were also the same as those found in the WT docking experiment in f. For clarity, only the transmembrane domain of the channel is shown with individual subunits colored in slate, light grey, green, and red-brown. Docked propofol molecules are in red.
Extended Data Fig. 5 Perfusion of propofol to HCN1 M305L recovers voltage dependent gating.
a, Schematic of perfusion experiment design. Two electrode voltage clamp recordings were performed pre- and post-perfusion with 30 µM propofol for 10 min. To verify inward HCN1 currents, recording solution supplemented with 1 mM caesium chloride was perfused on and off the cell for 5 min. Shown are representative traces of n = 3 and 4 similar recordings with b, WT and c, M305L, respectively. d, For WT and M305L, the inward current is blocked by caesium while the outward depolarized tails remain intact. Corresponding Boltzmann fits are also shown for WT and M305L. Controls demonstrating inward current caesium block in the absence of 30 µM propofol are shown for e, WT and f, M305L and are representative traces of n = 3 similar recordings. Voltage clamp ranged from +45 mV to −125 mV with tail currents measured at +50 mV. The current response at −85 mV is highlighted in red. Empty and filled symbols with error bars represent mean ± s.d. for normalized apo and propofol data, respectively. n represents the number of biological replicates. HEK293S GnTI- cells transfected with HCN1 g, WT and h, M305L using Lipofectamine 2000 (Invitrogen). Nuclei are in blue, the plasma membrane in red, and HCN1 in green. Expression at the plasma membrane is demonstrated by colocalization (yellow). Shown is a representative cell of WT n = 20 and M305L n = 10 similar cells, over 3 independent transfections. Plotted to the right are intensity values across the dashed orange line. The scale bar represents 10 µm. For microscopy source data, see Supplementary Fig. 2.
Extended Data Fig. 6 Cryo-EM data processing of HCN1 M305L nanodisc in the absence and presence of 1 mM propofol.
a, SEC chromatograms and SDS-PAGE of HCN1 M305L nanodisc purifications in the presence and absence of propofol. The nanodiscs for HCN1 M305L holo without propofol were made using MSP1E3 while the ones for HCN1 M305L with propofol were made using MSP2N2. For gel source data, see Supplementary Fig. 1. The cryoEM processing schematics are shown for b, apo HCN1 M305L nanodisc and c, HCN1 M305L nanodisc + pfl. Micrograph scalebar represents 50 nm. d, Pore diagram comparison between holo M305L nanodisc, M305L + pfl, and holo WT detergent (PDB 5U6P9) structures using HOLE63. Red indicates regions that are smaller than a single water molecule to pass, green for a single water molecule, and blue is double the radius of a single water molecule. Both holo structures contain cAMP. e, Backbone r.m.s.d. deviations of the voltage sensing domain (S1-S4) between holo M305L, holo WT (PDB 5U6P9), and M305L HCN1 propofol structures.
Extended Data Fig. 7 Voltage-independent spHCN-M375L channels are blocked by the specific HCN channel blocker ZD7288 and the Met375-Phe459 interaction is important to close spHCN channels at positive voltages.
a, Representative current traces from spHCN M375L channels before (left) and after (right) the application of 100 µM ZD7288. Dashed lines indicate no currents. Met375 and Phe459 mutants show currents at positive voltages and similar voltage sensor movement. b, GV and c, FV relations from WT (black), M375L (blue), M375F (purple), M375A (green), M375C (orange) and M375S (pink) mutant spHCN channels. d, GV and e, FV relations from WT (black), F459Y (pink), F459C (orange), F459M (purple), F459E (cyan), F459A (green), F459L (blue), F459Q (gray), F459V (magenta) and F459W (dark yellow) mutant spHCN channels. f, Representative current traces from oocytes expressing WT, M375F, F459M and M375F/F459M spHCN channels. Dashed lines indicate no currents. g, GV relations from WT (black), M375F (green), F459M (orange) and M375F/F459M (red) spHCN channels. All GV1/2, FV1/2 and n numbers are shown in Extended Data Table 2. Data are represented as mean ± s.e.m. n indicates the number of biological replicates.
Extended Data Fig. 8 Met-aromatic interactions occur in voltage-gated HCN1 channels.
a, Local structure of HCN1 + propofol, HCN1 closed (PDB 5U6O), and HCN1 crosslinked (PDB 6UQF) around the Met305-Phe389 interaction. The homologous positions Ile307-Ile392 for the CNGA1 structure (PDB 7LFT) are also shown. Approximate distances between atoms (dashed yellow lines) are labeled between methionine, isoleucine, and the adjacent aromatic rings (purple). Propofol is colored in pink and adjacent protomers are in blue and yellow. b, Multiple sequence alignment between human HCN and CNG isoforms. Residue numbering follows the HCN1 amino acid sequence. The methionine, isoleucine, and aromatic positions labeled in panel a are highlighted in red, orange, and blue. A single aliphatic-aromatic interaction (1-bridge) exists in CNG channels which are ligand gated. In contrast, an interaction between methionine with two aromatic residues (2-bridge) occurs in HCN channels which are voltage gated.
Supplementary information
Supplementary Information
Supplementary Fig. 1. Uncropped HCN1 WT and M305L purifications. Supplementary Fig. 2. Uncropped HCN1 WT and M305L confocal images. Supplementary Appendix.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, E.D., Wu, X., Lee, S. et al. Propofol rescues voltage-dependent gating of HCN1 channel epilepsy mutants. Nature 632, 451–459 (2024). https://doi.org/10.1038/s41586-024-07743-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07743-z
This article is cited by
-
Extracellular activation of HCN4 by a subtype-specific nanobody
Nature Communications (2025)
-
Membrane-spanning aromatic foldamers with tunable pore diameters synthesized via Suzuki coupling for mass transport
Science China Chemistry (2025)