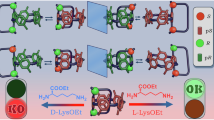
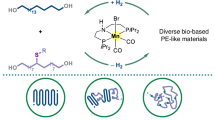
Abstract
Natural chiral polymers, such as DNA, proteins, cellulose and poly[(R)-3-hydroxybutyrate] ((R)-P3HB), are prevalent in their enantiopure forms1,2. Existing methods to synthesize enantiopure polymers focus on enantiospecific polymerization, in which only one specific enantiomer is obtained from the corresponding chiral monomer3,4,5,6. Here we introduce a catalytic stereodivergent synthetic strategy to access all enantiopure di-isotactic poly(3-hydroxyalkanoate) (PHA) diastereomers from bacterial (R)-P3HB as the single chiral source. A series of enantiopure (R,R)-α-alkylated-β-butyrolactones are obtained from (R)-P3HB and then subjected to the catalyst-controlled diastereodivergent ring-opening polymerization (ROP) to enantiopure di-isotactic α-alkylated PHAs. Metal-catalysed coordination–insertion ROP results in threo-(R,R)-di-isotactic PHAs with chiral retention, whereas anionic ROP catalysed by an organic superbase produces erythro-(R,S)-di-isotactic PHAs with chiral inversion, achieving precision di-isotactic PHAs with exclusive regio- and stereoregularity. This strategy has also enabled the stereodivergent synthesis of all four [(R,R), (S,S), (R,S) and (S,R)] stereoisomers of α,α-dialkylated PHAs from (R)-P3HB, which can be depolymerized to chiral α,α-dialkylated-β-butyrolactones with high stereoselectivity. Overall, this catalyst-controlled regio- and stereoselective, stereodivergent synthetic methodology provides access to 16 enantiopure stereoisomers of α(α)-(di)substituted PHAs and enables the stereochemistry-defined structure–property relationship study of the di-isotactic PHAs, providing insights into the effects of main-chain stereoconfigurations and alkyl side chains on their thermal properties, melt processability, mechanical performance and supramolecular stereocomplexation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available in the Article and its Supplementary Information.
References
Vishakha, S. K., Kishor, D. B. & Sudha, S. R. Natural polymers–a comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 3, 1597–1613 (2012).
Muhammadi, Shabina, Afzal, M. & Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 8, 56–77 (2015).
Tutoni, G. G. et al. Microfluidic assembly of degradable, stereocomplexed hydrogel Microparticles. J. Am. Chem. Soc. 146, 14705–14714 (2024).
Popowski, Y., Lu, Y., Coates, G. W. & Tolman, W. B. Stereocomplexation of stereoregular aliphatic polyesters: change from amorphous to semicrystalline polymers with single stereocenter inversion. J. Am. Chem. Soc. 144, 8362–8370 (2022).
Auriemma, F. et al. Stereocomplexed poly(limonene carbonate): a unique example of the cocrystallization of amorphous enantiomeric polymers. Angew. Chem. Int. Ed. 54, 1215–1218 (2015).
Hori, Y., Suzuki, M., Yamaguchi, A. & Nishishita, T. Ring-opening polymerization of optically active β-butyrolactone using distannoxane catalysts: synthesis of high-molecular-weight poly(3-hydroxybutyrate). Macromolecules 26, 5533–5534 (1993).
Zheng, Y. & Pan, P. Crystallization of biodegradable and biobased polyesters: polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 109, 101291 (2020).
Worch, J. C. et al. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 3, 514–535 (2019).
García, F., Gómez, R. & Sánchez, L. Chiral supramolecular polymers. Chem. Soc. Rev. 52, 7524–7548 (2023).
Shen, J. & Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 116, 1094–1138 (2016).
Kakuchi, T. & Sakai, R. in Encyclopedia Polymer Science Technology Vol. 3, 1–32 (Wiley, 2014).
Yashima, E., Maeda, K., Iida, H., Furusho, Y. & Nagai, K. Helical polymers: synthesis, structures, and functions. Chem. Rev. 109, 6102–6211 (2009).
Westlie, A. H., Quinn, E. C., Parker, C. R. & Chen, E. Y.-X. Synthetic biodegradable polyhydroxyalkanoates (PHAs): recent advances and future challenges. Prog. Polym. Sci. 134, 101608 (2022).
Anjum, A. et al. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int. J. Biol. Macromol. 89, 161–174 (2016).
Sudesh, K., Abe, H. & Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25, 1503–1555 (2000).
Shoda, S. I., Uyama, H., Kadokawa, J. I., Kimura, S. & Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 116, 2307–2413 (2016).
Kobayashi, S. & Makino, A. Enzymatic polymer synthesis: an opportunity for green polymer chemistry. Chem. Rev. 109, 5288–5353 (2009).
Xu, G. et al. Asymmetric kinetic resolution polymerization. Coord. Chem. Rev. 414, 213296 (2020).
Young, M. S., LaPointe, A. M., MacMillan, S. N. & Coates, G. W. Highly enantioselective polymerization of β-butyrolactone by a bimetallic magnesium catalyst: an interdependent relationship between favored and unfavored enantiomers. J. Am. Chem. Soc. 146, 18032–18040 (2024).
Huang, H.-Y. et al. Spiro-salen catalysts enable the chemical synthesis of stereoregular polyhydroxyalkanoates. Nat. Catal. 6, 720–728 (2023).
Beletskaya, I. P., Najera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Krautwald, S. & Carreira, E. M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 139, 5627–5639 (2017).
Quinn, E. C. et al. Installing controlled stereo-defects yields semicrystalline and biodegradable poly(3-hydroxybutyrate) with high toughness and optical clarity. J. Am. Chem. Soc. 145, 5795–5802 (2023).
Bruckmoser, J., Pongratz, S., Stieglitz, L. & Rieger, B. Highly isoselective ring-opening polymerization of rac-β-butyrolactone: access to synthetic poly(3-hydroxybutyrate) with polyolefin-like material properties. J. Am. Chem. Soc. 145, 11494–11498 (2023).
Tang, X. et al. Biodegradable polyhydroxyalkanoates by stereoselective copolymerization of racemic diolides: stereocontrol and polyolefin-like properties. Angew. Chem. Int. Ed. 59, 7881–7890 (2020).
Grassie, N., Murray, E. J. & Holmes, P. A. The thermal degradation of poly(-(D)-β-hydroxybutyric acid): part 3—the reaction mechanism. Polym. Degrad. Stab. 6, 127–134 (1984).
Morikawa, H. & Marchessault, R. H. Pyrolysis of bacterial polyalkanoates. Can. J. Chem. 59, 2306–2313 (1981).
Zhou, L. et al. Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates. Science 380, 64–69 (2023).
Zhou, Z., LaPointe, A. M., Shaffer, T. D. & Coates, G. W. Nature-inspired methylated polyhydroxybutyrates from C1 and C4 feedstocks. Nat. Chem. 15, 856–861 (2023).
Zhou, Z., LaPointe, A. M. & Coates, G. W. Atactic, isotactic, and syndiotactic methylated polyhydroxybutyrates: an unexpected series of isomorphic polymers. J. Am. Chem. Soc. 145, 25983–25988 (2023).
Yang, J.-C., Yang, J., Li, W.-B., Lu, X.-B. & Liu, Y. Carbonylative polymerization of epoxides mediated by tri-metallic complexes: a dual catalysis strategy for synthesis of biodegradable polyhydroxyalkanoates. Angew. Chem. Int. Ed. 61, e202116208 (2022).
Furutate, S. et al. Superior thermal stability and fast crystallization behavior of a novel, biodegradable α-methylated bacterial polyester. npg Asia Mater. 13, 31 (2021).
Tanahashi, N. & Doi, Y. Thermal properties and stereoregularity of poly(3-hydroxybutyrate) prepared from optically active β-butyrolactone with a zinc-based catalyst. Macromolecules 24, 5732–5733 (1991).
Zhang, Y., Gross, R. A. & Lenz, R. W. Stereochemistry of the ring-opening polymerization of (S)-β-butyrolactone. Macromolecules 23, 3206–3212 (1990).
Rieth, L. R., Moore, D. R., Lobkovsky, E. B. & Coates, G. W. Single-site β-diiminate zinc catalysts for the ring-opening polymerization of β-butyrolactone and β-valerolactone to poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 124, 15239–15248 (2002).
Shakaroun, R. M., Jéhan, P., Alaaeddine, A., Carpentier, J. F. & Guillaume, S. M. Organocatalyzed ring-opening polymerization (ROP) of functional β-lactones: new insights into the ROP mechanism and poly(hydroxyalkanoate)s (PHAs) macromolecular structure. Polym. Chem. 11, 2640–2652 (2020).
Jedlinski, Z. et al. Stereochemical control in the anionic polymerization of β-butyrolactone initiated with alkali-metal alkoxides. Macromolecules 29, 3773–3777 (1996).
Kurcok, P., Kowalczuk, M., Hennek, K. & Jedlinski, Z. Anionic polymerization of β-lactones initiated with alkali-metal alkoxides: reinvestigation of the polymerization mechanism. Macromolecules 25, 2017–2020 (1992).
Li, Z., Zhao, D., Shen, Y. & Li, Z. Ring-opening polymerization of enantiopure bicyclic ether-ester monomers toward closed-loop recyclable and crystalline stereoregular polyesters via chemical upcycling of bioplastic. Angew. Chem. Int. Ed. 62, e202302101 (2023).
Amgoune, A., Thomas, C. M., Ilinca, S., Roisnel, T. & Carpentier, J. Highly active, productive, and syndiospecific yttrium initiators for the polymerization of racemic β‐butyrolactone. Angew. Chem. Int. Ed. 45, 2782–2784 (2006).
De Winter, J., Coulembier, O., Gerbaux, P. & Dubois, P. High molecular weight poly(α,α′,β-trisubstituted β-lactones) as generated by metal-free phosphazene catalysts. Macromolecules 43, 10291–10296 (2010).
Fráter, G., Müller, U. & Günther, W. The stereoselective α-alkylation of chiral β-hydroxy esters and some applications thereof. Tetrahedron 40, 1269–1277 (1984).
Tutoni, G. & Becker, M. L. Underexplored stereocomplex polymeric scaffolds with improved thermal and mechanical properties. Macromolecules 53, 10303–10314 (2020).
Im, S. H. et al. Stereocomplex polylactide for drug delivery and biomedical applications: a review. Molecules 26, 2846 (2021).
Tang, X. & Chen, E. Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 9, 2345 (2018).
Amgoune, A., Thomas, C. M., Roisnel, T. & Carpentier, J.-F. Ring‐opening polymerization of lactide with group 3 metal complexes supported by dianionic alkoxy‐amino‐bisphenolate ligands: combining high activity, productivity, and selectivity. Chem. Eur. J. 12, 169–179 (2006).
Acknowledgements
The work by J.-J.T., R.L., J.N. and E.Y.-X.C. was supported in part by RePLACE (Redesigning Polymers to Leverage a Circular Economy), funded by the Office of Science of the US Department of Energy (award no. DE-SC0022290). The work by E.C.Q., E.R.C., Z.Z., L.Z., R.R.G. and E.Y.-X.C. was supported in part by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office (AMMTO) and Bioenergy Technologies Office (BETO), performed as part of the BOTTLE Consortium, which includes members from Colorado State University and funded under contract no. DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy. We thank J. Boes of the Kennan group for assistance in CD measurements.
Author information
Authors and Affiliations
Contributions
E.Y.-X.C. conceived the project, acquired the funding and directed the research. J.-J.T. and R.L. led the experimental design and study. J.-J.T., R.L., E.C.Q., J.N., E.R.C., Z.Z., L.Z. and R.R.G. designed and conducted experiments as well as acquired, analysed and interpreted data or results. J.-J.T. and R.L. wrote the original draft and revised the subsequent manuscript versions. E.Y.-X.C. edited the initial and subsequent versions of the paper. All authors have reviewed and approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Yohei Ogiwara, Rong Tong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The structures of 22 enantiopure diisotactic PHAs synthesized and investigated in this study.
Sixteen enantiopure diisotactic PHAs were derived from (R)-P3HB and six enantiopure diisotactic PHAs were derived from ethyl-(S)-3-hydroxybutanoate.
Extended Data Fig. 2 Synthesis and absolute stereoconfiguration determination of (R)- and (S)-[P3H(Me)2B].
a, Catalytic stereodivergent polymerization of (R)-[(Me)2BL] leads to enantiopure (R)- and (S)-[P3H(Me)2B], and their hydrolysis gives the corresponding enantiopure (R)- and (S)-hydroxyacids. b, Comparative 13C NMR spectra (CDCl3) of atactic P3H(Me)2B and enantiopure (R)- and (S)-[P3H(Me)2B].
Extended Data Fig. 3 Chemical recycling of enantiopure P3H(Me)2B to chiral lactones.
a, Catalytic stereodivergent polymerization of virgin (R)-(Me)2BL and NaOH-catalyzed depolymerization of enantiopure P3H(Me)2B. b, Comparative crude 1H NMR spectra (CDCl3) of virgin (R)-(Me)2BL, recycled (S)-(Me)2BL, and recycled (R)-(Me)2BL.
Extended Data Fig. 4 Chemical recycling of diastereomeric P(M5) to chiral lactones.
a, Catalytic stereodivergent polymerization of virgin (R,R)-M5 and chemical recycling of (R,R)-P(M5) and (R,S)-P(M5). b, Catalytic stereodivergent polymerization of virgin (S,R)-M5 and chemical recycling of (S,R)-P(M5) and (S,S)-P(M5). c, Comparative crude 1H NMR spectra (CDCl3) of virgin (R,R)-M5, recycled (R,S)-M5 and recycled (R,R)-M5. d, Comparative crude 1H NMR spectra (CDCl3) of virgin (S,R)-M5, recycled (S,S)-M5 and recycled (S,R)-M5.
Extended Data Fig. 5 Stereocomplexation behavior of enantiomeric P(M2).
a, DSC second heating scans (10 °C/min) for (R,R)-P(M2), (S,S)-P(M2), and their 1:1 physical blend. b, DSC second heating scans (10 °C/min) for (R,S)-P(M2), (S,R)-P(M2), and stereocomplex, sc-PHEBRS/SR. c, DSC first cooling scans (10 °C/min) for (R,S)-P(M2), (S,R)-P(M2), and sc-PHEBRS/SR. d, Stacked WAXS profiles of (R,S)-P(M2), (S,R)-P(M2), and sc-PHEBRS/SR.
Extended Data Fig. 6 Stacked DSC crystallization thermograms of P(M3) enantiomers and their stereocomplexes.
a, DSC first cooling scans (10 °C/min) for (R,R)-P(M3), (S,S)-P(M3), and sc-PHPBRR/SS. b, DSC first cooling scans (10 °C/min) for (R,S)-P(M3), (S,R)-P(M3), and sc-PHPBRS/SR.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figs. 1–122, Supplementary Tables 1–12 and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, JJ., Li, R., Quinn, E.C. et al. Stereodivergent transformation of a natural polyester to enantiopure PHAs. Nature 643, 967–974 (2025). https://doi.org/10.1038/s41586-025-09220-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09220-7