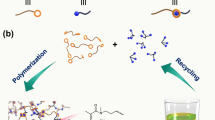
Abstract
Limited strategies exist for chemical recycling of commodity diene polymers, like those found in tyres1,2,3. Here we apply C–H amination and backbone rearrangement of polymers to deconstruct these materials into precursors for epoxy resins. Specifically, we develop a sulfur diimide reagent4,5 that enables up to about 35% allylic amination of diene polymers and rubber. Then, we apply the cationic 2-aza-Cope rearrangement to deconstruct aminated diene polymers. In a model system, we see molecular weight reduction from 58,100 to approximately 400 g mol−1, and aminated post-consumer rubber is deconstructed over 6 hours into soluble amine-functionalized polymers, which can be utilized to prepare epoxy thermosets with similar stiffnesses to commercial bisphenol A-derived resins6. Altogether, this work demonstrates the power of C–H amination and backbone rearrangement to enable chemical recycling of post-consumer materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Provisional patent numbers of applications associated with this work are as follows: 63/454,452; Filing Date: 03-27-2023 (M.R., A.V.Z.) and 63/710,916; Filing Date: 10-24-2024 (S.E.T., A.V.Z.).
References
Kim, J. K. et al. (eds) Rubber Recycling: Challenges and Developments (Royal Society of Chemistry, 2018).
Andler, R., Valdés, C., Díaz-Barrera, A. & Steinbüchel, A. Biotransformation of poly(cis-1,4-isoprene) in a multiphase enzymatic reactor for continuous extraction of oligo-isoprenoid molecules. New Biotechnol. 58, 10–16 (2020).
Wolf, S. & Plenio, H. On the ethenolysis of end-of-life tire granulates. Green Chem. 15, 315–319 (2013).
Sharpless, K. B. & Hori, T. Allylic amination of olefins and acetylenes by imido sulfur compounds. J. Org. Chem. 41, 176–177 (1976).
Schönberger, N. & Kresze, G. Zur chemie der schwefeldiimide, VI. Enreaktionen und [2+2]-cycloadditionen von N,N′-ditosylschwefeldiimid und N-sulfinyl-p-toluolsulfonamid. Justus Liebigs Ann. Chem. 1975, 1725–1731 (1975).
Ellis, B. Chemistry and Technology of Epoxy Resins (Springer, 2015).
2021 US Scrap Tire Management Summary (US Tire Manufacturer's Association, 2022); www.ustires.org/system/files/files/2024-02/21%20US%20Scrap%20Tire%20Management%20Report%20101722.pdf.
Xu, J. et al. Rubber antioxidants and their transformation products: environmental occurrence and potential impact. Int. J. Environ. Res. Public Health 19, 14595 (2022).
Gomes, F. O., Rocha, M. R., Alves, A. & Ratola, N. A review of potentially harmful chemicals in crumb rubber used in synthetic football pitches. J. Hazard. Mater. 409, 124998 (2021).
Singh, A. et al. Uncontrolled combustion of shredded tires in a landfill–part 2: population exposure, public health response, and an air quality index for urban fires. Atmos. Environ. 104, 273–283 (2015).
Ditzler, R. A. J. & Zhukhovitskiy, A. V. Sigmatropic rearrangements of polymer backbones: vinyl polymers from polyesters in one step. J. Am. Chem. Soc. 143, 20326–20331 (2021).
Ratushnyy, M. & Zhukhovitskiy, A. V. Polymer skeletal editing via anionic Brook rearrangements. J. Am. Chem. Soc. 143, 17931–17936 (2021).
Ditzler, R. A. J., King, A. J., Towell, S. E., Ratushnyy, M. & Zhukhovitskiy, A. V. Editing of polymer backbones. Nat. Rev. Chem. 7, 600–615 (2023).
Overman, L. E., Humphreys, P. G. & Welmaker, G. S. in Organic Reactions (ed. Denmark, S. E.) 747–820 (Wiley, 2011).
Abu-Rayyan, A. et al. Recent progress in the development of organic chemosensors for formaldehyde detection. ACS Omega 8, 14859–14872 (2023).
Brewer, T. F. & Chang, C. J. An aza-Cope reactivity-based fluorescent probe for imaging formaldehyde in living cells. J. Am. Chem. Soc. 137, 10886–10889 (2015).
Jones, A. C., May, J. A., Sarpong, R. & Stoltz, B. M. Toward a symphony of reactivity: cascades involving catalysis and sigmatropic rearrangements. Angew. Chem. Int. Ed. Engl. 53, 2556–2591 (2014).
Johannsen, M. & Jørgensen, K. A. Allylic amination. Chem. Rev. 98, 1689–1708 (1998).
Hodges, M. N. et al. Upcycling of polybutadiene facilitated by selenium‐mediated allylic amination. Angew. Chem. Int. Ed. Engl. 62, e202303115 (2023).
Kresze, G. & Muensterer, H. Bis(methoxycarbonyl)sulfur diimide, a convenient reagent for the allylic amination of alkenes. J. Org. Chem. 48, 3561–3564 (1983).
Bao, H. & Tambar, U. K. Catalytic enantioselective allylic amination of unactivated terminal olefins via an ene reaction/[2,3]-rearrangement. J. Am. Chem. Soc. 134, 18495–18498 (2012).
Campbell, T. W., Monagle, J. J., Foldi, V. S. & Carbodiimides, I. Conversion of isocyanates to carbodiimides with phospholine oxide catalyst. J. Am. Chem. Soc. 84, 3673–3677 (1962).
Bruncko, M., Khuong, T.-A. V. & Sharpless, K. B. Allylic amination and 1,2-diamination with a modified diimidoselenium reagent. Angew. Chem. Int. Ed. Engl. 35, 454–456 (1996).
Natsugari, H., Whittle, R. R. & Weinreb, S. M. Stereocontrolled synthesis of unsaturated vicinal diamines from Diels–Alder adducts of sulfur dioxide bis(imides). J. Am. Chem. Soc. 106, 7867–7872 (1984).
Morgan, K. R., Hemmingson, J. A., Furneaux, R. H. & Stanley, R. A. A 13C solid-state NMR study of ion-exchange resins derived from natural polysaccharides. Carbohydr. Res. 262, 185–194 (1994).
Clarke, C. J., Tu, W.-C., Levers, O., Bröhl, A. & Hallett, J. P. Green and sustainable solvents in chemical processes. Chem. Rev. 118, 747–800 (2018).
Engels, H. et al. in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2011).
Subba Reddy, B. V., Nair, P. N., Antony, A., Lalli, C. & Grée, R. The aza-Prins reaction in the synthesis of natural products and analogues. Eur. J. Org. Chem. 2017, 1805–1819 (2017).
Martinez, H., Ren, N., Matta, M. E. & Hillmyer, M. A. Ring-opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 5, 3507–3532 (2014).
Kobayashi, S., Pitet, L. M. & Hillmyer, M. A. Regio- and stereoselective ring-opening metathesis polymerization of 3-substituted cyclooctenes. J. Am. Chem. Soc. 133, 5794–5797 (2011).
Gaborieau, M. & Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 399, 1413–1423 (2011).
Tanaka, Y. & Kakiuchi, H. Study of epoxy compounds. Part VI. Curing reactions of epoxy resin and acid anhydride with amine, acid, alcohol, and phenol as catalysts. J. Polym. Sci. A Gen. Pap. 2, 3405–3430 (1964).
Okabe, T. et al. Curing reaction of epoxy resin composed of mixed base resin and curing agent: experiments and molecular simulation. Polymer 54, 4660–4668 (2013).
Kim, S. L., Skibo, M. D., Manson, J. A., Hertzberg, R. W. & Janiszewski, J. Tensile, impact and fatigue behavior of an amine‐cured epoxy resin. Polym. Eng. Sci. 18, 1093–1100 (1978).
Odagiri, N. et al. Amine/epoxy stoichiometric ratio dependence of crosslinked structure and ductility in amine-cured epoxy thermosetting resins. J. Appl. Polym. Sci. 138, 50542 (2021).
Daghyani, H. R., Ye, L., Mai, Y.-W. & Wu, J. Fracture behaviour of a rubber-modified tough epoxy system. J. Mater. Sci. Lett. 13, 1330–1333 (1994).
Daly, J., Pethrick, R. A., Fuller, P., Cunliffe, A. V. & Datta, P. K. Rubber-modified epoxy resins: 1. Equilibrium physical properties. Polymer 22, 32–36 (1981).
Roschangar, F., Sheldon, R. A. & Senanayake, C. H. Overcoming barriers to green chemistry in the pharmaceutical industry–the Green Aspiration LevelTM concept. Green Chem. 17, 752–768 (2015).
Sheldon, R. A. The E factor 25 years on: the rise of green chemistry and sustainability. Green Chem. 19, 18–43 (2017).
Katz, T. J. & Shi, S. A simple allylic amination procedure and the metathesis of N-sulfinylcarbamates. J. Org. Chem. 59, 8297–8298 (1994).
Acknowledgements
This research was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Catalysis Science Program, under award DE-SC0022898. S.E.T. is also supported by the National Science Foundation Graduate Research Fellowship. We thank F. Leibfarth (UNC-CH) for discussion and for the use of his group’s ATR-FTIR and dogbone cutter. We thank the Sheiko group and J.-J. Wang (UNC-CH) for help with DMA data collection. We thank the Becker laboratory (Duke University) for the use of their cryomill. We thank M. ter Horst (UNC-CH) for NMR-related discussion, C. Chen (UNC-CH) for X-ray diffraction advice and B. Ehrmann (UNC-CH) for MS advice and assistance with the MALDI-TOF. We thank the UNC Chemistry NMR facility (NSF grant no. CHE-0922858), UNC Chemistry X-Ray Diffraction facility (NSF grant no. CHE-2117287) and the UNC Chemistry Mass Spectrometry facility (NIH grant no. R35GM118055, DOD grant no. FA9550-23-1-0077) for the use of their instrumentation. We thank K. Sharp-Knott (Virginia Tech) and the Virginia Tech Chemistry NMR Facility for collection of all solid-state NMR spectroscopy data. We thank the physics machine shop (UNC-CH) for creating our Teflon moulds for materials testing.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.V.Z. Methodology: S.E.T., M.R., L.S., G.M.L. Investigation: S.E.T., M.R., L.S., G.M.L. Visualization: S.E.T., M.R., L.S., A.V.Z. Funding acquisition: A.V.Z. Project administration: A.V.Z. Supervision: A.V.Z. Writing—original draft: S.E.T., M.R., A.V.Z. Writing—review and editing: S.E.T., M.R., L.S., G.M.L., A.V.Z.
Corresponding author
Ethics declarations
Competing interests
Two patent applications have been filed on sulfur diimide-mediated amination and aza-Cope deconstruction.
Peer review
Peer review information
Nature thanks Ciaran Lahive, Christophe Thomas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Results from ACR deconstruction studies.
ACR of 15 showing deconstruction products 16a–20a. (Left) 1H NMR spectra of deconstruction of 15 at zero h and 48 h; (Right) Associated GPC differential refractive index (dRI) traces of 1H NMR samples after Boc-protection using conditions c.
Supplementary information
Supplementary Information
This file contains Supplementary materials, methods, figures and data.
Supplementary Data
Check-CIF file for the X-ray crystal structure of S31.
Supplementary Video 1
PBD rubber deconstruction.
Supplementary Video 2
Post-consumer rubber deconstruction.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Towell, S.E., Ratushnyy, M., Cooke, L.S. et al. Deconstruction of rubber via C–H amination and aza-Cope rearrangement. Nature 640, 384–389 (2025). https://doi.org/10.1038/s41586-025-08716-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08716-6