Abstract
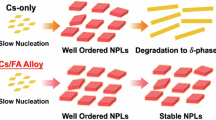
Colloidal perovskite nanocrystal (PeNC) has long been synthesized using the hot-injection method and room-temperature ligand-assisted reprecipitation as the prominent techniques1,2. However, both methods have challenges for industrial-scale production3,4,5: the hot-injection method requires high temperatures, an inert gas environment and rapid cooling, which raise safety concerns, whereas ligand-assisted reprecipitation can exhibit limited productivity on scale-up. Here we present a cold-injection method based on pseudo-emulsion, enabling scalable synthesis of PeNCs with near-unity photoluminescence quantum yield (PLQY, ~100%) and enhanced stability by injecting precursor solution below 4 °C. In the cold-injection method, PeNCs grow through the assembly of fully coordinated plumbates out of the pseudo-emulsion with the assistance of a demulsifier. We discovered that slow assembly of polybromide plumbates, assisted by cold temperature, is essential for defect suppression, resulting in reproducible, stable and pure-green-emitting PeNCs with near-unity PLQY. Furthermore, this method enables efficient large-scale production, achieving 20-l-scale synthesis with remarkable batch weight while maintaining near-unity PLQY. Our findings represent a substantial advancement in synthesis of high-quality PeNCs, offering potential for broad applications in display and lighting industries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during the current study are included in the Article and Supplementary Information.
References
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Zhang, F. et al. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: potential alternatives for display technology. ACS Nano 9, 4533–4542 (2015).
Dey, A. et al. State of the art and prospects for halide perovskite nanocrystals. ACS Nano 15, 10775–10981 (2021).
Han, T. H. et al. A roadmap for the commercialization of perovskite light emitters. Nat. Rev. Mater. 7, 757–777 (2022).
Kim, J. I. et al. Strategies to extend the lifetime of perovskite downconversion films for display applications. Adv. Mater. 35, 2209784 (2023).
Sun, X. et al. Diffusion-mediated synthesis of high-quality organic–inorganic hybrid perovskite nanocrystals. Nat. Synth. 4, 167–176 (2025).
Tan, Z. K. et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 9, 687–692 (2014).
Cho, H. et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 350, 1222–1225 (2015).
Dong, Y. et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 15, 668–674 (2020).
Shamsi, J., Rainò, G., Kovalenko, M. V. & Stranks, S. D. To nano or not to nano for bright halide perovskite emitters. Nat. Nanotechnol. 16, 1164–1168 (2021).
Jiang, Y. et al. Synthesis-on-substrate of quantum dot solids. Nature 612, 679–684 (2022).
Chu, Z. et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 22% via small-molecule passivation. Adv. Mater. 33, 2007169 (2021).
Vighnesh, K., Wang, S., Liu, H. & Rogach, A. L. Hot-injection synthesis protocol for green-emitting cesium lead bromide perovskite nanocrystals. ACS Nano 16, 19618–19625 (2022).
Huang, H., Susha, A. S., Kershaw, S. V., Hung, T. F. & Rogach, A. L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2, 1500194 (2015).
Li, X. et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 26, 2435–2445 (2016).
Kim, Y. H. et al. Highly efficient light-emitting diodes of colloidal metal–halide perovskite nanocrystals beyond quantum size. ACS Nano 11, 6586–6593 (2017).
Rahimnejad, S., Kovalenko, A., Forés, S. M., Aranda, C. & Guerrero, A. Coordination chemistry dictates the structural defects in lead halide perovskites. ChemPhysChem 17, 2795–2798 (2016).
Stamplecoskie, K. G., Manser, J. S. & Kamat, P. V. Dual nature of the excited state in organic–inorganic lead halide perovskites. Energy Environ. Sci. 8, 208–215 (2015).
Yoon, S. J., Stamplecoskie, K. G. & Kamat, P. V. How lead halide complex chemistry dictates the composition of mixed halide perovskites. J. Phys. Chem. Lett. 7, 1368–1373 (2016).
Yan, K. et al. Hybrid halide perovskite solar cell precursors: colloidal chemistry and coordination engineering behind device processing for high efficiency. J. Am. Chem. Soc. 137, 4460–4468 (2015).
Sun, S., Yuan, D., Xu, Y., Wang, A. & Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 10, 3648–3657 (2016).
Ahmed, G. H. et al. Pyridine-induced dimensionality change in hybrid perovskite nanocrystals. Chem. Mater. 29, 4393–4400 (2017).
Kim, Y. H. et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 15, 148–155 (2021).
Bodnarchuk, M. I. et al. Rationalizing and controlling the surface structure and electronic passivation of cesium lead halide nanocrystals. ACS Energy Lett. 4, 63–74 (2019).
Fiuza-Maneiro, N. et al. Ligand chemistry of inorganic lead halide perovskite nanocrystals. ACS Energy Lett. 8, 1152–1191 (2023).
Chen, Y. et al. Surface termination of CsPbBr3 perovskite quantum dots determined by solid-state NMR spectroscopy. J. Am. Chem. Soc. 142, 6117–6127 (2020).
Maes, J. et al. Light absorption coefficient of CsPbBr3 perovskite nanocrystals. J. Phys. Chem. Lett. 9, 3093–3097 (2018).
Smock, S. R., Williams, T. J. & Brutchey, R. L. Quantifying the thermodynamics of ligand binding to CsPbBr3 quantum dots. Angew. Chem. Int. Ed. 57, 11711–11715 (2018).
Kazes, M., Udayabhaskararao, T., Dey, S. & Oron, D. Effect of surface ligands in perovskite nanocrystals: extending in and reaching out. Acc. Chem. Res. 54, 1409–1418 (2021).
Pan, A. et al. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: the role of organic acid, base, and cesium precursors. ACS Nano 10, 7943–7954 (2016).
Otero-Martínez, C. et al. Colloidal metal–halide perovskite nanoplatelets: thickness-controlled synthesis, properties, and application in light-emitting diodes. Adv. Mater. 34, 2107105 (2022).
Akkerman, Q. A. et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. J. Am. Chem. Soc. 138, 1010–1016 (2016).
Pan, J. et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 28, 8718–8725 (2016).
Ding, S. et al. In situ bonding regulation of surface ligands for efficient and stable FAPbI3 quantum dot solar cells. Adv. Sci. 9, 2204476 (2022).
Toso, S., Baranov, D., Filippi, U., Giannini, C. & Manna, L. Collective diffraction effects in perovskite nanocrystal superlattices. Acc. Chem. Res. 56, 66–76 (2023).
Toso, S., Baranov, D., Giannini, C., Marras, S. & Manna, L. Wide-angle X-ray diffraction evidence of structural coherence in CsPbBr3 nanocrystal superlattices. ACS Mater. Lett. 1, 272–276 (2019).
Kim, Y. H., Wolf, C., Kim, H. & Lee, T. W. Charge carrier recombination and ion migration in metal-halide perovskite nanoparticle films for efficient light-emitting diodes. Nano Energy 52, 329–335 (2018).
Ma, K. et al. Multifunctional conjugated ligand engineering for stable and efficient perovskite solar cells. Adv. Mater. 33, 2100791 (2021).
Peng, J., Chen, Y., Zheng, K., Pullerits, T. & Liang, Z. Insights into charge carrier dynamics in organo-metal halide perovskites: From neat films to solar cells. Chem. Soc. Rev. 46, 5714–5729 (2017).
Kim, J. S. et al. Ultra-bright, efficient and stable perovskite light-emitting diodes. Nature 611, 688–694 (2022).
Jeong, S. H. et al. Characterizing the efficiency of perovskite solar cells and light-emitting diodes. Joule 4, 1206–1235 (2020).
Li, W. et al. Relationship of giant dielectric constant and ion migration in CH3NH3PbI3 single crystal using dielectric spectroscopy. J. Phys. Chem. C 124, 13348–13355 (2020).
Kim, Y.-H. et al. Exploiting the full advantages of colloidal perovskite nanocrystals for large-area efficient light-emitting diodes. Nat. Nanotechnol. 17, 590–597 (2022).
Yang, Q. et al. Surface polarization and recombination in organic–inorganic hybrid perovskite solar cells based on photo- and electrically induced negative capacitance studies. Org. Electron. 62, 203–208 (2018).
Acknowledgements
We thank M. V. Kovalenko (ETH) for valuable discussions and insights regarding the NMR analysis. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2025-00560490), the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (RS-2022-NR067540), the Technology Innovation Program (RS-2025-25393382, ‘Development of perovskite nano emissive material for AR/VR near-eye displays’) funded By the Ministry of Trade, Industry and Resources (MOTIR, Korea), and the Technology Innovation Program (RS-2024-00425883/Optimized Perovskite Nanocrystal Resin Composition and Prototyping Large-Area Continuous Color Conversion Film) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea). G.-S.P. was supported by the NRF grant by the Ministry of Science, ICT and Future Planning (No. RS-2023-00258732). S.K.L. was supported by the NRF grant funded by the Ministry of Science and ICT (2020R1A3B2079815).
Author information
Authors and Affiliations
Contributions
S. Kim, S.-A.K., S.-C.L. and T.-W.L. conceived the research idea. S. Kim, S.-A.K., E.K. and Y.J. synthesized the PeNCs and fabricated LED devices and analysed data. G.-S.P. performed the STEM measurement and analysis. S. Kim, S.-A.K., E.K., D.-H.K. and Y.J. conducted PLQY measurements. D.-H.K. conducted temperature-dependent PL. S.-J.W. conducted in situ PL measurement and optical simulation. J.J.K. conducted solid-state 1H MAS NMR. S. Kang conducted TEM measurement and analysis. M.L. conducted the cryo-STEM measurement and analysis. H.J.Y. conducted the XPS measurement. S. Kim, S.-A.K., S.P. and H.-J.S. conducted PeNC mass production. J.S.K. and K.Y.J. conducted transient electroluminescence measurements. M.-J.S., C.-Y.P. and S.-A.K. conducted grazing-incidence wide-angle X-ray scattering and analysed data. S.E.C. conducted scanning electron microscopy. D.-H.K. and Jinwoo Park assisted with the fabrication of LED devices. Jungwon Park analysed TEM and cryo-STEM data. S.K.L. analysed solid-state 1H MAS NMR data and provided assistance in NMR interpretation. S. Kim drafted the first version of the manuscript, with assistance from S.-A.K. and T.-W.L. All authors contributed to the final manuscript. T.-W.L. supervised the research project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kumar Sudhir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Expansion of Ci-method to various A-site cation compositions.
a-f, Normalized PL spectra and solution PLQY of FAPbBr3 (a,b), MAPbBr3 (c,d) and CsPbBr3 (e,f) synthesized at various TINJ.
Extended Data Fig. 2 Expansion of Ci-method to various halide compositions.
a-f, Normalized PL spectra solution PLQY of red-emitting PeNCs; FA0.9Cs0.1PbBr1.2I1.8 (a,b), blue-emitting PeNCs; FAPbCl1.2Br1.8 and (c,d) FAPbI3 PeNCs (e,f) synthesized at various TINJ.
Extended Data Fig. 3
Normalized PL spectra and CIE coordinates (inset) of PeNCs synthesized at various TINJ.
Extended Data Fig. 4 Variation in the size of corner-shared polybromide plumbates with increasing ABr:PbBr2 ratio measured by dynamic light scattering.
As the ABr:PbBr2 ratio increases, stronger isolation by A-site cations leads to a reduction in plumbate size.
Extended Data Fig. 5 Variation in perovskite precipitation behaviour depending on the ABr:PbBr2 ratio of the precursor solution.
Precursor solutions with varying ABr:PbBr2 ratios were injected into toluene, showing distinct precipitation behaviours. At low ABr:PbBr2 ratio (≤1), large-sized perovskites with an orange coloration formed, whereas PeNC formation was gradually suppressed at ratios above 1.5. Given that DMF, the polar solvent used for the precursor solution, and toluene, the nonpolar solvent, are miscible and that no ligands were included, it can be inferred that an excess A-site cations isolated the plumbates, thus preventing their assembly.
Extended Data Fig. 6 Surface of PeNCs.
a, Solid-state 1H MAS NMR spectra of PeNCs synthesized at various TINJ. b, Quantitative analysis of DAmH+ to A-site cation in PeNCs synthesized at various TINJ, as determined by solid-state 1H MAS NMR.
Extended Data Fig. 7 Enhanced charge-carrier confinement in Ci-PeNCs.
a, PL lifetime of PeNCs synthesized at various TINJ. b-d, PL lifetime of Ci-PeNCs (TINJ = 0 °C) (b), RT-PeNCs (TINJ = 20 °C) (c) and average PL lifetime (d) at varying excitation intensities.
Extended Data Fig. 8 Improved thermal activation energy for PL quenching in Ci-PeNCs.
a-c, Temperature-dependent integrated PL intensity (a) and two-dimensional (2D) map of temperature-dependent PL spectra of Ci-PeNCs (TINJ = 0 °C) (b) and RT-PeNCs (TINJ = 20 °C) (c).
Extended Data Fig. 9 Suppressed defect formation in Ci-PeNCs.
a-c, Full transient electroluminescence signal in the timescale of 10 ms (a), magnified transient electroluminescence signal rise region (b) and capacitance–frequency (c) characteristics of Ci-PeLEDs and RT-PeLEDs.
Extended Data Fig. 10 Color conversion film (CCF) using PeNCs.
a, Structure of CCF. b, Stability of CCF under 60 °C and 90% humidity (inset: fabricated CCF with a scale bar of 3 cm). c, Fabricated CCF mounted on an actual tablet. Photograph on the display in c by Jacob Yavin, reproduced from Pexels (https://www.pexels.com/).
Supplementary information
Supplementary Information
Supplementary Notes 1–6, which include Figs. 1–39 and Tables 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Kim, SA., Park, GS. et al. Cold-injection synthesis of highly emissive perovskite nanocrystals. Nature (2026). https://doi.org/10.1038/s41586-026-10117-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41586-026-10117-2