Abstract
The naming of Australopithecus deyiremeda1 from Woranso-Mille (less than 3.59 to more than 3.33 million years) indicated the presence of a species contemporaneous with Australopithecus afarensis in the Ethiopian Afar Rift. A partial foot (BRT-VP-2/73)2 and several isolated teeth from two Burtele (BRT) localities, however, were not identified to the species level. Recently recovered dentognathic specimens clarify not only the taxonomic affinity of the BRT hominin specimens but also shed light on the diet and locomotion of A. deyiremeda. Here we present a comparative description of these specimens and show that they are attributable to A. deyiremeda. We also find it parsimonious to attribute the BRT foot to this species based on the absence of other hominin species at BRT. The new material demonstrates that overall, A. deyiremeda was dentally and postcranially more primitive than A. afarensis, particularly in aspects of canine and premolar morphology, and in its retention of pedal grasping traits. Furthermore, the low and less variable distributions of its dental enamel δ13C values are similar to those from Ardipithecus ramidus and Australopithecus anamensis, indicating a reliance on C3 foods. This suggests that A. deyiremeda had a dietary strategy similar to the earlier A. ramidus and A. anamensis. The BRT foot and its assignment to A. deyiremeda provides conclusive evidence that arboreality was a significant component of the positional behaviour of this australopith, further corroborating that some degree of arboreality persisted among Pliocene hominins1,3,4,5,6,7.
Similar content being viewed by others
Main
Hominin fossil discoveries in the past two decades suggest that multiple contemporaneous species existed in eastern Africa during the mid-Pliocene1,2,8, but their validity, except for A. afarensis (a well-known Pliocene hominin species) has been questioned on various grounds including inadequate morphological distinction, small sample size or poor preservation9,10,11. There appears to be a consensus, however, that the BRT foot (BRT-VP-2/73) from Woranso-Mille2, which was not assigned to a species at the time of its initial publication, is the strongest evidence for the presence of a non-A. afarensis species during the mid-Pliocene of eastern Africa1,2,12,13,14. Some researchers have argued that the presence of human-like feet in some members of early Homo, variability in the foot morphology of australopiths and a foot with an opposable hallux in some of the earliest hominins such as A. ramidus all suggest diversity in pedal adaptations for bipedal locomotion15. More recent studies have further suggested that the BRT foot demonstrated the presence of variability in foot morphology and bipedal locomotor adaptation among species of Australopithecus9,16,17, whereas others have hinted that the BRT foot belonged to a late-surviving species of Ardipithecus18 despite there being differences in the morphology of the first metatarsal and metatarsophalangeal joints19.
One of the reasons why the BRT foot and several isolated teeth from the BRT localities were not assigned to A. deyiremeda, despite their provenience from the same horizon as one of the paratypes of the species, was the lack of diagnosable dentognathic specimens that could be directly compared with the holotype and paratype specimens of the species2. In the initial description of A. deyiremeda, it has been shown that some aspects of its maxillary and mandibular morphology were more derived than A. afarensis1. However, its second and third molars, including other teeth from BRT, overlapped, both in size and shape, with A. afarensis and other Pliocene hominins. Further fieldwork at Woranso-Mille, particularly at the BRT localities, has now resulted in the recovery of additional, and more diagnostic, dentognathic specimens from the same horizon as and immediately below the BRT foot (Fig. 1). On the basis of the provenience (vertical and horizontal), it is more than likely that the dentognathic specimens from the BRT localities and the BRT foot belong to the same species: A. deyiremeda. The BRT hominins retain more primitive (A. anamensis-like) dental features than A. afarensis, and there is now evidence that like A. anamensis, they also had a C3-dominated dietary adaptation.
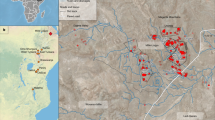
a, Map of Ethiopia showing the location of the Woranso-Mille site. GPTS, geomagnetic polarity time scale. b, Satellite image showing the location of the BRT area (white rectangle) relative to other nearby mid-Pliocene localities. KSD, Korsi Dora (3.6 Myr); LDD, Leado Dido’a (3.4 Myr); NFR, Nefuraytu (3.3 Myr). The black lines indicate observed fault lines. x-axis, latitude; y-axis, longitude. c, A closeup view of the BRT area showing the distribution of fossil hominins on the landscape. The squares represent the holotype and paratype specimens of A. deyiremeda. The circles represent previously and newly discovered specimens including BRT-VP-2/73 and BRT-VP-2/135 shown by the white arrows. The white line is the boundary between BRT-VP-1 and BRT-VP-2, and the black lines are the faults shown in panel b. d, Composite stratigraphical section of the BRT and WYT localities. The coloured vertical bars show the vertical extent of each locality in the section. The maximum age of the hominins from these localities is 3.55 ± 0.05 Myr based on a new 40Ar/39Ar age from K-feldspar dating of grains in a detrital sandstone (see Supplementary Information, Supplementary Fig. 1 and Supplementary Data 1 for details). The entire stratigraphical sequence exhibits normal paleomagnetic polarity, suggesting that the hominins are 3.596–3.330 Myr (chron C2An.3n). Panels b,c were modified from Google Earth (Data SIO, NOAA. U/S/ Navy, NGA, GEBCO; Image IBCAO; Image Landsat/Copernicus).
Provenience and geological age
Most of the hominin specimens from BRT (BRT-VP-1 and BRT-VP-2) and Waytaleyta (WYT-VP-2) are surface finds that have weathered out of sandstone horizons along an approximately 30-m-thick section above a thin tuff, which has been radiometrically dated to 3.469 ± 0.008 million years ago (Ma)1,2 (Fig. 1, Supplementary Fig. 1 and Supplementary Data 1). Further detail is provided in Supplementary Information. Within this section, multiple fossiliferous sandstone horizons have been identified. The uppermost sandstone at BRT-VP-2, approximately 24 m above the dated tuff, yielded the BRT foot (BRT-VP-2/73). A lateral continuation of this sandstone is also exposed at the nearby WYT-VP-2 locality, where one of the paratype specimens of A. deyiremeda (WYT-VP-2/10) was found, less than 1 km southeast of the BRT foot. Other specimens from BRT-VP-2 and BRT-VP-1 (which samples the same section as BRT-VP-2 but goes slightly deeper) described in this and previous studies were recovered from a slope below the uppermost sandstone (green circles in Fig. 1c), suggesting that they probably eroded out from the upper sandstone. Only one hominin specimen at BRT-VP-1 was found at the lower level below two layers of sandstone horizons (blue circle in Fig. 1c) and it may have weathered out of any of the sandstones above it. The minimum age of the hominins from BRT-VP-1, BRT-VP-2 and WYT-VP-2 is 3.33 million years (Myr), as determined by paleomagnetic data1,2 (Supplementary Information). Therefore, they are close in age to the nearby A. afarensis-bearing localities such as Leado Dido’a (LDD-VP-1; 3.42–3.330 Myr)20 and Nefuraytu (NFR-VP-1; 3.330–3.207 Myr)21, which are located 4.5–5 km to the south and east, respectively (Fig. 1b).
Comparative description
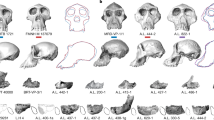
A total of 25 hominin specimens, mostly isolated teeth, have been recovered from three BRT localities (BRT-VP-1, BRT-VP-2 and BRT-VP-3) thus far. Fourteen of these specimens, including the holotype and paratypes of A. deyiremeda, were reported in Haile-Selassie et al.2. Continued fieldwork since 2015 resulted in the discovery of additional, mostly dentognathic specimens (Table 1). A brief description of these specimens is provided in the Supplementary Information (also see Extended Data Figs. 1 and 2). One of the newly recovered specimens is a juvenile mandible, BRT-VP-2/135 (Fig. 2), which most likely eroded out from the same stratigraphic horizon as the BRT foot (BRT-VP-2/73; Fig. 1d). It was recovered approximately 300 m northeast of BRT-VP-2/73, and the difference in developmental age and distance between the two specimens shows that they did not belong to the same individual.
a, Micro-CT scan rendering showing the occlusal view of the mandible. Tooth crowns are shown in white and preserved roots in yellow. b, Left lateral view of the mandible. c, Left lateral view of the mandible with crowns, roots and unerupted permanent dentition visible. d, Right lateral view of the mandible. e, Right lateral view of the mandible with crowns, roots and unerupted permanent dentition visible. Occlusal and labial or buccal views of permanent dentition are shown. f, Left I1. g, Left I2. h, Left P3 (mesial view included). i, Left P4. j, Left M1. k, Right M1. l, Right P4. m, Right P3 (mesial view included). n, Right canine. o, Right I2. p, Right I1.
BRT-VP-2/135 consists of most of the corpus and a near-complete deciduous dentition, with the deciduous incisors (di1–2) having already been exfoliated. The mandible also retains a total of 12 permanent teeth: the right and left incisors (I1 and I2; both erupting); the right canine still in the crypt and isolated mesial half of the left canine; the right and left third and fourth premolars (P3 and P4) still in the crypt; and the right and left first molars (M1s). Both M1s show slight wear facets on the protoconid and hypoconid, indicating that they were in occlusion with the maxillary teeth even though their root apices are still open. This mixed dentition allows an evaluation of the pattern of dental development as well as an estimate of the age of death of BRT-VP-2/135. When compared with other infant-to-juvenile hominin mandibular specimens at the same M1 developmental state (the root three-quarters complete), the developmental status of BRT-VP-2/135 is similar to other early australopiths, specifically LH 3 (A. afarensis) and KNM-KP 34725 (A. anamensis), both of which evince a great ape-like pattern of relatively delayed incisor formation22 (Extended Data Fig. 3 and Supplementary Table 1). An age at death estimate for BRT-VP-2/135 matches a chimpanzee-equivalent age of 4.47 years (range of 3.48–5.46 years), which is similar to age at death estimates for geologically younger hominins from South Africa including DNH 107 (4.82 years; Paranthropus robustus), Sts 24 (4.35 years; Australopithecus africanus) and StW 151 (4.65 years; early Homo) and to the geologically older KNM-KP 34725 (3.94 years; A. anamensis) from eastern Africa23. Details are provided in Supplementary Information.
The base of the BRT-VP-2/135 corpus and cortical bone in the symphyseal region are both missing. The lateral face of the corpus on the left side, however, is well preserved, showing the absence of the lateral corpus hollow (Fig. 2b), which is present in and diagnostic of both juvenile and adult mandibles of A. afarensis24. BRT-VP-2/258, a left edentulous mandible recovered during the 2025 field season, is about the same ontogenetic age and size of BRT-VP-2/135 and it also lacks the lateral corpus hollow (Extended Data Fig. 2l–m). BRT-VP-2/135, as does BRT-VP-2/258, lacks a deciduous canine (dc)–deciduous first molar (dm1) jugum (equivalent of C1–P3 jugum in adults) which is characteristic of A. afarensis14,15. A. africanus juvenile mandibles such as Taung also lack these features. A single mental foramen is positioned low on the corpus at the level of the distal dm2, slightly posterior than in most A. afarensis juveniles where it is positioned at dm1 or dm1–dm2. BRT-VP-2/258 is similar to the A. afarensis condition in this regard where the mental foramen is positioned mid-corpus at the mesial dm2 level. The ascending ramus rises at the posterior M1 level. The symphysis is damaged, although the preserved part shows that it is more posteriorly receding than in A. afarensis juveniles of comparable age.
Deciduous teeth
The left dc, distal half of the left dm1 crown and both dm2s of BRT-VP-2/135 are preserved. The occlusal crown outline of the dc is oval, comparable with the deciduous canines of A. afarensis and A. anamensis25,26. The heavily damaged dm1 crown obscures any taxonomically informative morphology. The left dm2 is preserved intact, whereas the enamel surfaces on the hypoconid, metaconid and mesial face of the right dm2 are missing. The protoconid, hypoconid and hypoconulid of dm2 all have variably sized dentine pits on their cusp tips. Morphologically, the occlusal outline of both dm2s is broadly similar to those of A. afarensis and A. anamensis (there are no dm2s reported for A. ramidus). The dm2s of BRT-VP-2/135 are, however, mesiodistally shorter and much smaller in crown area, falling outside the known range of A. afarensis27 and A. africanus28, but within the known range of A. anamensis29 (Fig. 3a and Supplementary Table 2).
a–f, Box-and-whisker plots of crown area of the dm2 (a), C1 (b), P3 (c), C1:P3 ratio (d), P4 (e) and M1 (f) in A. ramidus, A. anamensis, A. afarensis and A. africanus compared with A. deyiremeda. The box in each plot represents the first to third quartiles of the dimensions, the line inside the box shows the median, and the horizontal lines outside the box represent the maximum and minimum values. The sample size is indicated by n. A statistical summary of the dental metrics used for this figure is provided in Supplementary Table 2 and Supplementary Data 2. The data were compiled from refs. 18,30 (A. ramidus), refs. 26,29 (A. anamensis), refs. 10,20,21,27 (A. afarensis) and ref. 28 (A. africanus).
Incisors
The central incisors of BRT-VP-2/135 are emerging and close to the level of the occlusal plane, whereas the lateral incisors are only slightly beyond alveolar emergence. The incisor crowns are absolutely larger than those of A. ramidus (n = 4)18,30 but fall within the known range of A. anamensis26,29 and A. afarensis10,27.
Canines
The canines of BRT-VP-2/135, generated from high-resolution micro-computerized tomography (micro-CT) visualization, lack prominent lingual relief with only a thin, posteriorly positioned vertical ridge present, demarcating a distally placed vertical lingual groove (Extended Data Fig. 4). This is in stark contrast to the lower canines of A. afarensis that have prominent lingual relief with a centrally positioned thick vertical lingual ridge25 creating prominent mesial and distal vertical lingual grooves (Extended Data Fig. 4d,e). The lack of lingual relief on the canines has been described as characteristic of both upper and lower canines of A. deyiremeda1. The lingual face and wear pattern of an isolated upper canine, BRT-VP-1/380, is also similar to A. anamensis canines such as KNM-KP 58309 and MRD-VP-1/1 in its lingual profile and wear pattern (Extended Data Fig. 5). Generally, the upper and lower canine crowns of A. deyiremeda are absolutely smaller than A. ramidus and other australopiths (see Fig. 4a–c for C1 dimensions). Morphologically, however, the height of the mesial crown shoulders relative to the overall crown height on both upper and lower canines is within the range of variation seen in A. afarensis.
a–f, Box-and-whisker plots showing upper canine mesiodistal (MD) length (a), buccolingual (BL) breadth (b), the square root of MD multiplied by BL (c), M1 MD length (d), BL breadth (e) and the square root of MD multiplied by BL (f) in A. ramidus, A. anamensis, A. afarensis and A. deyiremeda. Note that A. deyiremeda has the smallest upper canines and M1s in the comparative sample. The box in each plot represents the first to third quartiles of the dimensions, the line inside the box shows the median, and the horizontal lines outside the box represent the maximum and minimum values. The sample size is indicated by n. A statistical summary of dental metrics used for this figure is provided in Supplementary Table 3, and the data were compiled from refs. 18,30 (A. ramidus), refs. 26,29 (A. anamensis), refs. 10,20,21,27 (A. afarensis) and ref. 28 (A. africanus).
Premolars
Both P3s of BRT-VP-2/135, also generated from micro-CT visualization, and BRT-VP-1/324 have large anterior foveae and diminutive to absent metaconids. Furthermore, in both left and right P3s of BRT-VP-2/135 and BRT-VP-1/324, the protoconid is positioned centrally and the transverse crest is oriented distolingually, whereas in A. afarensis, the protoconid apex is positioned more mesially in most P3s even though some specimens such as A.L. 128-23 and A.L. 288-1i have centrally positioned protoconid apices (Extended Data Fig. 6). However, these specimens—like all other known A. afarensis P3s—possess a transverse crest that forms less than 90° with the mesial protoconid crest (64.3–88.6° (n = 12) with an average of 76° and standard deviation of 7.6°), whereas in A. ramidus, A. anamensis and the P3s of BRT-VP-2/135, it is more than 90° (Extended Data Fig. 6). In the P3s from BRT, the mesial marginal ridge is also weak, and when viewed mesially, the anterior fovea is diamond shaped because of the steeply sloped buccal and weakly developed lingual segments of the mesial marginal ridge (Fig. 2h,m). All of these features are frequently seen in the P3s of A. anamensis from Kanapoi as well as in Pan troglodytes31. The P3 of the paratype mandible of A. deyiremeda (BRT-VP-3/14) also has a weakly developed metaconid, poorly definable lingual segment of the mesial marginal ridge whose mesial and distal segments are angled to the base of the crown, and distolingually oriented transverse ridge (Extended Data Fig. 6b). It is also high-crowned relative to its mesiodistal dimension, similar to the BRT-VP-2/135 P3s. Although substantial intraspecific variation in the P3 crown shape and occlusal morphology has been documented in A. anamensis and A. afarensis27,29, as well as in A. ramidus30, a few features distinguish the P3s of these two species. For example, the P3s of early A. anamensis from Kanapoi have relatively large anterior fovea and the metaconid is either absent or incipient when present26. Such intraspecific variation is also observed in the P3s from BRT. Overall, however, the occlusal morphology and shape of the BRT P3s are more similar to those of A. ramidus and A. anamensis than of A. afarensis (Extended Data Fig. 6). In terms of crown size, the P3s from BRT are on the lower size range of australopiths but larger than those of A. ramidus (Fig. 3c). However, the latter species has larger canines relative to the size of the P3 (Fig. 3d).
The P4 crowns of BRT-VP-2/135 (also generated from micro-CT visualization) and other isolated P4s (for example, BRT-VP-1/120; Extended Data Fig. 6b) have an oval occlusal crown outline, similar to A. ramidus and most A. anamensis P4s (for example, KNM-KP 53160, KNM-KP 29286 and KNM-ER 20342). Another P4, BRT-VP-1/363, is more likely the same individual as BRT-VP-1/2 based on its provenience and matches the latter in its preserved occlusal surface (Extended Data Figs. 1c and 2f). The preserved P4 on one of the paratypes of A. deyiremeda (BRT-VP-3/14) also appears to have had an oval outline even though it is moderately damaged. By contrast, most A. afarensis P4s have either a rhomboidal or square occlusal outline (Extended Data Fig. 6). In terms of their crown size, however, the BRT P4s fall within the known range of A. anamensis, A. afarensis and A. africanus (Fig. 3e and Supplementary Data 2). BRT-VP-2/89 (Extended Data Fig. 1j) is a complete P4 crown germ that is morphologically indistinguishable from the previously reported P4 (BRT-VP-3/37) of A. deyiremeda1 and the P4s of A. anamensis and A. afarensis. Its preserved crown dimensions are much larger than A. ramidus30 but fall within the range of A. anamensis26 and A. afarensis10 (Extended Data Fig. 1j and Supplementary Data 2).
Molars
Upper and lower molars of mid-Pliocene australopiths do not show significant metric and morphological variation. The size of two measurable M1s from BRT fall within the known range of Australopithecus species but larger than those of A. ramidus (Fig. 3f). This was also the case with the molars associated with the paratype mandible of A. deyiremeda1. However, upper molars of the holotype of A. deyiremeda (BRT-VP-3/1) have been shown to be smaller than other australopiths1. BRT-VP-1/365 is one of the newly found M1s and its size is only slightly larger than the M1 of BRT-VP-3/1 (Fig. 4d–f and Supplementary Data 2). Both molars are also buccolingually narrower than all known australopiths (Fig. 4e). This is one of the diagnostic features of A. deyiremeda.
Postcranial evidence
Two postcranial specimens have been recovered from BRT-VP-2. One of these is the partial foot (BRT-VP-2/73) previously described by Haile-Selassie et al.2. The second specimen (BRT-VP-2/87) is a right ischium of a juvenile individual found from the same locality and horizon as the partial foot. A detailed description of BRT-VP-2/87 is provided in Supplementary Information (see also Extended Data Figs. 7 and 8). The partial foot displays morphological features that suggest a greater ability for grasping than modern humans and most fossil hominins2,32. These features include relatively long, sagittally curved pedal proximal phalanges; increased diaphyseal torsion and curvature in the transverse plane curvature of the second metatarsal (MT2); increased curvature in the sagittal plane of the MT1 diaphysis; increased mediolateral curvature of the MT1 proximal articular facet; and a gracile hallucal proximal phalanx with diaphyseal curvature also in the sagittal plane. This is in stark contrast to the foot morphology of A. afarensis33 and provides further evidence for Pliocene hominin locomotor diversity (see Supplementary Information for further discussion).
The divergent hallux of A. ramidus (ARA-VP-6/500)34 clearly differs from the comparatively more adducted, less mobile, hallux of A. afarensis, principally based on the morphology of the medial cuneiform (A.L. 333-28) and its articular relationship with the MT1 (A.L. 333-54). The MT1s assigned to A. ramidus from Aramis (ARA-VP-6/500-089)34 and As Duma (GWM67/P2k)35 display paired articular facets on the dorsal side of the bone divided by what Lovejoy et al.34 refer to as a nonsubchondral isthmus (Extended Data Fig. 9). The expression of this MT1 head articular surface variation is hypothesized to reflect a divergent hallux as observed among extant African apes with arboreal foot-grasping adaptations34. Although there are subtle differences between the MT1 heads of the Aramis and As Duma specimens, including slightly increased dorsal doming in the latter, the BRT MT1 head is derived towards later hominins in lacking a nonsubchondral isthmus combined with mild dorsal doming and a more mediolaterally continuous MT1 head contour that bears resemblance to StW 595 (A. africanus) from Sterkfontein8. The MT2 of the BRT foot is also morphologically similar to StW 89 (refs. 2,32), another presumed A. africanus specimen from Sterkfontein. A multivariate analysis of 16 MT1 and fourth proximal phalanx (PP4) variables preserved across the partial feet of early hominins demonstrates that the A. afarensis (A.L. 333-115) and the BRT foot are both derived in the direction of modern humans relative to A. ramidus (ARA-VP-6/500). The placement of the BRT foot in the multivariate analysis is consistent with our observations of its more derived MT1 morphology than A. ramidus (Fig. 5).
a, The first three dimensions of the linear discriminant (LD1–3) analysis represent 98.9% of the sample variance. b, BRT-VP-2/73 has a relatively shallow MT1 head similar to ARA-VP-6/500 and African apes, and unlike A.L. 333-115 and Homo sapiens. c, BRT-VP-2/73 has a relatively wide MT1 head, overlapping with H. sapiens, whereas A.L. 333-115 and ARA-VP-6/500 have narrower, more African ape-like MT1 heads. d, The relative length of the BRT-VP-2/73 PP4 is reduced compared with ARA-VP-6/500 and A.L. 333-115, falling at the low end of the Gorilla distributions. e, The relative depth of the PP4 trochlea is reduced in BRT-VP-2/73 compared with ARA-VP-6/500 and A.L. 333-115. All linear distances are scaled by the geometric mean. The box-and-whisker plots display the median, interquartile range and range, along with individual values. The star and dashed vertical line indicate the values for BRT-VP-2/73, whereas the black circles are the other fossil hominins in panels b–e.
The presence of metatarsal head dorsal doming—albeit minimal—and non-hallucal proximal phalanges with dorsally oriented proximal articular facets indicate an increased range of dorsiflexion at the metatarsophalangeal joint, which occurs during push-off among plantigrade bipeds36 and digitigrade quadrupeds33. The combination of a relatively long MT4 and short MT1 implies that the oblique axis of the foot might have been used during the push-off period of the bipedal stance phase of the BRT hominin2,37. Although the torsion of MT4 of the BRT foot is consistent with the presence of a transverse arch that enhances midfoot stiffness during push-off38, its diaphysis is relatively long and slender and it preserves features that do not align with a rigid midfoot, such as a dorsal portion of the proximal articular surface that is mediolaterally curved, and a diaphysis that lacks a plantar orientation relative to its base. Likewise, the distal end displays a dorsomedial metatarsal head ridge that recalls the condition observed in some gorillas. Collectively, these lateral forefoot and midfoot traits suggest that the BRT hominin lacked the structural longitudinal arch and derived push-off morphology of the A. afarensis specimen A.L. 333-160 (ref. 39) and might have retained some midfoot mobility useful for climbing32.
Taxonomy and phylogenetic relationships
A. afarensis and A. deyiremeda have been identified from 3.5 to 3.3 Myr localities at Woranso-Mille1,20,21. The specimens assigned to A. deyiremeda derive from one of the localities at BRT (BRT-VP-3) and a slightly younger locality (WYT-VP-2), providing an age to the species between 3.5 and 3.3 Myr1. The specimens from BRT-VP-1 and BRT-VP-2 described here and in Haile-Selassie et al.1,2 are from a horizon that is contemporaneous with WYT-VP-2 (3.47–3.33 Myr) where one of the paratype specimens of A. deyiremeda has been found. Furthermore, most of the newly recovered dental and mandibular remains retain morphological features that are diagnostic of A. deyiremeda. Among the dentognathic features shared with A. deyiremeda are premolars with more primitive occlusal morphology compared with the contemporaneous A. afarensis, canines with less lingual relief, small M1s that are buccolingually narrow, mandibular body lacking lateral corpus hollow, and an anteriorly positioned ascending ramus root. The new dentognathic remains described here derive from the same stratigraphical unit as, and in close spatial proximity to, BRT-VP-2/73 (Fig. 1), suggesting that the latter can be confidently assigned to the same species. The absence of compelling evidence for the presence of more than one hominin species at the BRT localities also supports this assignment. The primitive aspects of the BRT foot could suggest a taxonomic attribution to a late-surviving species of Ardipithecus, but our understanding of the foot morphology of Pliocene hominins such as A. ramidus and A. afarensis is far from complete, and the pedal remains of A. anamensis are virtually unknown except for a fragmentary metatarsal shaft and an eroded distal phalanx from Asa Issie40.
The inclusion of the BRT foot in A. deyiremeda further demonstrates that the latter species is also more primitive in its foot morphology than A. afarensis. Conversely, some aspects of the MT1 and MT2 of BRT-VP-2/73 are similar to A. africanus from Sterkfontein than to A. afarensis. A. deyiremeda and A. africanus have also previously been shown to be similar in maxillary shape than either is to A. afarensis41,42. A parsimony analysis to assess the phylogenetic position of A. deyiremeda has previously shown that it is a sister to a clade containing A. africanus, Paranthropus and Homo1 with some dentognathic homoplasy. The inclusion of BRT-VP-2/73 in the A. deyiremeda hypodigm and its morphological similarity in some aspects of its morphology to A. africanus further highlights close relationship between these two taxa.
Diet in A. deyiremeda
Habitat reconstruction of Pliocene hominins using different paleontological and geological proxies have shown that different hominins lived in a range of habitats. Although morphological approaches have traditionally been used for dietary reconstructions43,44, carbon isotope data have been shown to provide a refined dietary signal43,44,45,46,47, particularly powerful when the number of dental specimens is limited. Among Pliocene hominins, dietary adaptations of A. deyiremeda remain unknown even though two previously analysed isolated hominin teeth from BRT-VP-1 yielded δ13C values < −10‰, indicative of a reliance on C3 foods47. For this study, an additional six A. deyiremeda teeth from the two BRT localities (BRT-VP-1 and BRT-VP-2) were sampled for their isotopic composition. Combined with previously published data from isolated hominin teeth from BRT-VP-1 (n = 2), presumably belonging to A. deyiremeda47, the mean δ13C value for the BRT teeth is −10.2 ± 1.2‰ and range from −12.4 to −8.8‰ (n = 8; Table 2 and Fig. 6a). Compared with other mammal teeth sampled for isotopic study from these localities (n = 10), the hominins yield some of the lowest δ13C values and indicate a diet dominated by C3 resources, despite the presence of other mammals that depended on either a mix of C3–C4 resources (for example, Theropithecus oswaldi cf. darti) or pure C4 graze (Notochoerus euilus; Fig. 6b).
a, δ13C values of new A. deyiremeda teeth from BRT-VP-1 and BRT-VP-2 plotted with published data from other early and middle Pliocene hominins from eastern Africa. b, Other mammal teeth analysed from BRT-VP-1 and BRT-VP-2. For panel a, δ13C data came from the following sites and studies: for A. ramidus, Aramis, Sagantole Fm48; for A. anamensis, Turkana (Koobi Fora Formation and Kanapoi Fms)44; for A. afarensis, Hadar Fm at Hadar and Dikika46 and from two sites at Woranso-Mille (LDD and NFR)47; and for K. playtops, Nachukui Fm44. The hominin data from other study areas are plotted using the compilation by Levin et al.47. The previously published data from other mammal teeth analysed from BRT-VP-1 and BRT-VP-2 also came from ref. 47. The boxplots represent median values with the middle line, and the interquartile ranges as the horizontal edges of the box, and the minimum and maximum values are plotted as lines extending from the boxes, with outliers plotted outside these lines. Jitter points are plotted to visualize the distribution of all data points and the number of analyses. Only single points are plotted for cases where n = 1. VPDB, Vienna Pee Dee Belemnite international reference standard.
Compared with other early and mid-Pliocene hominins at Woranso-Mille and elsewhere in the Afar, and from eastern Africa more broadly, the δ13C values from the BRT hominin teeth are indistinct from distributions of δ13C values from A. ramidus and A. anamensis teeth, but distinct from δ13C values for A. afarensis and Kenyanthropus playtops (Table 3 and Fig. 6a). A. anamensis (n = 12)44,45,46 and earlier hominins such as A. ramidus (n = 5)48 relied mainly on C3 food resources even though A. anamensis may also have consumed a substantial amount of C4 resources49. Species such as A. afarensis and Kenyanthropus platyops utilized a wide range of both C3 and C4 resources44,46. The lower δ13C values of the BRT hominin teeth, with more limited distributions, and their similarity to those from A. ramidus and A. anamensis, indicate that the BRT hominins may have retained dietary strategies of these older (more primitive) hominins, corroborating morphological observations.
Discussion and conclusion
Newly discovered 3.47–3.33 Myr fossil hominin specimens from mid-Pliocene Woranso-Mille have been described. Comparative analysis of these mostly dentognathic remains shows that they can confidently be assigned to A. deyiremeda, based on features diagnostic of the species (for example, lack of lingual relief on both upper and lower canines and relatively smaller teeth). Although A. deyiremeda appears to be more derived than A. afarensis in certain maxillary (low, anterior zygomatic origin)1,41,42 and mandibular traits (lack of lateral corpus hollow)1, this study found that its premolar occlusal morphology (oval P4 outline and reduced or absent P3 metaconid) resembles the earlier A. anamensis and A. ramidus, indicating that it is dentally more primitive than A. afarensis.
Enamel isotope analysis also suggests that A. deyiremeda had a C3-dominated diet, similar to A. anamensis and A. ramidus. The BRT partial foot (BRT-VP-2/73) is assigned to A. deyiremeda due to its temporal and spatial association with dentognathic materials assigned to the species. This assignment provides the most compelling evidence for multiple bipedal adaptations in Australopithecus, despite shared postcanine megadontia. Although the exact timing of dental enlargement in australopiths remains uncertain, it probably reflects adaptation to changing environments and diverse dietary niches. Previous studies have highlighted variability in early australopith foot morphology, indicating diverse bipedal forms, each with variable pelvic, limb and foot morphology50. Current evidence has suggested that some species—such as A. deyiremeda, A. africanus and Australopithecus sediba—remained capable climbers, whereas others such as A. afarensis were more terrestrial. The mosaic craniodental and postcranial morphology of A. deyiremeda raises questions about skeletal integration, and confirms that postcanine megadontia and obligate, human-like bipedality did not evolve simultaneously. The origin of Australopithecus correlates with increased craniodental size—possibly linked to selection for smaller canines and premolar molarization—preceding full obligate bipedality.
Although mid-Pliocene hominin paleobiology and locomotion remain incompletely understood40, the BRT foot confirms the presence of multiple bipedal forms during the Pliocene. Further postcranial fossil discoveries, particularly of early species such as A. anamensis, will be crucial to fully understanding the origins of obligate human-like bipedality.
Methods
Micro-CT scanning and visualization protocols
The BRT mandible (BRT-VP-2/135) was scanned (with permit from the Ethiopian Heritage Authority to Y.H.-S.) using a GE v|tome|x L300 industrial multiscale micro-CT scanner at the Pennsylvania State University Center for Quantitative Imaging with the following scan parameters: 150 kV, 200 μA, 0.2-mm Al filter, 2,400 projections, 5 frames averages per projection, detector timing of 500 ms and reconstructed with an isotropic voxel size of 32.5 μm. The data were segmented and a three-dimensional isosurface of the unerupted teeth and external surface of the mandible were created in Avizo v9.5 (Thermo Fisher Scientific). Enamel and dentine surfaces of erupted teeth were segmented using automated thresholding, whereas dental tissue boundaries for the unerupted dentition were segmented manually. All dental tissue boundaries (enamel, dentine and pulp) were manually traced approximately every ten slices and filled in using the interpolation tool. These interpolated regions were then visually inspected for boundary integrity and continuity and manually edited if needed. Cross-sectional images of each tooth were generated in Avizo v9.5 (Thermo Fisher Scientific) using the slice tool so that each cross-section captured the maximum extent of the dentine horn as close to the cusp tip as possible. Surface models of each tooth were exported and processed (spikes and small holes removed) in GeoMagic Wrap 2017.
Enamel isotope sampling
Enamel from isolated fossil teeth sampled with a hand-held dental drill and diamond-burred bits, targeting broken surfaces where possible. Sample powders were treated with 3% hydrogen peroxide for 15 min and then with 0.1 M buffered acetic acid, rinsing three times after each treatment, and then dried at 60 °C overnight before analysis. Treated powders were then analysed on Kiel IV (15 min reaction in H3PO4 at 75 °C), coupled to a Thermo Fisher MAT 253 mass spectrometer at the University of Michigan Stable Isotope Laboratory and calibrated using calcite standards. Oxygen isotope values were calculated assuming a temperature-dependent acid fractionation factor for fossil teeth51. External precision of working enamel standards during the analysis were less than 0.4‰ and less than 0.15‰ for δ13C and δ18O values, respectively. Isotopic data are reported relative to the Vienna Pee Dee Belemnite standard.
Statistics were calculated in R (2024.04.2 + 764). The ± symbol indicates standard deviation. Statistics of difference were calculated using the unpaired Wilcoxon–Mann–Whitney rank-sum test, with significant distinctions set with P = 0.05.
Multivariate analysis of partial feet
Linear discriminant analysis was used to test whether extant taxa (H. sapiens n = 52, P. troglodytes n = 39, Pan paniscus n = 18, Gorilla gorilla n = 30, Gorilla beringei n = 31 and Pongo spp. n = 22) could be separated using 16 MT1 and PP4 variables preserved among BRT-VP-2/73, A.L. 333-115 and ARA-VP-6/500. The variables include 5 MT1 variables (midshaft mediolateral width, midshaft dorsoplantar depth, dorsal head width, plantar head width and head dorsoplantar depth) and 11 PP4 variables (length, maximum dorsoplantar depth of base, dorsoplantar depth of proximal articular facet, maximum mediolateral width of base, mediolateral width of the proximal articular facet, midshaft mediolateral width, midshaft dorsoplantar depth, mediolateral width of trochlea, dorsoplantar depth of trochlea, diaphyseal curvature and a dorsal canting ratio). Diaphyseal curvature was calculated using measurement photographs, and the dorsal canting ratio is the ratio of the dorsal interarticular length to the plantar interarticular length. All linear measurements were scaled by a geometric mean composed of 14 measurements per individual. The pooled covariance matrix of the 16 variables was analysed using linear discriminant analysis in R v4.4.0.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data used in this article are presented in the Supplementary Information. Request to access the three-dimensional scans of specimens included in this article should be sent to the Ethiopian Heritage Authority at yonasyilma2627@gmail.com.
References
Haile-Selassie, Y. et al. New species from Ethiopia further expands middle Pliocene hominin diversity. Nature 521, 483–488 (2015).
Haile-Selassie, Y. et al. A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature 483, 565–569 (2012).
Stern, J. T. Jr & Susman, R. L. The locomotor anatomy of Australopithecus afarensis. Am. J. Phys. Anthropol. 60, 279–317 (1983).
Clarke, R. J. & Tobias, P. V. Sterkfontein member 2 foot bones of the oldest South African hominid. Science 269, 521–524 (1995).
Zipfel, B. et al. The foot and ankle of Australopithecus sediba. Science 333, 1417–1420 (2011).
DeSilva, J. M., Proctor, D. J. & Zipfel, B. A complete second metatarsal (StW 89) from Sterkfontein member 4, South Africa. J. Hum. Evol. 63, 487–496 (2012).
McNutt, E. J. et al. Footprint evidence of early hominin locomotor diversity at Laetoli, Tanzania. Nature 600, 468–471 (2021).
Leakey, M. G. et al. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature 410, 433–440 (2001).
White, T. Early hominids — diversity or distortion? Science 299, 1994–1997 (2003).
Kimbel, W. H., Rak, Y. & Johanson, D. C. The Skull of Australopithecus afarensis (Oxford Univ. Press, 2004).
Kimbel, W. H. et al. Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. J. Hum. Evol. 51, 134–152 (2006).
McNutt, E. J., Zipfel, B. & DeSilva, J. M. The evolution of the human foot. Evol. Anthropol. 27, 197–217 (2018).
Wood, B. & Boyle, E. K. Hominin taxic diversity: fact or fantasy? Am. J. Phys. Anthropol. 159, 37–78 (2016).
Alemseged, Z. Reappraising the palaeobiology of Australopithecus. Nature 617, 45–54 (2023).
Harcourt-Smith, W. E. & Aiello, L. C. Fossils, feet and the evolution of human bipedal locomotion. J. Anat. 204, 403–416 (2004).
DeSilva, J. M. et al. Australopithecus sediba — the anatomy of the lower limb skeleton of Australopithecus sediba. Paleoanthropology 2018, 357–405 (2018).
DeSilva, J. M., McNutt, E. J. & Zipfel, B. in The Evolution of the Primate Foot: Anatomy, Function, and Palaeontological Evidence (eds Zeininger, A. et al.) 361–385 (Springer, 2022).
White, T. D., Lovejoy, C. O., Asfaw, B., Carlson, J. P. & Suwa, G. Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proc. Natl Acad. Sci. USA 112, 4877–4884 (2015).
Prang, T. C., Ramirez, K., Grabowski, M. & Williams, S. A. Ardipithecus hand provides evidence that humans and chimpanzees evolved from an ancestor with suspensory adaptations. Sci. Adv. 7, eabf2474 (2021).
Melillo, S. M. et al. New Pliocene hominins from the Leado Dido’a area of Woranso-Mille, Ethiopia. J. Hum. Evol. 153, 102956 (2021).
Haile-Selassie, Y. et al. Dentognathic remains of Australopithecus afarensis from Nefuraytu (Woranso-Mille, Ethiopia): comparative description, geology, and paleoecological context. J. Hum. Evol. 100, 35–53 (2016).
Dean, M. C., Lim, S. Y. & Liversidge, H. M. Patterns of permanent incisor, canine and molar development in modern humans, great apes and early fossil hominins. Arch. Oral Biol. 143, 105549 (2022).
Smith, T. M. et al. Dental ontogeny in Pliocene and early Pleistocene hominins. PLoS ONE 10, e0118118 (2015).
Glowacka, H., Kimbel, W. H. & Johanson, D. C. in Human Paleontology and Prehistory: Vertebrate Paleobiology and Paleoanthropology (eds Marom, A. & Hovers, E.) 127–144 (Springer, 2017).
Johanson, D. C., White, T. D. & Coppens, Y. Dental remains from the Hadar Formation, Ethiopia: 1974–1977 collections. Am. J. Phys. Anthropol. 57, 545–603 (1982).
Ward, C. V., Leakey, M. G. & Walker, A. Morphology of Australopithecus anamensis from Kanapoi and Allia Bay, Kenya. J. Hum. Evol. 41, 255–368 (2001).
White, T. D. New fossil hominids from Laetoli, Tanzania. Am. J. Phys. Anthropol. 46, 197–230 (1977).
Moggi-Cecchi, J., Grine, F. E. & Tobias, P. V. Early hominid dental remains from member 4 and 5 of the Sterkfontein Formation (1966–1996 excavations): catalogue, individual associations, morphological descriptions and initial metrical analysis. J. Hum. Evol. 50, 239–328 (2006).
Ward, C. V., Manthi, F. K. & Plavcan, J. M. New fossils of Australopithecus anamensis from Kanapoi, West Turkan, Kenya (2003–2008). J. Hum. Evol. 65, 501–524 (2013).
Suwa, G. et al. Paleobiological implications of the Ardipithecus ramidus dentition. Science 326, 69–99 (2009).
Delezene, L. K. & Kimbel, W. H. Evolution of the mandibular third premolar crown in early Australopithecus. J. Hum. Evol. 60, 711–730 (2011).
DeSilva, J., McNutt, E., Benoit, J. & Zipfel, B. One small step: a review of Plio-Pleistocene hominin foot evolution. Am. J. Phys. Anthropol. 168, 63–140 (2019).
Latimer, B. & Lovejoy, C. O. Metatarsophalangeal joints of Australopithecus afarensis. Am. J. Phys. Anthropol. 83, 13–23 (1990).
Lovejoy, C. O., Latimer, B., Suwa, G., Asfaw, B. & White, T. D. Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science 326, 72–72.e8 (2009).
Simpson, S. W., Levin, N. E., Quade, J., Rogers, M. J. & Semaw, S. Ardipithecus ramidus postcrania from the Gona Project area, Afar regional state, Ethiopia. J. Hum. Evol. 129, 1–45 (2019).
Fernández, P. J. et al. Evolution and function of the hominin forefoot. Proc. Natl Acad. Sci. USA 115, 8746–8751 (2018).
Latimer, B. & Lovejoy, C. O. Hallucal tarsometatarsal joint in Australopithecus afarensis. Am. J. Phys. Anthropol. 82, 125–133 (1990).
Venkadesan, M. et al. Stiffness of the human foot and evolution of the transverse arch. Nature 579, 97–100 (2020).
Ward, C. V., Kimbel, W. H. & Johanson, D. C. Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science 331, 750–753 (2011).
White, T. D. et al. Asa Issie, Aramis and the origin of Australopithecus. Nature 440, 883–889 (2006).
Hanegraef, H., Leakey, M. G., Leakey, L. N. & Spoor, F. Mid-Pliocene hominin diversity revisited. C. R. Paleovol. 23, 453–464 (2024).
Spoor, F., Leakey, M. G. & O’Higgins, P. Middle Pliocene hominin diversity: Australopithecus deyiremeda and Kenyanthropus platyops. Phil. Trans. R. Soc. B 371, 20150231 (2016).
Bobe, R., Manthi, F. K., Ward, C. V., Plavcan, J. M. & Carvalho, S. The ecology of Australopithecus anamensis in the early Pliocene of Kanapoi, Kenya. J. Hum. Evol. 140, 102717 (2020).
Cerling, T. E. et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc. Natl Acad. Sci. USA 110, 10501–10506 (2013).
Sponheimer, M. et al. Isotopic evidence of early hominin diets. Proc. Natl Acad. Sci. USA 110, 10513–10518 (2013).
Wynn, J. G. et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc. Natl Acad. Sci. USA 110, 10495–10500 (2013).
Levin, N. E., Haile-Selassie, Y., Frost, S. R. & Saylor, B. Z. Dietary change among hominins and cercopithecids in Ethiopia during the early Pliocene. Proc. Natl Acad. Sci. USA 112, 12304–12309 (2015).
White, T. D. et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326, 67–93 (2009).
Quinn, R. L. Isotopic equifinality and rethinking the diet of Australopithecus anamensis. Am. J. Phys. Anthropol. 169, 403–421 (2019).
Prang, T. C. The subtalar joint complex of Australopithecus sediba. J. Hum. Evol. 90, 105–119 (2016).
Passey, B. H., Cerling, T. E. & Levin, N. E. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun. Mass Spectrom. 21, 2853–2859 (2007).
Haile-Selassie, Y. et al. A 3.8-million-year-old hominin cranium from Woranso-Mille, Ethiopia. Nature 573, 214–219 (2019).
Acknowledgements
We thank the Ethiopian Heritage Authority of the Ethiopian Ministry of Tourism for permission to conduct field and laboratory work; the Afar people of Woranso-Mille and the Mille District administration for their hospitality; M. Mengesha for assisting in the geological work at BRT; the project’s fieldwork crew members for their tireless support of field activities; T. White, G. Suwa and B. Asfaw for access to the original A. ramidus material and for providing images; C. Campisano for access to unpublished A. afarensis material; S. Melillo, C. Taylor, A. Slotter and D. Bernardoni for fossil recovery; S. Melaku of the Ethiopian Heritage Authority for access to the fossil collections housed in the Paleoanthropology Laboratory in Addis Ababa and facilitating isotope sampling; and T. Ryan and T. Stecko from the Penn State Center for Quantitative Imaging for assistance with computed tomography scanning. N.E.L. thanks K. Lohmann at the University of Michigan for assistance with isotope analysis. This research was supported by previous grants from the US National Science Foundation (BCS-1124705, BCS-1124713, BCS-1124716, BCS-1125157 and BCS-1125345) and The W. M. Keck Foundation.
Author information
Authors and Affiliations
Contributions
Y.H.-S., B.Z.S., A.D., L.G. and N.E.L. conducted the fieldwork and collected primary data. Y.H.-S., G.T.S. and T.C.P. did the comparative description of the hominins. A.R. performed the three-dimensional segmentation. N.E.L. carried out the isotopic analysis. A.D., L.G., B.Z.S. and N.E.L. worked on the stratigraphy, geochronology and magnetostratigraphy. All co-authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Thure Cerling and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Dental specimens from BRT-VP-1 and BRT-VP-2.
a, BRT-VP-1/234 in occlusal, mesial, and distal views. b, BRT-VP-1/256 in occlusal, lingual and mesial views. c, BRT-VP-1/363 in occlusal, distal, lingual, and mesial views. d, BRT-VP-1/365 in occlusal, mesial, distal, lingual, and buccal views. e, BRT-VP-1/1 in occlusal view. f, BRT-VP-1/2 in occlusal and buccal views. g, BRT-VP-1/13 in occlusal, lingual, buccal, mesial, and distal views. h, BRT-VP-1/18 in occlusal and mesial views. i, BRT-VP-1/118 in occlusal view. j, BRT-VP-2/89 in occlusal and mesial views. k, BRT-VP-1/324 in occlusal, buccal, lingual, mesial, and distal views. l, BRT-VP-1/380 in occlusal, buccal, lingual, mesial, and distal views. Specimens e-j were listed in Table 1 in Haile-Selassie et al.1. Their brief description is provided in Supplementary Information.
Extended Data Fig. 2 Cranial, dental and mandibular specimens from BRT-VP-1 and BRT-VP-2.
BRT-VP-2/10-150 in ectocranial (a), endocranial (b), anterior (c), and posterior (d) views. BRT-VP-1/345 in occlusal (e), buccal (f), and lingual (g) views. BRT-VP-1/271 in occlusal (h), lingual (i), buccal (j), and anterior (k) views. BRT-VP-2/258 in occlusal (l), buccal (m), and lingual (n) views. BRT-VP-2/200 in ectocranial (o), endocranial (p), posterior (q), and anterior (r) views.
Extended Data Fig. 3 Fractional stages of mandibular incisor formation.
This figure (adapted from ref. 22) shows the frequency distribution of the fractional stages of mandibular central incisor (crown & root) formation in extant great ape and modern human individuals where the M1 is at stage R¾. Aside from fractional staging, the crown, root, or the root apex of the incisor can be initiating (“i”), complete (“c”), or closed (“cl”). Relative to M1 development, gracile (i.e., chronologically older) australopiths are delayed in incisor formation relative to chronologically younger early Homo. Incisor development being at least one half complete (i.e., R½ or less) characterizes all great apes and occurs in only 20.4% of all modern humans sampled. Central incisors of BRT-VP2 (orange polygon) are between R¼ and R½ (slightly closer to R½) which, among early hominins that died with their first molar roots three-quarters formed, is most similar to specimens from Laetoli (LH3; Au. afarensis) and Kanapoi (KNM-KP 34725; Au. anamensis), and dissimilar to early Homo (StW 151 and KNM-ER 820). Thus, BRT-VP-2/135 evinces a state of mandibular central incisor development observed in a relatively small fraction of modern humans, but one that is common in great apes. Silhouettes were created using PhyloPic (http://phylopic.org): Pan troglodytes by T. M. Keesey under a Creative Commons licence CC BY 3.0; Gorilla gorilla by M. Michaud under a Creative Commons licence CCO 1.0; Pongo by D. J. Ardesch under a Creative Commons licence CCO 1.0; and Homo sapiens by A. Farke under a Creative Commons licence CC BY 3.0.
Extended Data Fig. 4 BRT-VP-2/135 canines compared to Au. afarensis canines.
a, the right canine of BRT-VP-2/135, unreconstructed and reconstructed lingual views, and buccal view. b, occlusal, mesial and distal views of the right canine of BRT-VP-2/135. c, labial, mesial, distal, and lingual views of the preserved distal half of the left canine of BRT-VP-2/135. d, A.L. 400-1b lower canine with prominent vertical lingual ridge (black arrow). e, A.L. 200-1 upper canine with prominent vertical lingual ridge. Note that the BRT-VP-2/135 right canine does not have a prominent lingual ridge. Rather, it has a thin crest-like structure that bounds the distal lingual groove mesially.
Extended Data Fig. 5 Upper canine crown shape and wear pattern.
Black and grey arrows show the length and orientation of the mesial and distal crown shoulders, respectively. Note that the length and orientation of the mesial crown shoulder in BRT-VP-1/380 is similar to Au. afarensis, whereas the distal crown shoulder is short and oriented obliquely as in the Au. anamensis canines. The wear pattern in BRT-VP-1/380 is also similar to Au. anamensis canines where the wear, as shown by the red stars, extends to the lingual face and basal tubercle of the crown. Figure was adapted from ref. 52, Springer Nature.
Extended Data Fig. 6 Early hominin lower premolar morphology.
Relatively unworn lower premolars of Au. afarensis (a), BRT-VP-2/135 (b), Au. anamensis (c), and Ar. ramidus (d). Some P3s of Au. afarensis have more primitive occlusal morphology (e.g., metaconid non-existent or incipient, centrally positioned protoconid (white arrow)). However, like the derived P3s with well-developed metaconid, they are associated with a P4 that has a rhomboidal occlusal outline. The mesial protoconid crest-transverse crest angle is <90° as shown in A.L. 128-23 and A.L. 288-1i. On the other hand, The P3s of BRT-VP-2/135 (b), have diminutive to absent metaconid with a centrally positioned protoconid and the mesial protoconid crest-transverse crest angle is >90°. The P4s are also oval in occlusal outline. This is the combination of traits seen in the earlier hominins such as Au. anamensis (c) and Ar. ramidus (d).
Extended Data Fig. 7 BRT-VP-2/87.
Lateral (a), medial (b), and posterior (c) views.
Extended Data Fig. 8 Ontogenetic series of juvenile human ischia.
Fourteen modern human ischia ranging in age from 1 to 6 years are shown. The age and sex of each individual are provided. y = year; (M) = male; (F) = female. These specimens were sampled from the Hamann-Todd human skeletal collection at the Cleveland Museum of Natural History, Cleveland, OH, USA.
Extended Data Fig. 9 Hallucal metatarsophalangeal joint morphology in early hominins.
Note the presence of a nonsubchondral isthmus in Gorilla gorilla and Ardipithecus ramidus (ARA-VP-6/500-089, GWM67/P2k). In contrast, BRT-VP-2/73c, A.L. 333-115, and A.L. 333-21, StW 562, and StW 595 possess more rounded articular facets, which is the condition derived toward later hominins. Note that StW 562 and StW 595 have bony growths on the dorsolateral region of the head, which are non-articular and do not contribute to the formation of a nonsubchondral isthmus. Also note the extreme dorsal head doming of AL. 333-115 and A.L. 333-21 compared to the Homo sapiens MT1. The asterisk signifies the specimen was mirrored for comparison to BRT-VP-2/73c. The ARA-VP-6/500-089 image was reproduced from ref. 34, AAAS. The GWM67/P2k image was provided by S. W. Simpson. All other scans were generated by T.C.P.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures and Supplementary Tables 1–3.
Supplementary Data 1
40Ar/39Ar analytical data of geological samples from Burtele (BRT) locality.
Supplementary Data 2
Statistical summary of early hominin lower dentition measurements used in the comparative analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haile-Selassie, Y., Schwartz, G.T., Prang, T.C. et al. New finds shed light on diet and locomotion in Australopithecus deyiremeda. Nature 648, 640–648 (2025). https://doi.org/10.1038/s41586-025-09714-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09714-4