Abstract
Flowering plant sexual reproduction requires double fertilization, yielding embryo and endosperm seed compartments: the latter supports embryo growth and seed germination. In an experiment to generate haploid embryos through inhibition of pollen phospholipase activity in sunflower (Helianthus annus), we serendipitously discovered that emasculated sunflowers spontaneously form parthenogenic haploid seed. Exploration of genetic, chemical and environmental factors demonstrated that a specific genotype background enabled high parthenogenesis and that full spectrum high-intensity light supplementation boosted parthenogenesis, yielding hundreds of haploid seeds per head. Induction of doubled haploid plants can greatly accelerate plant breeding efficiency; however, despite successful engineering of haploid induction in many crops, few reported systems are commercially scalable1. Here we report efficient methods of chemical emasculation and genome doubling to produce fertile plants and enable a scalable sunflower doubled haploid system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available within this article and its Supplementary Information. Source data for Fig. 2a can be found in Supplementary Table 2.
References
Jacquier, N. M. A. et al. Puzzling out plant reproduction by haploid induction for innovations in plant breeding. Nat. Plants 6, 610–619 (2020).
Fujita, M. K., Singhal, S., Brunes, T. O. & Maldonado, J. A. Evolutionary dynamics and consequences of parthenogenesis in vertebrates. Annu. Rev. Ecol. Evol. Syst. 51, 191–214 (2020).
Darwin, C. The Different Forms of Flowers on Plants of the Same Species (D. Appleton, 1897).
Hojsgaard, D. & Hörandl, E. The rise of apomixis in natural plant populations. Front. Plant Sci. 10, 436713 (2019).
Majeský, Ľ., Vašut, R. J., Kitner, M. & Trávníček, B. The pattern of genetic variability in apomictic clones of Taraxacum officinale indicates the alternation of asexual and sexual histories of apomicts. PLoS ONE https://doi.org/10.1371/journal.pone.0041868 (2012).
Khanday, I., Skinner, D., Yang, B., Mercier, R. & Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565, 91–95 (2019).
Underwood, C. J. et al. A PARTHENOGENESIS allele from apomictic dandelion can induce egg cell division without fertilization in lettuce. Nat. Genet. 54, 84–93 (2022).
Fu, J. et al. Integration of genomic selection with doubled-haploid evaluation in hybrid breeding: from GS 1.0 to GS 4.0 and beyond. Mol. Plant 15, 577–580 (2022).
Kelliher, T. et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 542, 105–109 (2017).
Liu, C. et al. A 4-bp insertion at ZmPLA1 encoding a putative phospholipase A generates haploid induction in maize. Mol. Plant 10, 520–522 (2017).
Gilles, L. M. et al. Loss of pollen‐specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 36, 707–717 (2017).
Chaikam, V. et al. Analysis of effectiveness of R1-nj anthocyanin marker for in vivo haploid identification in maize and molecular markers for predicting the inhibition of R1-nj expression. Theor. Appl. Genet. 128, 159–171 (2015).
Sunflowerseed Explorer. USDA Foreign Agriculture Service https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2224000 (2024).
Jiang, C. et al. A reactive oxygen species burst causes haploid induction in maize. Mol. Plant 15, 943–955 (2022).
Leclercq, P. Une stérilité mâle cytoplasmique chez le Tournesol. In Annales de l’Amélioration des Plantes (ĽInstitut National de la Recherche Agronomique, 1969).
Bracey, M. H., Hanson, M. A., Masuda, K. R., Stevens, R. C. & Cravatt, B. F. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science 298, 1793–1796 (2002).
Newcomb, W. The development of the embryo sac of sunflower Helianthus annuus after fertilization. Can. J. Bot. 51, 879–890 (1973).
Miller, J. & Fick, G. Adaptation of reciprocal full‐sib selection in sunflower breeding using gibberellic acid induced male sterility 1. Crop Sci. 18, 161–162 (1978).
Wang, H., Hou, H., Jan, C. C. & Chao, W. S. Irradiated pollen-induced oarthenogenesis for doubled haploid oroduction in sunflowers (Helianthus spp.). Plants 12, 2430 (2023).
Laurie, D. & Bennett, M. Early post-pollination events in hexaploid wheat × maize crosses. Sexual Plant Reprod. 3, 70–76 (1990).
Patial, M., Pal, D., Thakur, A., Bana, R. S. & Patial, S. Doubled haploidy techniques in wheat (Triticum aestivum L.): an overview. Proc. Natl Acad. Sci. USA 89, 27–41 (2019).
Dordas, C. Foliar boron application improves seed set, seed yield, and seed quality of alfalfa. Agron. J. 98, 907–913 (2006).
Xin, P., Li, B., Zhang, H. & Hu, J. Optimization and control of the light environment for greenhouse crop production. Sci. Rep. 9, 8650 (2019).
Rieu, I., Twell, D. & Firon, N. Pollen development at high temperature: from acclimation to collapse. Plant Physiol. 173, 1967–1976 (2017).
Cashmore, A. R., Jarillo, J. A., Wu, Y.-J. & Liu, D. Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765 (1999).
Melchinger, A. E., Molenaar, W. S., Mirdita, V. & Schipprack, W. Colchicine alternatives for chromosome doubling in maize haploids for doubled‐haploid production. Crop Sci. 56, 559–569 (2016).
Blakeslee, A. F. & Avery, A. G. Methods of inducing doubling of chromosomes in plants: by treatment with colchicine. J. Hered. https://doi.org/10.1093/oxfordjournals.jhered.a104294 (1937).
Hardham, A. & Gunning, B. Structure of cortical microtubule arrays in plant cells. J. Cell Biol. 77, 14–34 (1978).
Manzoor, A., Ahmad, T., Bashir, M. A., Hafiz, I. A. & Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 8, 194 (2019).
Verdeil, J.-L., Alemanno, L., Niemenak, N. & Tranbarger, T. J. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci. 12, 245–252 (2007).
Yu, J.-K. Advanced breeding technologies for accelerating genetic gain. Plant Breed. Biotechnol. 8, 203–210 (2020).
Yao, L. et al. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 4, 530–533 (2018).
Lv, J. et al. Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nat. Biotechnol. 38, 1397–1401 (2020).
Wang, N., Gent, J. I. & Dawe, R. K. Haploid induction by a maize cenh3 null mutant. Sci. Adv. 7, eabe2299 (2021).
Zhong, Y. et al. In vivo maternal haploid induction in tomato. Plant Biotechnol. J. 20, 250–252 (2022).
Rao, K. S. & Rohini, V. Agrobacterium-mediated transformation of sunflower (Helianthus annuus L.): a simple protocol. Ann. Bot. 83, 347–354 (1999).
Qu, Y. et al. Mapping of QTL for kernel abortion caused by in vivo haploid induction in maize (Zea mays L.). PLoS ONE 15, e0228411 (2020).
Shen, K., Qu, M. & Zhao, P. The roads to haploid embryogenesis. Plants 12, 243 (2023).
Lv, J. & Kelliher, T. Recent advances in engineering of in vivo haploid induction systems. Methods Mol. Biol. 2653, 365–383 (2023).
Ferrie, A. & Caswell, K. Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tiss. Org. Cult. 104, 301–309 (2011).
Todorova, M., Ivanov, P., Shindrova, P., Christov, M. & Ivanova, I. Doubled haploid production of sunflower (Helianthus annuus L.) through irradiated pollen-induced parthenogenesis. Euphytica 97, 249–254 (1997).
Davis, G. L. The life history of Podolepis jaceoides (Sims) Voss-II. Megasporogenesis, female gametophyte and embryogeny. Phytomorphology 11, 206–219 (1961).
Cyprys, P., Lindemeier, M. & Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 5, 253–257 (2019).
Kallamadi, P. R. & Mulpuri, S. Ploidy analysis of Helianthus species by flow cytometry and its use in hybridity confirmation. Nucleus 59, 123–130 (2016).
Garcés, R. et al. Characterization of sunflower seed and oil wax ester composition by GC/MS, a final evaluation. LWT 173, 114365 (2023).
Van Rossum, G. & Drake, F. L. Python 3 Reference Manual (CreateSpace, 2009).
Silverman, B. W. Density Estimation for Statistics and Data Analysis (Routledge, 2018).
Duncan, K. E., Czymmek, K. J., Jiang, N., Thies, A. C. & Topp, C. N. X-ray microscopy enables multiscale high-resolution 3D imaging of plant cells, tissues, and organs. Plant Physiol. 188, 831–845 (2022).
Deng, J. et al. Concept and methodology of characterising infrared radiative performance of urban trees using tree crown spectroscopy. Build. Environ. 157, 380–390 (2019).
Aznar‐Moreno, J. A. et al. Sunflower (Helianthus annuus) long‐chain acyl‐coenzyme A synthetases expressed at high levels in developing seeds. Physiol. Plant. 150, 363–373 (2014).
Acknowledgements
We thank M. Marion, B. Lise, P. Joel and Y. Ma for whole-genome 33-SNP-maker analysis; J. Comadran Trabal and R. Sensolini for genetic distance check; J. Fabish for statistical support; W. Huang from the China Agriculture University and B. Ma for technical support; Y. Gao and F. Zhu for pipeline supports; V. Walbot for manuscript editing and consultation; A. Brosnan and M. Zong for ploidy analysis support; W. Shi and Y. Song from Zeiss for microcomputed tomography analysis; S. Tian and S. Ni for confocal microscopy studies; A. Bharti, A. Farmer and J. Curley for SY58 genome sequencing; E. Cheek for lighting infrastructure support; the Duke University Phytatron team, M. Betts, G. Piotrowski, A. Eddings and T. Smith; X. Zhang, B. Zhang, X. Chen, I. Jepson, C. Baxter, E. Dunder, R. Egger, J.-C. Rousseaux, C. Zuo, F. Kong, J. Zhang and O. Sauvageot for project guidance and support; B. Bao, Y. He, L. Li and M. Lopez-Sendon for sunflower lines; and C. Leming and M. Bublitz for intellectual property guidance. Syngenta Crop Protection provided internal funding.
Author information
Authors and Affiliations
Contributions
J.L., D.L. and T.K. conceptualized the study. E.B. developed the emasculation method. V.M.T., D.S., P.W., X.C. and J.C. conducted the phenotyping trials in field locations. H.J., H.D. and E.B. undertook greenhouse plant care and phenotyping. B.C., P.W. and Z.L. conceptualized and performed the light treatments. Y.D., Changbao Li, Chao Li, R.C. and P.D. performed the tissue culture and genome doubling. C.P., A.T. and P.D. performed the marker analysis and fingerprinting. X.T. and J.L. performed the microscopy. B.S. and W.C. performed the seed lipid profile check. F.B. and V.M.T. provided guidance and methodological considerations. J.L. and T.K. wrote the original draft of the manuscript, and reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.L., D.L., T.K., E.B., Changbao Li, Y.D., F.B., V.M.T., C.P. and P.D. have filed the patent application PCT/CN2024/090146, which covers the haploid parthenogenesis phenomenon, information on germplasm testing, doubling methods, in-medium seed culture processes to enhance germination, and environmental response of haploid seed setting. Assignees of PCT/CN2024/090146 are Syngenta Group Co., Ltd and Syngenta Crop Protection AG. The status of the application is not yet published. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Loren Rieseberg, Charles Underwood and Thomas Widiez for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Biology and seed phenotypes of corn and sunflower haploids.
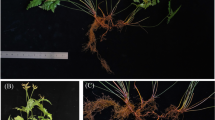
a, Typical corn colorless haploid and diploid embryos (center) dissected from haploid-induced ears (left) expressing purple anthocyanin pigment from transmission of the R1-nj marker in sperm. Haploid embryos are selected, genome-doubled, and cultured into DH plantlets (right). b, Disc floret from SY54 and SY54_CMS, after removing corolla. Anthers from SY54_CMS are shorter and not able to produce pollen, due to the CMS. c, Developing parthenogenic embryo in a SY54_CMS ovary ~20 days post-anthesis. d, An ovary from the same head lacking a parthenogenic embryo. Evidence of autonomous endosperm is not found in either image. e, SY54 (diploid) self-pollinated embryo with cellularized endosperm ~15 days post-anthesis. Results in c to e are representative of four to six replicate images of each. f, Normal diploid embryos from SY58 at 22 days post-anthesis. g, Normal diploid embryo from SY58 excised from the seed coat at 16 d post-anthesis. Two cotyledons are marked (^). h and i, Parthenogenic haploid embryos from SY58_CMS at 22 days post-anthesis. j and k, Transverse micro-computed tomography (microCT) sections of mature parthenogenic haploid (j) and normally fertilized diploid (k) seed from a SY54_CMS head. Subsets, green horizontal line identified the position of the section in the seed. l, Germplasm test in Toulouse field in 2021. SY54-CMS heads bagged as they start to flower. m, Close up image of the flowering stage with the outermost rings at pollination stage, surrounding the inner rings of immature flowers. n, Bagging and crossing plants in the field. PI, Propidium iodide stain; UV auto, autofluorescence visualized using UV light excitation (405 nm).
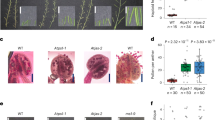
Extended Data Fig. 2 Parent emasculation and treatment to induce parthenogenic haploids.
a, R1 stage for GA3 treatment. b, R1 stage bud just following GA3 application. c, GA3-sterilized SY58 head showing absence of anther exertion and pollen shed. d, Parthenogenesis stimulation test (with maize pollen and other AIs treatment) with SY54_CMS sunflower head adding ZM01 maize pollen (e) painted onto the SY54 stigmas (f). g, Hormone was injected into the spongy layer of sunflower head (green arrow). h, Germination of SY58_CMS derived haploids in soil. Two hundred seeds yielded 80 seedings, of which roughly half were normal looking. i, and half were abnormal (j, k).
Extended Data Fig. 3 Parthenogenic haploid germination and doubling.
a-c, Impact of semi-solid formulation on tissue penetrance, effective treatment duration, and plant vigor. a, Liquid control formulation with DMSO and fluorescein showing some leaf penetration at 4 h but no penetration into the meristem (red circle). b, Semi-solid formulation with DMSO and fluorescein, showing deep tissue penetration at 5 h, and full saturation of the meristem region at 48 h (red circle). c, Liquid formulation with colchicine. d, Semi-solid formulation with colchicine, showing reduction of leaf growth compared to (c). This indicates that the colchicine is having a greater impact (limiting) growth in the semi-solid treatment. e and f, Growth of colchicine-induced doubled haploid plants (e) and leaves (f) derived from germinated parthenogenic haploid seeds. g, Spontaneous doubled haploids among parthenogenic (PRG) haploid progenies. Out of thirty-nine viable plants derived from the planting of 200 haploid seed, four appeared larger and scored as diploids in the ploidy analysis (flow cytometry) data. h, Sunflower parthenogenic doubled haploid (DH) process. Left, plants are emasculated manually or with a GA3 treatment. Center, seeds are separated using a blower and germinated in soil (bottom) or in tissue culture (top); a doubling treatment is provided to produce DH plants (right).
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–6 and Supplementary Tables 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, J., Liang, D., Bumann, E. et al. Haploid facultative parthenogenesis in sunflower sexual reproduction. Nature 641, 732–739 (2025). https://doi.org/10.1038/s41586-025-08798-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08798-2