Abstract
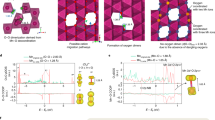
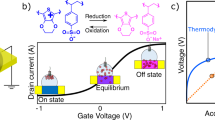
Structural disorder within materials gives rise to fascinating phenomena, attributed to the intricate interplay of their thermodynamic and electrochemical properties1,2. Oxygen-redox (OR) electrochemistry offers a breakthrough in capacity limits, while inducing structural disorder with reduced electrochemical reversibility3,4,5. The conventional explanation for the thermal expansion of solids relies on the Grüneisen relationship, linking the expansion coefficient to the anharmonicity of the crystal lattice6. However, this paradigm may not be applicable to OR materials due to the unexplored dynamic disorder–order transition in such systems7,8. Here we reveal the presence of negative thermal expansion with a large coefficient value of −14.4(2) × 10−6 °C−1 in OR active materials, attributing this to thermally driven disorder–order transitions. The modulation of OR behaviour not only enables precise control over the thermal expansion coefficient of materials, but also establishes a pragmatic framework for the design of functional materials with zero thermal expansion. Furthermore, we demonstrate that the reinstatement of structural disorder within the material can also be accomplished through the electrochemical driving force. By adjusting the cut-off voltages, evaluation of the discharge voltage change indicates a potential for nearly 100% structure recovery. This finding offers a pathway for restoring OR active materials to their pristine state through operando electrochemical processes, presenting a new mitigation strategy to address the persistent challenge of voltage decay.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Simonov, A. & Goodwin, A. L. Designing disorder into crystalline materials. Nat. Rev. Chem. 4, 657 (2020).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519 (2014).
Kim, B. et al. A theoretical framework for oxygen redox chemistry for sustainable batteries. Nat. Sustain. 5, 708–716 (2022).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305 (2022).
Sleight, A. Zero-expansion plan. Nature 425, 674–675 (2003).
Qiu, B. et al. Metastability and reversibility of anionic redox-based cathode for high-energy rechargeable batteries. Cell Rep. Phys. Sci. 1, 100028 (2020).
Singer, A. et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat. Energy 3, 641–647 (2018).
Bennett, T. D., Cheetham, A. K., Fuchs, A. H. & Coudert, F. X. Interplay between defects, disorder and flexibility in metal-organic frameworks. Nat. Chem. 9, 11–16 (2016).
Ma, E. Tuning order in disorder. Nat. Mater. 14, 547 (2015).
Keen, D. & Goodwin, A. L. The crystallography of correlated disorder. Nature 521, 303–309 (2015).
Onsager, L. Crystal statistics I. A two-dimensional model with an order-disorder transition. Phys. Rev. 65, 117 (1944).
Evans, J. S. O. et al. Compressibility, phase transitions, and oxygen migration in zirconium tungstate, ZrW2O8. Science 275, 61 (1996).
Mary, T. A., Evans, J. S., Vogt, T. & Sleight, A. W. Negative thermal expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 272, 90 (1996).
Lunkenheimer, P., Loidl, A., Riechers, B., Zaccone, A. & Samwer, K. Thermal expansion and the glass transition. Nat. Phys. 19, 694–699 (2023).
Zhang, L. et al. Giant polarization in super-tetragonal thin films through interphase strain. Science 361, 494 (2018).
Zheng, X. G. et al. Giant negative thermal expansion in magnetic nanocrystals. Nat. Nanotech. 3, 724–726 (2008).
Liao, J. et al. Thermally boosted upconversion and downshifting luminescence in Sc2(MoO4)3:Yb/Er with two-dimensional negative thermal expansion. Nat. Commun. 13, 2090 (2022).
Yamamoto, H., Imai, T., Sakai, Y. & Azuma, M. Colossal negative thermal expansion in electron-doped PbVO3 perovskites. Angew. Chem. Inter. Ed. 57, 8170–8173 (2018).
Azuma, M. et al. Colossal negative thermal expansion in BiNiO3 induced by intermetallic charge transfer. Nat. Commun. 2, 347 (2011).
Wang, W. et al. Local orthorhombic lattice distortions in the paramagnetic tetragonal phase of superconducting NaFe1-xNixAs. Nat. Commun. 9, 3128 (2018).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Ulvestad, A. et al. Topological defect dynamics in operando battery nanoparticles. Science 348, 1344 (2015).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827 (2013).
McCalla, E. et al. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Qiu, B. et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 7, 12108 (2016).
Frati, F., Hunault, M. O. J. Y. & de Groot, F. M. F. Oxygen K-edge X-ray absorption spectra. Chem. Rev. 120, 4056–4110 (2020).
Assat, G., Iadecola, A., Foix, D., Dedryvère, R. & Tarascon, J.-M. Direct quantification of anionic redox over long cycling of Li-rich NMC via hard X-ray photoemission spectroscopy. ACS Energy Lett. 3, 2721–2728 (2018).
Dai, K. et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 1–24 (2019).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Zhou, Y. et al. Sufficient oxygen redox activation against voltage decay in Li-rich layered oxide cathode materials. ACS Mater. Lett. 3, 433–441 (2021).
Gent, W. E. et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 8, 2091 (2017).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Hong, J. et al. Metal-oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat. Mater. 18, 256 (2019).
Yin, W. et al. Structural evolution at the oxidative and reductive limits in the first electrochemical cycle of Li1.2Ni0.13Mn0.54Co0.13O2. Nat. Commun. 11, 1252 (2020).
Csernica, P. M. et al. Persistent and partially mobile oxygen vacancies in Li-rich layered oxides. Nat. Energy 6, 642–652 (2021).
Cupid, D. M. et al. Interlaboratory study of the heat capacity of LiNi1/3Mn1/3Co1/3O2 (NMC111) with layered structure. Int. J. Mater. Res. 108, 1008–1021 (2017).
Yin, C. et al. Boosting energy efficiency of Li-rich layered oxide cathodes by tuning oxygen redox kinetics and reversibility. Energy Storage Mater. 35, 388 (2021).
Li, X. et al. Rational design of thermally stable polymorphic layered cathode materials for next generation lithium rechargeable batteries. Mater. Today 61, 91 (2022).
Yin, C. et al. Structural insights into composition design of Li-rich layered cathode materials for high-energy rechargeable battery. Mater. Today 51, 15 (2021).
Zhang, M. et al. High pressure effect on structural and electrochemical properties of anionic redox-based lithium transition metal oxides. Matter 4, 164–181 (2021).
Zhou, X., Wang, F., Zhu, Y. & Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 21, 3353–3358 (2011).
Yang, T.-Y., Wen, W. & Yin, G.-Z. Introduction of the X-ray diffraction beamline of SSRF. Nucl. Sci. Tech. 26, 020101 (2015).
Chen, J. et al. Tunable thermal expansion in framework materials through redox intercalation. Nat. Commun. 8, 14441 (2017).
Casas-Cabanas, M., Reynaud, M., Rikarte, J., Horbach, P. & Rodríguez-Carvajal, J. FAULTS: a program for refinement of structures with extended defects. J. Appl. Crystallogr. 49, 2259–2269 (2016).
Shunmugasundaram, R., Arumugam, R. S. & Dahn, J. A study of stacking faults and superlattice ordering in some Li-rich layered transition metal oxide positive electrode materials. J. Electrochem. Soc. 163, A1394–A1400 (2016).
Toby, B. H. & Dreele, R. B. V. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52272253, 52472266), the External Cooperation Program of the Chinese Academy of Sciences (181GJHZ2024126MI), the ‘Lingyanʼ Research and Development Plan of Zhejiang Province (2022C01071), a Low-Cost Cathode Material (TC220H06P), the R&D Project of Jiangsu Province (BKBG2024021), The “Innovation Yongjiang 2035” Key R&D Program (2025Z063), the Zhongke Hangzhou Bay Institute (Ningbo) New Materials Co. Ltd. (NIMTE-61-2024-2), the Natural Science Foundation of Ningbo (2024QL041) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2022299). M.Z. and Y.S.M. acknowledge support from the University of Chicago through startup funding. W.W. acknowledges support from the Photon Science Research Center for Carbon Dioxide. We thank beamline BL14B1 of the Shanghai Synchrotron Radiation Facility for providing the beamtime. The authors appreciate the neutron beamtime at the High-resolution Neutron Diffractometer (TREND) (https://cstr.cn/31113.02.CSNS.TREND) and General Purpose Powder Diffractometer (GPPD) (https://csns.cn/31113.02.CSNS.GPPD) at the China Spallation Neutron Source (CSNS) (https://cstr.cn/31113.02.CSNS), and thank W. Ji, S. Deng, F. Shen, Z. Tan, W. Xie, Q. Ma and D. Zhang for their technical assistance during the neutron scattering experiments.
Author information
Authors and Affiliations
Contributions
B.Q. conceived the project. B.Q., M.Z., Y.S.M. and Z.L. supervised the project. M.Z. and B.Q. performed the mechanism analysis. Y.Z., K.G., Z.Z. and B.Q. performed the synthesis work. Y.Z. performed the Rietveld refinement for the X-ray and neutron diffraction. Y.Z., K.G. and W.W. performed the X-ray characterization. T.Z., P.M., L.H. and Y.X. performed the neutron characterization. H.L. and B.Q. conducted the voltage recovery. B.Q. and M.Z. prepared the initial draft of the manuscript. S.B. helped revise the manuscript. B.Q., M.Z., Y.S.M. and Z.L. organized the work and helped with the drafting of the manuscript. All the authors discussed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Takumi Nishikubo, Guixin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–40, Table 1 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, B., Zhou, Y., Liang, H. et al. Negative thermal expansion and oxygen-redox electrochemistry. Nature 640, 941–946 (2025). https://doi.org/10.1038/s41586-025-08765-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08765-x
This article is cited by
-
Advances in battery technologies for smart grids in 2025
Nature Reviews Clean Technology (2026)
-
BaTiO3 Nanoparticle-Induced Interfacial Electric Field Optimization in Chloride Solid Electrolytes for 4.8 V All-Solid-State Lithium Batteries
Nano-Micro Letters (2026)
-
Mechanistic insights into colossal negative thermal expansion in Ca2RuO4 mott insulator via computational modeling approaches
npj Computational Materials (2025)
-
Charge-polarized lanthanide coordination strategy enables activity-stability synergy in rare earth-tailored metallene electrocatalysts
Science China Materials (2025)
-
Low-frequency phonon driven enhancement of negative thermal expansion in Zn2−xMnxGeO4
Tungsten (2025)