Abstract
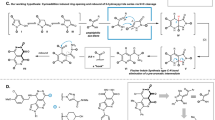
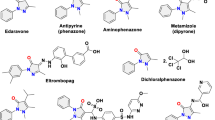
Pyrazoles are heterocycles commonly found as key substructures in agrochemicals and medicinally active compounds alike1,2. Despite their pervasiveness, established methods fall notably short in delivering complex pyrazoles selectively due to issues of differentiation during either assembly or N-functionalization3. This is a direct consequence of a dominant synthetic strategy that attempts to control selectivity-determining bonds between poorly differentiated starting materials. To overcome this longstanding challenge, we here describe a prototypical example of an alternative conceptual approach, ‘strategic atom replacement’, in which we synthesize N-alkyl pyrazoles from isothiazoles. The net forward transformation is a ‘swap’ of the isothiazole sulfur atom with a nitrogen atom and its associated alkyl fragment to deliver the alkylated pyrazole4,5. Linking the two azoles is an orphaned heterocycle class, 1,2,3-thiadiazine-S-oxides, whose synthetic potential has yet to be tapped6. By proceeding through these unusual heterocycles, the typical selectivity and separation challenges associated with exclusively bond-based pyrazole preparations are circumvented, and even minimally differentiated peripheral substituents can be discriminated to afford isomerically pure products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are in the Supplementary Information. NMR data are provided in image and peaklist format; original NMR spectra files are available from the corresponding author (M.D.L.) upon request. Crystallographic data have been deposited to the CCDC (2394327, 2394342, 2394344, 2394373, 2394381 and 2394383).
References
Li, G. et al. Pyrazole-containing pharmaceuticals: target, pharmacological activity, and their SAR studies. RSC Med. Chem. 13, 1300–1321 (2022).
Marshall, C. M., Federice, J. G., Bell, C. N., Cox, P. B. & Njardarson, J. T. An update on the nitrogen heterocycle compositions and properties of U.S. FDA-approved pharmaceuticals (2013–2023). J. Med. Chem. 67, 11622–11655 (2024).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Levin, M. D. Retrosynthetic simplicity. Synlett 35, 1471–1474 (2023).
Kolberg, A., Sieler, J. & Schulze, B. Synthesis of oxidized 1,2,3-thiadiazines by hydroperoxy-induced ring enlargement of 2-benzenesulfonylaminoisothiazolium salts. J. Heterocycl. Chem. 36, 1081–1086 (1999).
Trost, B. M. Selectivity: a key to synthetic efficiency. Science 219, 245–250 (1983).
Corey, E. J. The logic of chemical synthesis: multistep synthesis of complex carbogenic molecules (Nobel Lecture). Angew. Chem. Int. Ed. 30, 455–465 (1991).
Corey, E. J. & Cheng, X.-M. Logic of Chemical Synthesis (Wiley, 1995).
Nicolaou, K. C. & Sorensen, E. J. Classics in Total Synthesis: Targets, Strategies, Methods (Wiley, 1996).
Nogi, K. & Yorimitsu, H. Aromatic metamorphosis: conversion of an aromatic skeleton into a different ring system. Chem. Commun. 53, 4055–4065 (2017).
Yorimitsu, H. Aromatic metamorphosis of thiophenes: replacing endocyclic sulfur with a different atom or group of atoms. Phosphorus Sulfur Silicon Relat. Elem. 198, 478–480 (2023).
Pearson, T. J. et al. Aromatic nitrogen scanning by ipso-selective nitrene internalization. Science 381, 1474–1479 (2023).
Woo, J., Stein, C., Christian, A. H. & Levin, M. D. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 623, 77–82 (2023).
Cheng, Q. et al. Skeletal editing of pyridines through atom-pair swap from CN to CC. Nat. Chem. 16, 741–748 (2024).
Uhlenbruck, B. J. H., Josephitis, C. M., de Lescure, L., Paton, R. S. & McNally, A. A deconstruction–reconstruction strategy for pyrimidine diversification. Nature 631, 87–93 (2024).
Boswell, B. R., Zhao, Z., Gonciarz, R. L. & Pandya, K. M. Regioselective pyridine to benzene edit inspired by water-displacement. J. Am. Chem. Soc. 146, 19660–19666 (2024).
Kim, D. et al. Photocatalytic furan-to-pyrrole conversion. Science 386, 99–105 (2024).
Kanda, Y., Ishihara, Y., Wilde, N. C. & Baran, P. S. Two-phase total synthesis of taxanes: tactics and strategies. J. Org. Chem. 85, 10293–10320 (2020).
Zhang, Y.-A., Gu, X. & Wendlandt, A. E. A change from kinetic to thermodynamic control enables trans-selective stereochemical editing of vicinal diols. J. Am. Chem. Soc. 144, 599–605 (2022).
Zhang, Y.-A. et al. Stereochemical editing logic powered by the epimerization of unactivated tertiary stereocenters. Science 378, 383–390 (2022).
Gu, X. et al. Synthesis of non-canonical amino acids through dehydrogenative tailoring. Nature 634, 352–358 (2024).
Devasthale, P. & Wang, W. Azole amides and amines as alpha v integrin inhibitors. Patent WO 2018/089358 Al (2018).
Yang, E. & Dalton, D. M. N1-Selective methylation of pyrazoles via α-halomethylsilanes as masked methylating reagents. J. Org. Chem. 89, 4221–4224 (2024).
Wright, S. W., Arnold, E. P. & Yang, X. Steric redirection of alkylation in 1H-pyrazole-3-carboxylate esters. Tetrahedron Lett. 59, 402–405 (2018).
Bengel, L. L. et al. Engineered enzymes enable selective N-alkylation of pyrazoles with simple haloalkanes. Angew. Chem. Int. Ed. 60, 5554–5560 (2021).
Desai, S. P., Zambri, M. T. & Taylor, M. S. Borinic acid catalyzed regioselective N-alkylation of azoles. J. Org. Chem. 87, 5385–5394 (2022).
Wang, M. et al. Copper-catalyzed hydroamination: enantioselective addition of pyrazoles to cyclopropenes. J. Am. Chem. Soc. 145, 14573–14580 (2023).
Lee, S.-J., Bae, J.-Y. & Cho, C.-W. Phase-transfer-catalyzed asymmetric synthesis of chiral N-substituted pyrazoles by aza-Michael reaction. Eur. J. Org. Chem. 2015, 6495–6502 (2015).
Knorr, L. Einwirkung von Acetessigester auf Phenylhydrazin. Ber. Dtsch. Chem. Ges. 16, 2597–2599 (1883).
Aggarwal, R., Kumar, V., Kumar, R. & Singh, S. P. Approaches towards the synthesis of 5-aminopyrazoles. Beilstein J. Org. Chem. 7, 179–197 (2011).
Dalton, D. M., Walroth, R. C., Rouget-Virbel, C., Mack, K. A. & Toste, F. D. Utopia point Bayesian optimization finds condition-dependent selectivity for N-methyl pyrazole condensation. J. Am. Chem. Soc. 146, 15779–15786 (2024).
Kaberdin, R. V. & Potkin, V. I. Isothiazoles (1,2-thiazoles): synthesis, properties and applications. Russ. Chem. Rev. 71, 673–694 (2002).
Kletskov, A. V., Bumagin, N. A., Zubkov, F. I., Grudinin, D. G. & Potkin, V. I. Isothiazoles in the design and synthesis of biologically active substances and ligands for metal complexes. Synthesis 52, 159–188 (2019).
De Oliveira Silva, A., McQuade, J. & Szostak, M. Recent advances in the synthesis and reactivity of isothiazoles. Adv. Synth. Catal. 361, 3050–3067 (2019).
Roure, B. et al. Photochemical permutation of thiazoles, isothiazoles and other azoles. Nature 637, 860–867 (2025).
Liu, B.-B., Cao, W.-B., Wang, F., Wang, S.-Y. & Ji, S.-J. [4+1] Cycloaddition reaction of α,β-alkynic hydrazones and KSCN under transition-metal-free conditions: synthesis of N-iminoisothiazolium ylides. J. Org. Chem. 83, 11118–11124 (2018).
Loudon, J. D. in Organic Sulfur Compounds Vol. 1 (ed. Kharasch, N.) Ch. 26, 299–305 (Pergamon, 1961).
Boyd, G. V. et al. in Science of Synthesis Vol. 11 (ed. Schaumann, E.) 507 (Thieme Verlag, 2002).
Gasser, V. C. M., Makai, S. & Morandi, B. The advent of electrophilic hydroxylamine-derived reagents for the direct preparation of unprotected amines. Chem. Commun. 58, 9991–10003 (2022).
Porcs-Makkay, M. et al. Alkylation of 2H-1,2,3-benzothiadiazine 1,1-dioxides. Formation of a new family of mesoionic compounds. Tetrahedron 70, 2169–2174 (2014).
Joost, M. et al. Sulfur monoxide thermal release from an anthracene-based precursor, spectroscopic identification, and transfer reactivity. Proc. Natl Acad. Sci. USA 115, 5866–5871 (2018).
Lynch, C. F., Downey, J. W., Zhang, Y., Hooker, J. M. & Levin, M. D. Core-labeling (radio) synthesis of phenols. Org. Lett. 25, 7230–7235 (2023).
Huang, A. et al. Regioselective synthesis, NMR, and crystallographic analysis of N1-substituted pyrazoles. J. Org. Chem. 82, 8864–8872 (2017).
Escolástico, C., Blanco, M., Claramunt, R. M., Sanz, D. & Elguero, J. Microwave synthesis of arylmethyl substituted pyrazoles. Open Org. Chem. J. 2, 10–16 (2008).
Micetich, R. G. Lithiation of five-membered heteroaromatic compounds. The methyl substituted 1,2-azoles, oxadiazoles, and thiadiazoles. Can. J. Chem. 48, 2006–2015 (1970).
Mosrin, M. & Knochel, P. TMPZnCl·LiCl: a new active selective base for the directed zincation of sensitive aromatics and heteroaromatics. Org. Lett. 11, 1837–1840 (2009).
Olofson, R. A., Landesberg, J. M., Houk, K. N. & Michelman, J. S. The deprotonation of thiazole and related bases. J. Am. Chem. Soc. 88, 4265–4266 (1966).
Caton, M. P. L., Jones, D. H., Slack, R. & Wooldridge, K. R. H. 80. Isothiazoles. Part III. Reactions of isothiazol-5-yl-lithium compounds. J. Chem. Soc. https://doi.org/10.1039/JR9640000446 (1964).
Ziegler, K. & Zeiser, H. Untersuchungen über alkaliorganische Verbindungen, VII. mitteil.: alkalimetallalkyle und pyridin (vorläufige mitteilung). Ber. Dtsch. Chem. Ges. B Ser. 63, 1847–1851 (1930).
Wagen, C. & Wagen, A. Efficient and accurate pKa prediction enabled by pre-trained machine-learned interatomic potentials. Preprint at https://doi.org/10.26434/chemrxiv-2024-8489b (2024).
Zabierek, A. A., Konrad, K. M. & Haidle, A. M. A practical, two-step synthesis of 1-alkyl-4-aminopyrazoles. Tetrahedron Lett. 49, 2996–2998 (2008).
Mosallanejad, A. & Lorthioir, O. Application of Tsunoda reagent to the convenient synthesis of drug-like pyrazoles. Tetrahedron Lett. 59, 1708–1710 (2018).
Acknowledgements
The research reported in this work was supported by the Packard Foundation, the Bristol Myers Squibb Unrestricted Research Grant, and by Johnson and Johnson Innovative Medicine. University of Chicago Research Computing Center is thanked for computational resources. L.C. was supported by a University of Chicago Quad Scholars Grant for summer research funding. A.J.B. thanks the NSF Graduate Research Fellowship Program (grant no. 2140001) for fellowship support. P.Q.K. thanks the Seymour Goodman fund for fellowship support. Enamine is thanked for a generous donation of chemicals and B. Egle (Johnson & Johnson) is thanked for conducting safety testing.
Author information
Authors and Affiliations
Contributions
A.F., Y.A., L.C. and C.B.K. designed and conducted experiments, and collected and analysed the data. P.Q.K. and A.J.B. conducted computations of the mechanism. P.Q.K and A.J.B. conducted crystallographic measurements and analyses. M.D.L. and A.F. conceived of the project and wrote the manuscript with input from all authors. M.D.L. and C.B.K. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–25, including Figs. 1–31 and Tables 1–9. See the Table of Contents for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fanourakis, A., Ali, Y., Chen, L. et al. Strategic atom replacement enables regiocontrol in pyrazole alkylation. Nature 641, 646–652 (2025). https://doi.org/10.1038/s41586-025-08951-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08951-x
This article is cited by
-
Direct oxygen-to-sulfur single-atom substitution of oxazoles and isoxazoles
Nature Synthesis (2026)