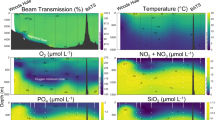
Abstract
Primary production in the sunlit surface ocean is regulated by the supply of key nutrients, primarily nitrate, phosphate and iron (Fe), required by phytoplankton to fix carbon dioxide into biomass1,2,3. Below the surface ocean, remineralization of sinking organic matter rapidly regenerates nutrients, and microbial metabolism in the upper mesopelagic ‘twilight zone’ (200–500 m) is thought to be limited by the delivery of labile organic carbon4,5. However, few studies have examined the role of nutrients in shaping microbial production in the mesopelagic6,7,8. Here we report the distribution and uptake of siderophores, biomarkers for microbial Fe deficiency9 across a meridional section of the eastern Pacific Ocean. Siderophore concentrations are high not only in chronically Fe-limited surface waters but also in the twilight zone underlying the North and South Pacific subtropical gyres, two key ecosystems for the marine carbon cycle. Our findings suggest that bacterial Fe deficiency owing to low Fe availability is probably characteristic of the twilight zone in several large ocean basins, greatly expanding the region of the marine water column in which nutrients limit microbial metabolism, with potential implications for ocean carbon storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Siderophore concentration data for the GP15 transect are publicly available through the BCODMO data archive (https://www.bco-dmo.org/dataset/875210 and https://www.bco-dmo.org/dataset/929884). Dissolved iron data for the GP15 transect have been deposited in BCODMO (https://www.bco-dmo.org/dataset/883862 and https://www.bco-dmo.org/dataset/884673). Nitrate data have been deposited in BCODMO (https://www.bco-dmo.org/dataset/777951 and https://www.bco-dmo.org/dataset/824867). The nitrate data in Fig. 3 are from the Hawaii Ocean Time-series (HOT) data archive at the University of Hawai’i at Manoa (https://hahana.soest.hawaii.edu/hot/methods/llnuts.html). Siderophore concentration data for the uptake experiment are provided at https://zenodo.org/records/12206828. The LC-ESIMS data for Extended Data Figs. 2 and 3 and Extended Data Table 1 are available at MassIVE (https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=7439be618f5949ecb1c2eac35978dba4).
References
Arrigo, K. R. Marine microorganisms and global nutrient cycles. Nature 437, 349–355 (2005).
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013).
Browning, T. J. & Moore, C. M. Global analysis of ocean phytoplankton nutrient limitation reveals high prevalence of co-limitation. Nat. Commun. 14, 5014 (2023).
Buesseler, K. O. et al. Metrics that matter for assessing the ocean biological carbon pump. Proc. Natl Acad. Sci. USA 117, 9679–9687 (2020).
Buesseler, K. O. et al. Revisiting carbon flux through the ocean’s twilight zone. Science 316, 567–570 (2007).
Baltar, F. et al. Specific effects of trace metals on marine heterotrophic microbial activity and diversity: key role of iron and zinc and hydrocarbon-degrading bacteria. Front. Microbiol. 9, 03190 (2018).
Bundy, R. M. et al. Distinct siderophores contribute to iron cycling in the mesopelagic at station ALOHA. Front. Mar. Sci. 5, 61 (2018).
Mazzotta, M. G., McIlvin, M. R. & Saito, M. A. Characterization of the Fe metalloproteome of a ubiquitous marine heterotroph, Pseudoalteromonas (BB2-AT2): multiple bacterioferritin copies enable significant Fe storage. Metallomics 12, 654–667 (2020).
Bagg, A. & Neilands, J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 51, 509–518 (1987).
Bressac, M. et al. Resupply of mesopelagic dissolved iron controlled by particulate iron composition. Nat. Geosci. 12, 995–1000 (2019).
Twining, B. S. et al. Differential remineralization of major and trace elements in sinking diatoms. Limnol. Oceanogr. 59, 689–704 (2014).
Bruland, K. W., Orians, K. J. & Cowen, J. P. Reactive trace metals in the stratified central North Pacific. Geochim. Cosmochim. Acta 58, 3171–3182 (1994).
Boyd, P. W., Ellwood, M. J., Tagliabue, A. & Twining, B. S. Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nat. Geosci. 10, 167–173 (2017).
Schlitzer, R. et al. The GEOTRACES Intermediate Data Product 2017. Chem. Geol. 493, 210–223 (2018).
Tortell, P. D., Maldonado, M. T. & Price, N. M. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature 383, 330–332 (1996).
Fourquez, M. et al. Effects of iron limitation on growth and carbon metabolism in oceanic and coastal heterotrophic bacteria. Limnol. Oceanogr. 59, 349–360 (2014).
van den Berg, C. M. Evidence for organic complexation of iron in seawater. Mar. Chem. 50, 139–157 (1995).
Rue, E. L. & Bruland, K. W. Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 50, 117–138 (1995).
Gledhill, M. & Buck, K. N. The organic complexation of iron in the marine environment: a review. Front. Microbiol. 3, 69 (2012).
Hassler, C. S., van den Berg, C. M. G. & Boyd, P. W. Toward a regional classification to provide a more inclusive examination of the ocean biogeochemistry of iron-binding ligands. Front. Mar. Sci. 4, 19 (2017).
Sexton, D. J. & Schuster, M. Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nat. Commun. 8, 230 (2017).
Sijerčić, A. & Price, N. M. Hydroxamate siderophore secretion by Pseudoalteromonas haloplanktis during steady-state and transient growth under iron limitation. Mar. Ecol. Prog. Ser. 531, 105–120 (2015).
Gauglitz, J. M. et al. Dynamic proteome response of a marine Vibrio to a gradient of iron and ferrioxamine bioavailability. Mar. Chem. 229, 103913 (2021).
Park, J. et al. Siderophore production and utilization by marine bacteria in the North Pacific Ocean. Limnol. Oceanogr. 68, 1636–1653 (2023).
Martin, J. H. et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature 371, 123–129 (1994).
Martinez, J. S. et al. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc. Natl Acad. Sci. USA 100, 3754–3759 (2003).
Martinez, J. S. et al. Self-assembling amphiphilic siderophores from marine bacteria. Science 287, 1245–1247 (2000).
Xu, G., Martinez, J. S., Groves, J. T. & Butler, A. Membrane affinity of the amphiphilic marinobactin siderophores. J. Am. Chem. Soc. 124, 13408–13415 (2002).
Kramer, J., Özkaya, Ö. & Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 18, 152–163 (2020).
Saha, R., Saha, N., Donofrio, R. S. & Bestervelt, L. L. Microbial siderophores: a mini review. J. Basic Microbiol. 53, 303–317 (2012).
Wilson, B. R., Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol. Med. 22, 1077–1090 (2016).
Schalk, I. J. & Guillon, L. Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 44, 1267–1277 (2013).
Greenwald, J. et al. Real time fluorescent resonance energy transfer visualization of ferric pyoverdine uptake in Pseudomonas aeruginosa: a role for ferrous iron. J. Biol. Chem. 282, 2987–2995 (2007).
Karl, D. M. & Church, M. J. Microbial oceanography and the Hawaii Ocean Time-series programme. Nat. Rev. Microbiol. 12, 699–713 (2014).
Hu, X. & Boyer, G. L. Siderophore-mediated aluminum uptake by Bacillus megaterium ATCC 19213. Appl. Environ. Microbiol. 62, 4044–4048 (1996).
Giering, S. L. C. et al. Reconciliation of the carbon budget in the ocean’s twilight zone. Nature 507, 480–483 (2014).
Steinberg, D. K. et al. Bacterial vs. zooplankton control of sinking particle flux in the ocean’s twilight zone. Limnol. Oceanogr. 53, 1327–1338 (2008).
Pakulski, J. D. et al. Iron stimulation of Antarctic bacteria. Nature 383, 133–134 (1996).
Granger, J. & Price, N. M. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol. Oceanogr. 44, 541–555 (1999).
Church, M. J., Hutchins, D. A. & Ducklow, H. W. Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl. Environ. Microbiol. 66, 455–466 (2000).
Mendonca, C. M. et al. Hierarchical routing in carbon metabolism favors iron-scavenging strategy in iron-deficient soil Pseudomonas species. Proc. Natl Acad. Sci. USA 117, 32358–32369 (2020).
Kirchman, D. L., Hoffman, K. A., Weaver, R. & Hutchins, D. A. Regulation of growth and energetics of a marine bacterium by nitrogen source and iron availability. Mar. Ecol. Prog. Ser. 250, 291–296 (2003).
Beier, S. et al. The transcriptional regulation of the glyoxylate cycle in SAR11 in response to iron fertilization in the Southern Ocean. Environ. Microbiol. Rep. 7, 427–434 (2015).
Kwon, E. Y., Primeau, F. & Sarmeento, J. L. The impact of remineralization depth on the air–sea carbon balance. Nat. Geosci. 2, 630–635 (2009).
Fitzsimmons, J. N. et al. Daily to decadal variability of size-fractionated iron and iron-binding ligands at the Hawaii Ocean Time-series Station ALOHA. Geochim. Cosmochim. Acta 171, 303–324 (2015).
Conway, T. M., Rosenberg, A. D., Adkins, J. F. & John, S. G. A new method for precise determination of iron, zinc and cadmium stable isotope ratios in seawater by double-spike mass spectrometry. Anal. Chim. Acta 793, 44–52 (2013).
Sieber, M. et al. Isotopic fingerprinting of biogeochemical processes and iron sources in the iron-limited surface Southern Ocean. Earth Planet. Sci. Lett. 567, 116967 (2021).
Middag, R. et al. Intercomparison of dissolved trace elements at the Bermuda Atlantic Time Series station. Mar. Chem. 177, 476–489 (2015).
Ellwood, M. J. et al. Distinct iron cycling in a Southern Ocean eddy. Nat. Commun. 11, 825 (2020).
Li, J. et al. Element-selective targeting of nutrient metabolites in environmental samples by inductively coupled plasma mass spectrometry and electrospray ionization mass spectrometry. Front. Mar. Sci. 8, 630494 (2021).
Boiteau, R. M., Fitzsimmons, J. N., Repeta, D. J. & Boyle, E. A. Detection of iron ligands in seawater and marine cyanobacteria cultures by high-performance liquid chromatography–inductively coupled plasma-mass spectrometry. Anal. Chem. 85, 4357–4362 (2013).
Boiteau, R. M. & Repeta, D. J. An extended siderophore suite from Synechococcus sp. PCC 7002 revealed by LC-ICPMS-ESIMS. Metallomics 7, 877–884 (2015).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Baars, O., Morel, F. M. & Perlman, D. H. ChelomEx: isotope-assisted discovery of metal chelates in complex media using high-resolution LC-MS. Anal. Chem. 86, 11298–11305 (2014).
Boiteau, R. M. Molecular Determination of Marine Iron Ligands by Mass Spectrometry. Thesis, Massachusetts Institute of Technology/Woods Hole Oceanographic Institution (2016).
Vraspir, J. M., Holt, P. D. & Butler, A. Identification of new members within suites of amphiphilic marine siderophores. BioMetals 24, 85–92 (2011).
Boiteau, R. M. et al. Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean. Proc. Natl Acad. Sci. 113, 14237–14242 (2016).
Kem, M. P. & Butler, A. Acyl peptidic siderophores: structures, biosyntheses and post-assembly modifications. BioMetals 28, 445–459 (2015).
GEOTRACES Intermediate Data Product Group. The GEOTRACES Intermediate Data Product 2021 version 2 (IDP2021v2). NERC EDS British Oceanographic Data Centre NOC. https://doi.org/10.5285/ff46f034-f47c-05f9-e053-6c86abc0dc7e (2023).
Xiang, Y. & Lam, P. J. Size-fractionated compositions of marine suspended particles in the Western Arctic Ocean: lateral and vertical sources. J. Geophys. Res. Oceans 125, e2020JC016144 (2020).
Acknowledgements
We thank P. Lam, K. Casciotti, G. Cutter, B. Summers and L. Jensen for their organization, assistance and support of the GP15 expedition. P. Morton provided assistance for the inductively coupled plasma mass spectrometry analyses at Texas A&M University. We also thank T. Burrell, R. Tabata, B. Brenes, D. Karl, A. White, P. Kong and M. Seixas for their support and assistance of the PARAGON 2022 cruise on the R/V Kilo Moana. Funding for this work was provided by National Science Foundation Chemical Oceanography Program (NSF awards OCE-1736280 and OCE-2045223 to D.J.R.; OCE-1737167 to J.N.F.; OCE-1737136 to T.M.C.; and OCE-1736896 to S.G.J.). Support was also provided for the Simons Collaboration on Ocean Processes and Ecology programme (SCOPE awards 721227 to D.J.R. and 721221 to M.J.C.), Simons Foundation Early Career Investigator Awards in Marine Microbial Ecology and Evolution (Life Sciences awards 621513 to R.M.Bo and 618401 to R.M.Bu.) and a Simons Foundation Postdoctoral Fellowship in Marine Ecology (award 729162 to L.E.M.).
Author information
Authors and Affiliations
Contributions
J.L., L.B.-A. and D.J.R. designed the project. J.L. and L.B.-A. collected and processed the GP15 samples. J.L., L.B.-A. and M.R.M. measured and characterized siderophores in the GP15 and PARAGON 2022 samples. D.J.R., J.N.F., R.M.Bo. and R.M.Bu. developed the liquid chromatography–mass spectrometry methods and data interrogation algorithms and purified marinobactins for the PARAGON 2022 experiments. J.L., L.E.M., I.-M.S., B.N.G. and M.J.C. conducted the siderophore uptake experiments during the PARAGON 2022 cruise. M.S., N.T.L., X.B., T.M.C., J.N.F. and S.G.J. measured dissolved iron in the GP15 samples. J.L. and D.J.R. performed the data analysis and wrote the first draft of the paper. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Martha Gledhill and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Molecular structures of representative marinobactins and amphibactins identified in GP15 samples.
Amphibactins and marinobactins are distinguished by the number and type of amino acids in the Fe-complexing peptide head group. Each family of siderophores has several homologues that differ in the number of CH2 groups and unsaturations in the fatty acid side chain.
Extended Data Fig. 2 Characterization of siderophores in GP15 samples.
a, The 56Fe chromatogram by LC-ICP-MS for sample at station 16, 400 m. b–e, Extracted ion chromatograms by LC-ESIMS from the same sample. Blue traces correspond to the 56Fe–siderophore [M + H]+ isotopologue and red traces correspond to the less abundant 54Fe–siderophore [M + H]+ isotopologue. The intensity of the 54Fe isotopologue has been scaled by the 56Fe/54Fe crustal abundance ratio of 15.7. Peaks were assigned as 56Fe–marinobactin A (m/z 985.401) (b), 56Fe–marinobactin B (m/z 1,011.416) (c), 56Fe–marinobactin C (m/z 1,013.432) (d) and 56Fe–marinobactin D (m/z 1,039.452) (e).
Extended Data Fig. 3 Validation of the 57Fe–siderophore amendment.
57Fe (dark blue trace) and 56Fe (light blue trace) chromatograms of amphibactin (a) and marinobactin (b) mixtures used for the siderophore uptake experiment. c, Extracted ion chromatograms for 57Fe–amphibactins in the amphibactin stock solution. Peaks were assigned as: A, 57Fe–amphibactin C10:0 (m/z 830.353); B, 57Fe–amphibactin C12:1 (m/z 856.368); C, 57Fe–amphibactin T (m/z 858.384); and D, 57Fe–amphibactin S (m/z 884.400). The peak with an m/z of 900.394 also represents an 57Fe–amphibactin but does not contribute to the main peaks in Fig. 4. d, Extracted ion chromatograms for 57Fe–marinobactins in the marinobactin stock solution. Peaks were assigned as: A, 57Fe–marinobactin A (m/z 986.406); B, 57Fe–marinobactin B (m/z 1,012.420); C, 57Fe–marinobactin C (m/z 1,014.437); and D, 57Fe–marinobactin D (m/z 1,040.452).
Extended Data Fig. 4 Sectional plots of nitrate:DFe ratio between the surface and at 500 m in different ocean basins.
a, GEOTRACES GP19 section in the South Pacific Ocean along 170° W. b, GEOTRACES GP16 section in the equatorial Pacific Ocean along 10–15° S. c, GEOTRACES GN01 section in the Arctic Ocean along 140° W–180° E. d, GEOTRACES GA03 section in the North Atlantic Ocean along 15–40° N. e, GEOTRACES GI04 section in the Indian Ocean along 40–85° E. f, GEOTRACES GA10 section in the South Atlantic Ocean along 35–40° S. The data were extracted from GEOTRACES Intermediate Data Product 2017 (ref. 14) and 2021 (ref. 59).
Extended Data Fig. 5 Sectional plots of DFe concentration between the surface and at 500 m in different ocean basins.
a, GEOTRACES GP19 section in the South Pacific Ocean along 170° W. b, GEOTRACES GP16 section in the equatorial Pacific Ocean along 10–15° S. c, GEOTRACES GN01 section in the Arctic Ocean along 140° W–180° E. d, GEOTRACES GA03 section in the North Atlantic Ocean along 15–40° N. e, GEOTRACES GI04 section in the Indian Ocean along 40–85° E. f, GEOTRACES GA10 section in the South Atlantic Ocean along 35–40° S. The data were extracted from GEOTRACES Intermediate Data Product 2017 (ref. 14) and 2021 (ref. 59).
Extended Data Fig. 6 Sectional plots of nitrate concentration between the surface and at 500 m in different ocean basins.
a, GEOTRACES GP19 section in the South Pacific Ocean along 170° W. b, GEOTRACES GP16 section in the equatorial Pacific Ocean along 10–15° S. c, GEOTRACES GN01 section in the Arctic Ocean along 140° W–180° E. d, GEOTRACES GA03 section in the North Atlantic Ocean along 15–40° N. e, GEOTRACES GI04 section in the Indian Ocean along 40–85° E. f, GEOTRACES GA10 section in the South Atlantic Ocean along 35–40° S. The data were extracted from GEOTRACES Intermediate Data Product 2017 (ref. 14) and 2021 (ref. 59).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Babcock-Adams, L., Boiteau, R.M. et al. Microbial iron limitation in the ocean’s twilight zone. Nature 633, 823–827 (2024). https://doi.org/10.1038/s41586-024-07905-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07905-z
This article is cited by
-
Siderophores produced by marine microorganisms
Antonie van Leeuwenhoek (2025)