Abstract
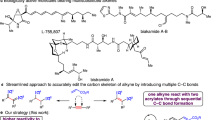
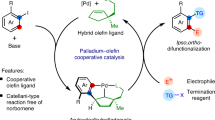
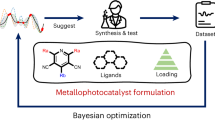
Cooperative catalysis, in which multiple catalytic units operate synergistically, underpins a variety of synthetically and mechanistically important organic reactions1,2,3,4. Despite its potential utility in new reactivity contexts, approaches to the discovery of cooperative catalysts have been limited, typically relying on serendipity or on previous knowledge of single-catalyst reactivity1,5. Systematic searches for unanticipated types of catalyst cooperativity must contend with vast combinatorial complexity and are therefore not undertaken6,7,8,9,10. Here we describe a pooling–deconvolution algorithm, inspired by group testing11, which identifies cooperative catalyst behaviours with low experimental cost while accommodating potential inhibitory effects between catalyst candidates. The workflow was validated first on simulated cooperativity data and then by experimentally identifying previously documented cooperativity between organocatalysts in an enantioselective oxetane-opening reaction. The workflow was then applied in a discovery context to a Pd-catalysed decarbonylative cross-coupling reaction, enabling the identification of several ligand pairs that promote the target transformation at substantially lower catalyst loading and temperature than previously reported with single-ligand systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text, in the SI, and on Zenodo (https://doi.org/10.5281/zenodo.17316238). Illustrations of “automated plating” and “high-throughput UPLC” in Fig. 4b were created in Adobe Illustrator.
Code availability
All code is available on Zenodo (https://doi.org/10.5281/zenodo.17316238). In addition to a persistent version on Zenodo, the Python library developed for simulation and execution is maintained on GitHub under the GPL 3.0 license (https://github.com/msh-yi/multicat-data).
References
Allen, A. E. & MacMillan, D. W. C. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 3, 633–658 (2012).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Romiti, F. et al. Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems. J. Am. Chem. Soc. 141, 17952–17961 (2019).
Matsunaga, S. & Shibasaki, M. Recent advances in cooperative bimetallic asymmetric catalysis: dinuclear Schiff base complexes. Chem. Commun. 50, 1044–1057 (2013).
Martínez, S., Veth, L., Lainer, B. & Dydio, P. Challenges and opportunities in multicatalysis. ACS Catal. 11, 3891–3915 (2021).
Reetz, M. T., Sell, T., Meiswinkel, A. & Mehler, G. A new principle in combinatorial asymmetric transition-metal catalysis: mixtures of chiral monodentate P ligands. Angew. Chem. Int. Ed. 42, 790–793 (2003).
Reetz, M. T. & Mehler, G. Mixtures of chiral and achiral monodentate ligands in asymmetric Rh-catalyzed olefin hydrogenation: reversal of enantioselectivity. Tetrahedron Lett. 44, 4593–4596 (2003).
Duursma, A. et al. First examples of improved catalytic asymmetric C−C bond formation using the monodentate ligand combination approach. Org. Lett. 5, 3111–3113 (2003).
Peña, D. et al. Improving conversion and enantioselectivity in hydrogenation by combining different monodentate phosphoramidites; a new combinatorial approach in asymmetric catalysis. Org. Biomol. Chem. 1, 1087–1089 (2003).
Schaufelberger, F. & Ramström, O. Dynamic covalent organocatalysts discovered from catalytic systems through rapid deconvolution screening. Chem. Eur. J. 21, 12735–12740 (2015).
Aldridge, M., Johnson, O. & Scarlett, J. Group testing: an information theory perspective. Found. Trends Commun. Inf. Theory 15, 196–392 (2019).
Berg, J. M., Gatto, G. G., Hines, J., Tymoczko, J. L. & Stryer, L. Biochemistry (W. H. Freeman, 2023).
Knowles, J. R. Enzyme catalysis: not different, just better. Nature 350, 121–124 (1991).
Wolfenden, R. & Snider, M. J. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 34, 938–945 (2001).
Kraut, D. A., Carroll, K. S. & Herschlag, D. Challenges in enzyme mechanism and energetics. Annu. Rev. Biochem. 72, 517–571 (2003).
Hansen, K. B., Leighton, J. L. & Jacobsen, E. N. On the mechanism of asymmetric nucleophilic ring-opening of epoxides catalyzed by (salen)CrIII complexes. J. Am. Chem. Soc. 118, 10924–10925 (1996).
Ready, J. M. & Jacobsen, E. N. Highly active oligomeric (salen)Co catalysts for asymmetric epoxide ring-opening reactions. J. Am. Chem. Soc. 123, 2687–2688 (2001).
White, D. E., Tadross, P. M., Lu, Z. & Jacobsen, E. N. A broadly applicable and practical oligomeric (salen)Co catalyst for enantioselective epoxide ring-opening reactions. Tetrahedron 70, 4165–4180 (2014).
Ford, D. D., Lehnherr, D., Kennedy, C. R. & Jacobsen, E. N. Anion-abstraction catalysis: the cooperative mechanism of α-chloroether activation by dual hydrogen-bond donors. ACS Catal. 6, 4616–4620 (2016).
DiRocco, D. A. et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science 356, 426–430 (2017).
Levi, S. M. & Jacobsen, E. N. in Organic Reactions 801–852 (Wiley, 2019).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Kim, U. B., Jung, D. J., Jeon, H. J., Rathwell, K. & Lee, S. Synergistic dual transition metal catalysis. Chem. Rev. 120, 13382–13433 (2020).
Taylor, C. J. et al. A brief introduction to chemical reaction optimization. Chem. Rev. 123, 3089–3126 (2023).
Ahneman, D. T., Estrada, J. G., Lin, S., Dreher, S. D. & Doyle, A. G. Predicting reaction performance in C–N cross-coupling using machine learning. Science 360, 186–190 (2018).
Zahrt, A. F., Athavale, S. V. & Denmark, S. E. Quantitative structure–selectivity relationships in enantioselective catalysis: past, present, and future. Chem. Rev. 120, 1620–1689 (2020).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Rinehart, N. I. et al. A machine-learning tool to predict substrate-adaptive conditions for Pd-catalyzed C–N couplings. Science 381, 965–972 (2023).
Wang, J. Y. et al. Identifying general reaction conditions by bandit optimization. Nature 626, 1025–1033 (2024).
Mallapaty, S. The mathematical strategy that could transform coronavirus testing. Nature 583, 504–505 (2020).
Wolf, E., Richmond, E. & Moran, J. Identifying lead hits in catalyst discovery by screening and deconvoluting complex mixtures of catalyst components. Chem. Sci. 6, 2501–2505 (2015).
Steimbach, R. R., Kollmus, P. & Santagostino, M. A validated “pool and split” approach to screening and optimization of copper-catalyzed C–N cross-coupling reactions. J. Org. Chem. 86, 1528–1539 (2021).
Fordham, J. M., Kollmus, P., Cavegn, M., Schneider, R. & Santagostino, M. A “pool and split” approach to the optimization of challenging Pd-catalyzed C–N cross-coupling reactions. J. Org. Chem. 87, 4400–4414 (2022).
Wieland, J. & Breit, B. A combinatorial approach to the identification of self-assembled ligands for rhodium-catalysed asymmetric hydrogenation. Nat. Chem. 2, 832–837 (2010).
Robbins, D. W. & Hartwig, J. F. A simple, multidimensional approach to high-throughput discovery of catalytic reactions. Science 333, 1423–1427 (2011).
Gordon, D. M., Patashnik, O. & Kuperberg, G. New constructions for covering designs. J. Comb. Des. 3, 269–284 (1995).
Gordon, D. M. & Stinton, D. in Handbook of Combinatorial Designs 365–372 (Chapman and Hall, 2006).
Schönheim, J. On coverings. Pac. J. Math. 14, 1405–1411 (1964).
Gordon, D. M., Patashnik, O., Kuperberg, G. & Spencer, J. H. Asymptotically optimal covering designs. J. Comb. Theory Ser. A 75, 270–280 (1996).
Gordon, D. M. Covering designs. https://www.dmgordon.org/cover/ (2025).
Hautus, M. J., Macmillan, N. A. & Creelman, C. D. Detection Theory: A User’s Guide (Routledge, 2022).
Yang, W., Wang, Z. & Sun, J. Enantioselective oxetane ring opening with chloride: unusual use of wet molecular sieves for the controlled release of HCl. Angew. Chem. Int. Ed. 55, 6954–6958 (2016).
Strassfeld, D. A., Wickens, Z. K., Picazo, E. & Jacobsen, E. N. Highly enantioselective, hydrogen-bond-donor catalyzed additions to oxetanes. J. Am. Chem. Soc. 142, 9175–9180 (2020).
Strassfeld, D. A., Algera, R. F., Wickens, Z. K. & Jacobsen, E. N. A case study in catalyst generality: simultaneous, highly-enantioselective Brønsted- and Lewis-acid mechanisms in hydrogen-bond-donor catalyzed oxetane openings. J. Am. Chem. Soc. 143, 9585–9594 (2021).
Strassfeld, D. A. Novel Transformations and Strategies in Enantioselective Catalysis Enabled by Non-Covalent Transition State Stabilization. PhD thesis, Harvard Univ. (2020).
Manville, N., Alite, H., Haeffner, F., Hoveyda, A. H. & Snapper, M. L. Enantioselective silyl protection of alcohols promoted by a combination of chiral and achiral Lewis basic catalysts. Nat. Chem. 5, 768–774 (2013).
Malapit, C. A., Ichiishi, N. & Sanford, M. S. Pd-catalyzed decarbonylative cross-couplings of aroyl chlorides. Org. Lett. 19, 4142–4145 (2017).
Zhou, T., Xie, P.-P., Ji, C.-L., Hong, X. & Szostak, M. Decarbonylative Suzuki–Miyaura cross-coupling of aroyl chlorides. Org. Lett. 22, 6434–6440 (2020).
Verbicky, J. W. Jr, Dellacoletta, B. A. & Williams, L. Palladium catalyzed decarbonylation of aromatic acyl chlorides. Tetrahedron Lett. 23, 371–372 (1982).
Obora, Y., Tsuji, Y. & Kawamura, T. Palladium-catalyzed decarbonylative coupling of acid chlorides, organodisilanes, and 1,3-dienes. J. Am. Chem. Soc. 115, 10414–10415 (1993).
Sugihara, T., Satoh, T. & Miura, M. Mizoroki–Heck type arylation of alkenes using aroyl chlorides under base-free conditions. Tetrahedron Lett. 46, 8269–8271 (2005).
King, R. P., Krska, S. W. & Buchwald, S. L. A ligand exchange process for the diversification of palladium oxidative addition complexes. Org. Lett. 23, 6030–6034 (2021).
Gensch, T. et al. A comprehensive discovery platform for organophosphorus ligands for catalysis. J. Am. Chem. Soc. 144, 1205–1217 (2022).
Dotson, J. J. et al. Data-driven multi-objective optimization tactics for catalytic asymmetric reactions using bisphosphine ligands. J. Am. Chem. Soc. 145, 110–121 (2023).
Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 19, 227–229 (2010).
Fors, B. P. & Buchwald, S. L. A multiligand based Pd catalyst for C−N cross-coupling reactions. J. Am. Chem. Soc. 132, 15914–15917 (2010).
Fan, Y., Cong, M. & Peng, L. Mixed-ligand catalysts: a powerful tool in transition-metal-catalyzed cross-coupling reactions. Chem. Eur. J. 20, 2698–2702 (2014).
Li, H. et al. Transformations of aryl ketones via ligand-promoted C−C bond activation. Angew. Chem. Int. Ed. 132, 14494–14499 (2020).
Wakioka, M. et al. Mixed-ligand approach to palladium-catalyzed direct arylation of heteroarenes with aryl chlorides: controlling reactivity of catalytic intermediates via dynamic ligand exchange. Organometallics 42, 3454–3465 (2023).
Wang, G.-Y., Ge, Z., Ding, K. & Wang, X. Cooperative bimetallic catalysis via one-metal/two-ligands: mechanistic insights of polyfluoroarylation-allylation of diazo compounds. Angew. Chem. Int. Ed. 135, e202307973 (2023).
Kaltenberger, S. & van Gemmeren, M. Controlling reactivity and selectivity in the nondirected C–H activation of arenes with palladium. Acc. Chem. Res. 56, 2459–2472 (2023).
Zhao, D., Xu, P. & Ritter, T. Palladium-catalyzed late-stage direct arene cyanation. Chem. 5, 97–107 (2019).
Sinha, S. K. et al. Dual ligand enabled nondirected C–H chalcogenation of arenes and heteroarenes. J. Am. Chem. Soc. 144, 12032–12042 (2022).
Meng, G. et al. Dual-ligand catalyst for the nondirected C–H olefination of heteroarenes. J. Am. Chem. Soc. 145, 8198–8208 (2023).
Wang, X.-X. & Jiao, L. Dual ligand enabled Pd-catalyzed ortho-alkylation of iodoarenes. J. Am. Chem. Soc. 146, 25552–25561 (2024).
Acknowledgements
This work was supported by Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, the National Science Foundation through grant no. CHE-2247494, and the National Institutes of Health through grant no. GM149244. We thank D. Strassfeld for documenting and investigating cooperativity in the TMSBr oxetane opening reaction and for their discussions. We thank G. Lovinger for early exploration of cooperativity in the TMSCl oxetane opening reaction and for early conceptual discussions. We thank S. Li for early exploration of Cu-based ligand cooperativity. We thank T. Adrianov, M. Brenner, D. X. Chen, D. Diaz, W. Goh, S. Gopalakrishnan, D. Gordon, A. LaPorte, S. Nistanaki, E. R. Raguram and C. Wagen for helpful discussions. We thank C. Yeung, N. Sciammetta, E. Edelstein, A. Neel, R. Ruck, S. Grosser, the Catalysis and Capabilities Network, Discovery Process Chemistry, and Data-Rich Experimentation at Merck & Co., Inc., Rahway, NJ, USA for generous experimental resources. We thank S. Miller for a generous donation of catalyst 1k. We thank an anonymous referee for proposing an operational, well-behaved definition of Q.
Author information
Authors and Affiliations
Contributions
M.H.S., E.E.K. and E.N.J. conceptualized the study. M.H.S. conducted the formal analysis. E.N.J. and E.E.K. helped with funding acquisition. M.H.S. conducted the investigation. M.H.S., E.N.J., E.E.K. and R.Y.L. devised the methodology. E.N.J. administered the project. E.E.K., E.N.J. and R.Y.L. provided the resources. M.H.S. wrote the software. E.N.J., E.E.K. and R.Y.L. supervised the project. M.H.S. validated the study, performed data visualization and wrote the original draft. M.H.S., E.N.J., E.E.K. and R.Y.L. reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Materials and Methods, Supplementary Text, Supplementary Figs. 1–12 and Supplementary Tables 1–21.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sak, M.H., Liu, R.Y., Kwan, E.E. et al. Accelerating the discovery of multicatalytic cooperativity. Nature 648, 333–340 (2025). https://doi.org/10.1038/s41586-025-09813-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09813-2