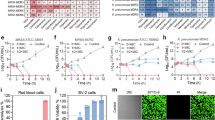
Abstract
The efficacy of antimicrobial peptides (AMPs) is limited by challenges of delivery and potency. We enhance AMP performance in the lung by converting AMPs to a peptibody format that fuses AMPs with fragment crystallizable domains to activate innate immunity and cathelin domains for infection-responsive activation, with their mRNA constructs delivered by anti-inflammatory lipid nanoparticles. The highest-scoring design outperforms antibiotic therapy approved by the US Food and Drug Administration in multidrug-resistant pneumonia models, eradicating representative MDR bacteria while mitigating inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Metlay, J. P. et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200, e45–e67 (2019).
Pragman, A. A., Berger, J. P. & Williams, B. J. Understanding persistent bacterial lung infections: clinical implications informed by the biology of the microbiota and biofilms. Clin. Pulm. Med. 23, 57–66 (2016).
Mettelman, R. C., Allen, E. K. & Thomas, P. G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 55, 749–780 (2022).
Hewitt, R. J. & Lloyd, C. M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 21, 347–362 (2021).
Cookson, W. O., Cox, M. J. & Moffatt, M. F. New opportunities for managing acute and chronic lung infections. Nat. Rev. Microbiol. 16, 111–120 (2018).
Parmanik, A. et al. Current treatment strategies against multidrug-resistant bacteria: a review. Curr. Microbiol. 79, 388 (2022).
Grief, S. N. & Loza, J. K. Guidelines for the evaluation and treatment of pneumonia. Prim. Care: Clin. Off. Pract. 45, 485–503 (2018).
Kellum, J. A. et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the genetic and inflammatory markers of sepsis (GenIMS) study. Arch. Intern. Med. 167, 1655–1663 (2007).
Kumar, V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol. 11, 1722 (2020).
Mookherjee, N., Anderson, M. A., Haagsman, H. P. & Davidson, D. J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19, 311–332 (2020).
Dijksteel, G. S., Ulrich, M. M., Middelkoop, E. & Boekema, B. K. Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 12, 616979 (2021).
Izadpanah, A. & Gallo, R. L. Antimicrobial peptides. J. Am. Acad. Dermatol. 52, 381–390 (2005).
Zhang, L.-j. & Gallo, R. L. Antimicrobial peptides. Curr. Biol. 26, R14–R19 (2016).
Di, Y. P., Kuhn, J. M. & Mangoni, M. L. Lung antimicrobial proteins and peptides: from host defense to therapeutic strategies. Physiol. Rev. 104, 1643–1677 (2024).
Mahlapuu, M., Björn, C. & Ekblom, J. Antimicrobial peptides as therapeutic agents: opportunities and challenges. Crit. Rev. Biotechnol. 40, 978–992 (2020).
Hou, X. et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15, 41–46 (2020).
Steinstraesser, L. et al. Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol. Ther. 22, 734–742 (2014).
Pyzik, M., Kozicky, L. K., Gandhi, A. K. & Blumberg, R. S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 23, 415–432 (2023).
Czajkowsky, D. M., Hu, J., Shao, Z. & Pleass, R. J. Fc-fusion proteins: new developments and future perspectives. EMBO Mol. Med. 4, 1015–1028 (2012).
Levin, D., Golding, B., Strome, S. E. & Sauna, Z. E. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 33, 27–34 (2015).
Dürr, U. H., Sudheendra, U. S. & Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425 (2006).
Sørensen, O. E. et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 (2001).
Park, M. D., Silvin, A., Ginhoux, F. & Merad, M. Macrophages in health and disease. Cell 185, 4259–4279 (2022).
Rosales, C. & Uribe-Querol, E. Phagocytosis: a fundamental process in immunity. BioMed. Res. Int. 2017, 9042851 (2017).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008).
Uribe-Querol, E. & Rosales, C. Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 11, 1066 (2020).
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 19, 177–191 (2022).
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P. & Malik, A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014).
Saxton, R. A., Glassman, C. R. & Garcia, K. C. Emerging principles of cytokine pharmacology and therapeutics. Nat. Rev. Drug Discov. 22, 21–37 (2023).
Huang, X., He, D., Pan, Z., Luo, G. & Deng, J. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater. Today Bio 11, 100124 (2021).
Wang, S. et al. Accelerating diabetic wound healing by ROS-scavenging lipid nanoparticle–mRNA formulation. Proc. Natl Acad. Sci. USA 121, e2322935121 (2024).
Zhang, F. et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 21, 1324–1332 (2022).
Maciá, M. D. et al. Efficacy and potential for resistance selection of antipseudomonal treatments in a mouse model of lung infection by hypermutable Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50, 975–983 (2006).
Lee, A. S. et al. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 4, 18033 (2018).
Zeng, C. et al. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Adv. Mater. 32, 2004452 (2020).
Chen, R. et al. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 41, 262–272 (2023).
Wang, C. et al. Blood–brain-barrier-crossing lipid nanoparticles for mRNA delivery to the central nervous system. Nat. Mater. 24, 1653–1663 (2025).
Kim, M. et al. Dual SORT LNPs for multi-organ base editing. Nat. Biotechnol. https://doi.org/10.1038/s41587-025-02675-z (2025).
Devkota, S. P., Onah, C., Joshi, P. R., Adhikari, S. & Baral, P. Optimized method for higher yield of alveolar macrophage isolation for ex vivo studies. Heliyon 10, e37221 (2024).
Xue, Y. et al. LNP–RNA-mediated antigen presentation leverages SARS-CoV-2-specific immunity for cancer treatment. Nat. Commun. 16, 2198 (2025).
Chen, S. et al. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Prim. 3, 63 (2023).
Mukherjee, A. et al. Engineered mutant α-ENaC subunit mRNA delivered by lipid nanoparticles reduces amiloride currents in cystic fibrosis-based cell and mice models. Sci. Adv. 6, eabc5911 (2020).
Witten, J. et al. Artificial intelligence-guided design of lipid nanoparticles for pulmonary gene therapy. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02490-y (2024).
Kim, J. et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mrna through inhalation. ACS Nano 16, 14792–14806 (2022).
Sun, Y. et al. In vivo editing of lung stem cells for durable gene correction in mice. Science 384, 1196–1202 (2024).
Facchini, M., De Fino, I., Riva, C. & Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 85, 51019 (2014).
van den Berg, E. et al. Role of the Fas/FasL system in a model of RSV infection in mechanically ventilated mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L451–L460 (2011).
Acknowledgements
Y.D. acknowledges support from the Maximizing Investigators’ Research Award (R35GM144117) from the National Institute of General Medical Sciences and institutional funds from the Icahn School of Medicine at Mount Sinai. We thank the Biorepository and Pathology CoRE Laboratory at the Icahn School of Medicine at Mount Sinai for their support. Cryo-electron microscopy was performed at the Center for Electron Microscopy and Analysis at The Ohio State University. We are grateful to E. Purisic and J. Dai for sharing the vibratome and providing instructions. Certain figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Y.X., X.H. and S.W. conceptualized the work, performed the experiments, analyzed the data and wrote the paper. Y. Zhang., C.W. and C.Y. contributed to the lipid synthesis. Y. Zhong., D.D.K. and Y.Y.Z. contributed to the mRNA synthesis. H.L., Z.L., M.T. and D.C. contributed to the animal studies. B.D. contributed to the cryo-electron microscopy imaging. P.H. and M.M. contributed to the collection of human lung tissues. Y.D. conceptualized and supervised the project and wrote the paper. The final paper was edited and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
Y.D. is a cofounder and holds equity in Immunanoengineering Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterizations of TS LNPs for pulmonary mRNA delivery.
a, Relative luminescence intensity of TS LNP-FLuc mRNA in MLG cells. Intensity was normalized to SM-102 LNP group. b, Relative luminescence intensity of TS LNP-FLuc mRNA in A549 cells. Intensity was normalized to SM-102 LNP group. c, Luminescence intensity in the lungs of mice intratracheally injected with TS LNPs-FLuc mRNA. d, Representative IVIS images of lung tissues from c. e, Luminescence intensity in the lungs of mice following IT injection of SM-102 LNP-FLuc mRNA, TS41 LNP-FLuc mRNA, or TS41S LNP-FLuc mRNA. f, Representative IVIS images of lung tissues from e. g, Schematic illustration showing that delivery of Cre recombinase mRNA induces tdTomato expression in Ai14 reporter mice through Cre-mediated recombination. h, Flow cytometry analysis of tdTomato expression in various lung cell types following IT injection of SM-102 LNP-Cre mRNA or TS41S LNP-Cre mRNA. All data are from n = 3 biologically independent samples and are presented as mean values ± s.d. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 2 Optimization of TS41 LNP formulation using orthogonal assay.
a, Table for two rounds of TS41 LNP optimization. Chol, cholesterol. b, Orthogonal assay to evaluate the impact trends of individual lipid component in TS41 LNP formulation at four levels in MLG cells. c, Luminescence intensity fold changes of the two rounds of optimization in MLG cells. d, Orthogonal assay to evaluate the impact trends of individual lipid component in TS41 LNP formulation at four levels in A549 cells. e, Luminescence intensity fold changes of the two rounds of optimization in A549 cells. f, Hydrodynamic diameter and PDI of TS41S LNP. PDI, polydispersity index. g, Zeta potential and encapsulation efficiency of TS41S LNP. h, Representative Cryo-TEM image of TS41S LNP. Scale bar = 100 nm. Data in b-g are from n = 3 biologically independent samples. Data in b and d are plotted as floating bar charts, where each bar represents the range of luminescence intensity from a start value (minimum intensity) to an end value (maximum intensity), with a line indicating the mean intensity. Data in c and e-g are presented as mean values ± SD. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. **P < 0.01, ****P < 0.0001.
Extended Data Fig. 3 Anti-inflammatory ability of TS41S LNP in pneumonia lung.
a, Flow cytometry analysis of ROS levels in neutrophils, alveolar macrophages and monocyte-derived macrophages in bronchoalveolar lavage fluid (BALF) from pneumonia lung treated with different LNPs. b, Percentage and cell number of infiltrating neutrophils among total immune cells in BALF. c, Expression level of inducible nitric oxide synthase (iNOS) in neutrophils. d, e, f, g, Expression level of IL-1β (d), IL-6 (e), TNF-α (f), and IFN-γ (g) in BALF. All data are from n = 5 biologically independent samples and are presented as mean values ± SD. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. n.s. not significant P > 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 4 Therapeutic effects of TS41S LNP-PB9 mRNA in chronic pneumonia models.
a, Treatment scheme for chronic pneumonia models induced by S. aureus infection or co-infection of S. aureus and P. aeruginosa. b, Relative body weight for each group in chronic pneumonia model of S. aureus infection. c, Quantification of bacteria load in the lung tissues of each group in chronic pneumonia model of S. aureus infection. d, Relative body weight for each group in chronic pneumonia model of S. aureus and P. aeruginosa co-infection. e, Quantification of bacteria load in the lung tissues of each group in chronic pneumonia model of S. aureus and P. aeruginosa co-infection. All data are from n = 5 (b and c) or 6 (d and e) biologically independent samples and are presented as mean values ± SD. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. **P < 0.01, ***P < 0.001, ****P < 0.0001. Panel a created using BioRender.com.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13.
Supplementary Data 1
Unprocessed western blots and statistical source data.
Source data
Source Data Fig. 1
Unprocessed western blots and statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Extended Data Figs. 1–4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, Y., Hou, X., Wang, S. et al. Antimicrobial peptide delivery to lung as peptibody mRNA in anti-inflammatory lipids treats multidrug-resistant bacterial pneumonia. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02928-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41587-025-02928-x