Abstract
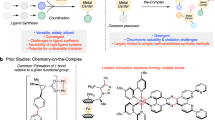
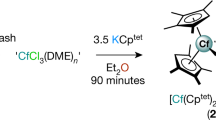
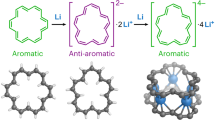
The discovery of ferrocene1 heralded the advent of modern organometallic chemistry. Characterized by the π-coordination of a metal by one or two planar annulene anions, ferrocenes and their analogues2,3,4 exemplify the archetype of out-of-plane annulene metal complexes. By contrast, the integration of metal within the annulene core to form in-plane annulene metal complexes featuring metal–carbon σ bonds has been obstructed not only by the synthetic difficulty and the non-planarity of annulenes with appropriate internal dimensions, but also by the difficulty of embedding the metal. These challenges have prevented the isolation of such in-plane annulene metal complexes. Here we report the preparation of three metal-centred planar [15]annulene frameworks. The most symmetrical fragment has D5h symmetry, with the metal centre shared by five identical five-membered rings. Density functional theory calculations demonstrate that metal d orbitals participate in conjugation with these five-membered rings, rendering all of them aromatic. The overall framework bears a loose structural and spectroscopic analogy to metallo-expanded porphyrins with multiple aza donors5, which thus provides a nexus between annulene chemistry and classic heteroatom-based coordination chemistry. The present systems display high stability and are easily functionalized. We thus suggest that metal-centred planar annulenes could emerge as promising building blocks for materials science.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data needed to evaluate the conclusions in this work are present in the article or Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2374570 (2c), 2374585 (3b), 2374576 (4a), 2374580 (4b), 2374584 (4c), 2374575 (5a), 2374581 (5b), 2374579 (7), 2374582 (8) and 2392762 (10). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Astruc, D. The numerous paths of ferrocene. Nat. Chem. 15, 1650 (2023).
Shima, T. et al. Hydroamination of alkenes with dinitrogen and titanium polyhydrides. Nature 632, 307–312 (2024).
Münzfeld, L. et al. Synthesis and properties of cyclic sandwich compounds. Nature 620, 92–97 (2023).
Malischewski, M., Adelhardt, M., Sutter, J., Meyer, K. & Seppelt, K. Isolation and structural and electronic characterization of salts of the decamethylferrocene dication. Science 353, 678–682 (2016).
Burrell, A. K., Hemmi, G., Lynch, V. & Sessler, J. L. Uranylpentaphyrin: an actinide complex of an expanded porphyrin. J. Am. Chem. Soc. 113, 4690–4692 (1991).
Faraday, M. On new compounds of carbon and hydrogen, and on certain other products obtained during the decomposition of oil by heat. Philos. Trans. R. Soc. London 115, 440–466 (1825).
Arndt, S. & Okuda, J. Mono(cyclopentadienyl) complexes of the rare-earth metals. Chem. Rev. 102, 1953–1976 (2002).
Pampaloni, G. Aromatic hydrocarbons as ligands. Recent advances in the synthesis, the reactivity and the applications of bis(η6-arene) complexes. Coord. Chem. Rev. 254, 402–419 (2010).
Mahieu, N., Piątkowski, J., Simler, T. & Nocton, G. Back to the future of organolanthanide chemistry. Chem. Sci. 14, 443–457 (2023).
Afanasyev, O. I. et al. Cyclobutadiene metal complexes: a new class of highly selective catalysts. An application to direct reductive amination. ACS Catal. 6, 2043–2046 (2016).
Zhou, Z. & Petrukhina, M. A. Adding multiple electrons to helicenes: how they respond. Chem. Sci. 16, 468–479 (2025).
Spitler, E. L., Johnson, C. A. II & Haley, M. M. Renaissance of annulene chemistry. Chem. Rev. 106, 5344–5386 (2006).
Masamune, S., Hojo, K., Hojo, K., Bigam, G. & Rabenstein, D. L. The geometry of [10]annulenes. J. Am. Chem. Soc. 93, 4966–4968 (1971).
Ajami, D., Oeckler, O., Simon, A. & Herges, R. Synthesis of a Möbius aromatic hydrocarbon. Nature 462, 819–821 (2003).
Moll, J. F., Pemberton, R. P., Gutierrez, M. G., Castro, C. & Karney, W. L. Configuration change in [14]annulene requires Möbius antiaromatic bond shifting. J. Am. Chem. Soc. 129, 274–275 (2007).
Stawski, W. et al. The anti-aromatic dianion and aromatic tetraanion of [18]annulene. Nat. Chem. 16, 998–1002 (2024).
Białek, M. J., Hurej, K., Furuta, H. & Latos-Grażyński, L. Organometallic chemistry confined within a porphyrin-like framework. Chem. Soc. Rev. 52, 2082–2144 (2023).
Vogel, E., Köcher, M., Schmickler, H. & Lex, J. Porphycene-a novel porphin isomer. Angew. Chem. Int. Ed. Engl. 25, 257–259 (1986).
Grover, V. & Ravikanth, M. Coordination chemistry of porphycenes. Coord. Chem. Rev. 516, 215999 (2024).
Thakur, M. S. et al. Metal coordinated macrocyclic complexes in different chemical transformations. Coord. Chem. Rev. 471, 214739 (2022).
Sessler, J. L., Gross, Z. & Furuta, H. Introduction: expanded, contracted, and isomeric porphyrins. Chem. Rev. 117, 2201–2202 (2017).
Lash, T. D. Carbaporphyrinoid systems. Chem. Rev. 117, 2313–2446 (2017).
Xu, B. et al. Syntheses, structures and reactivities of strained fused-ring metallaaromatics containing planar eleven-carbon chains with a shiftable metal carbyne bond. Nat. Commun. 14, 4378 (2024).
Byrne, P. A. & Gilheany, D. G. The mechanism of phosphonium ylide alcoholysis and hydrolysis: concerted addition of the O–H bond across the P=C bond. Chem. Eur. J. 22, 9140–9154 (2016).
Che, C.-M. et al. Triphenylphosphine reduction of dioxoosmium(VI) porphyrins. Crystal structures of bis(triphenylphosphine oxide) (octaethylporphinato)osmium(II) and bis(triphenylphosphine) (meso-tetraphenylporphinato)osmium(II). Inorg. Chem. 26, 3907–3911 (1987).
Zhu, C. et al. A metal-bridged tricyclic aromatic system: synthesis of osmium polycyclic aromatic complexes. Angew. Chem. Int. Ed. 53, 6232–6236 (2014).
Zhu, C. et al. σ-Aromaticity in an unsaturated ring: osmapentalene derivatives containing a metallacyclopropene unit. Angew. Chem. Int. Ed. 54, 3102–3106 (2015).
Schleyer, P. V. R. & Pühlhofer, F. Recommendations for the evaluation of aromatic stabilization energies. Org. Lett. 4, 2873–2876 (2002).
Fallah-Bagher-Shaidaei, H., Wannere, C. S., Corminboeuf, C., Puchta, R. & Schleyer, P. V. R. Which NICS aromaticity index for planar π rings is best? Org. Lett. 8, 863–866 (2006).
Geuenich, D., Hess, K., Köhler, F. & Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005).
Foroutan-Nejad, C. Is NICS a reliable aromaticity index for transition metal clusters? Theor. Chem. Acc. 134, 8 (2015).
Sundholm, D., Fliegl, H. & Berger, R. J. F. Calculations of magnetically induced current densities: theory and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 6, 639–678 (2016).
Knizia, G. & Klein, J. E. M. N. Electron flow in reaction mechanisms—revealed from first principles. Angew. Chem. Int. Ed. 54, 5518–5522 (2015).
Gross, C. L. & Girolami, G. S. Synthesis and characterization of osmium (IV) polyhydride complexes of stoichiometry (C5Me5)OsH3(L). Crystal structures of (C5Me5)OsH3(AsPh3) and (C5Me5)OsH3(PPh3). Organometallics 25, 4792–4798 (2006).
Chen, D., Hua, Y. & Xia, H. Metallaaromatic chemistry: history and development. Chem. Rev. 120, 12994–13086 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 92156021, 22350009, 21931002, 22371111 and 22071098), the Guangdong Provincial Key Laboratory of Catalysis (grant no. 2020B121201002), Introduction of Major Talent Projects in Guangdong Province (grant no. 2019CX01C079), Guangdong Grants (grant no. 2021ZT09C064), High Level of Special Funds (G03050K003), the Postdoctoral Fellowship Program of CPSF (GZB20240287) and the Outstanding Talents Training Fund in Shenzhen. The work in Austin was supported by the National Science Foundation (CHE-2304731 to J.L.S.) with further support provided by the Robert A. Welch Foundation (F-0018). The theoretical work was supported by the Center for Computational Science and Engineering at SUSTech. We thank J. Li of Tsinghua University, L. L. Liu, X. Jiang, X. Chang and Z. Dong of Southern University of Science and Technology, and H. Li of Zhejiang University for providing helpful suggestions in preparing the manuscript.
Author information
Authors and Affiliations
Contributions
D.C. and H.X. devised the project and supervised the experimental study. B.X., J.L.S. and H.X. analysed the experimental data. B.X., K.R., Y.C., J.Q., W.Z. and B.C. performed the experimental work. K.R., M.L. and Z.L. performed the computational analyses. B.X., D.C., K.R., M.L., J.L.S. and H.X. wrote the paper and prepared the supplementary information with input from all authors. All authors discussed the results in detail and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Fabrizio Ortu, Miquel Solà and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, characterization details, optimized Cartesian coordinates, Figs. 1–160, Tables 1–24 and references.
Crystallographic Data
Crystallographic data for complexes 2c, 2d, 3b, 4a, 4b, 4c, 5a, 5b, 7, 8 and 10.
Supplementary Video 1
Simulation of the gauge-including magnetically induced current (GIMIC) in the [55555] metallo-annulene model 5a′. This video shows the diatropic ring currents of 5a′, and suggests the [55555] metallo-annulene skeleton is aromatic.
Supplementary Video 2
Simulation of the gauge-including magnetically induced current (GIMIC) in benzene. This video shows the diatropic ring currents, and suggests benzene is aromatic.
Supplementary Video 3
Simulation of the gauge-including magnetically induced current (GIMIC) in the [55753] metallo-annulene model 2a′. This video shows the diatropic ring currents of 2a′, and suggests the [55753] metallo-annulene skeleton is aromatic.
Supplementary Video 4
Simulation of the gauge-including magnetically induced current (GIMIC) in the [55735] metallo-annulene model 3a′. This video shows the diatropic ring currents of 3a′, and suggests the [55735] metallo-annulene skeleton is aromatic.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, B., Chen, D., Ruan, K. et al. Metal-centred planar [15]annulenes. Nature 641, 106–111 (2025). https://doi.org/10.1038/s41586-025-08841-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08841-2
This article is cited by
-
In-plane aromatic metallo-annulenes: bridging annulene and coordination chemistry
Science China Chemistry (2026)
-
Metal-cored molecule is the first of its kind
Nature (2025)