Abstract
Follicular regulatory T (TFR) cells restrain follicular helper T (TFH) cell-mediated B cell responses to optimize humoral immunity while limiting autoimmunity. Here we assessed the developmental dynamics of TFR cells. We found that TFR cells undergo progressive differentiation through progenitor, early effector and late effector stages. Late effector TFR cells possessed inherent instability, and could lose expression of FoxP3 to become ExTFR cells. Expression of effector TFH programs in TFR cells preceded instability, a process that was mediated by Tcf7. A subset of ExTFR could be redeemed by re-expression of FoxP3. Extrinsically, TFH cells enhanced late effector TFR cell differentiation by diverting cells away from a default Prdm1/Blimp-1 fate to express Bcl6. Together, these data indicate that TFR cells are a dynamic and plastic cell subset, the differentiation of which is controlled by intrinsic and extrinsic programs that work together to form a feedback loop to control humoral immunity.
Similar content being viewed by others
Main
Antibodies protect against infection, but B cell dysregulation can lead to pathogenic antibodies. To prevent this, the immune system uses overlapping mechanisms to ensure antibodies are tightly controlled. Follicular helper T (TFH) cells promote antibody production by activating B cells and the germinal center (GC) reaction. In contrast, follicular regulatory T (TFR) cells limit B cells and GCs1,2,3,4. TFR cells attenuate foreign antigen-specific antibodies as well as pathogenic antibodies in autoimmunity, allergies and other diseases5,6,7. In addition, TFR cells regulate tertiary lymphoid structures (TLS) and control the efficacy of immune checkpoint blockade8,9. However, in some settings, TFR cells enhance antibodies10. This discrepancy can be partially explained by the finding that TFR cells may promote affinity maturation by limiting clonal competition within GCs11.
TFR cells differentiate from natural T regulatory (Treg) cell precursors, although some may arise from induced Treg cells12,13,14. Both dendritic cells and B cells are required for initial TFR differentiation, as is costimulation through CD28 and ICOS15,16,17,18. Similar to TFH, a subset of TFR cells circulate in the blood as memory cells14,16,19. TFR cells in GCs have lower CD25 and higher programmed cell death protein 1 (PD-1) expression indicating activation15,20,21,22. Despite clonal expansion, TFR cells proliferate less substantially than TFH cells12,23. Some TFR cells can also enter the GC reaction relatively late to contract GCs6,23. Like other Treg subsets, TFR coopt transcription factors of their target cells, such as TFH, to establish programs24,25. Some TFR can downregulate FoxP3 to become ExTFR with reduced suppressive capacity25. Recent data indicate TFH cells undergo progressive differentiation from stem-like progenitor to effector stages—a process highly regulated by the immune system26,27. Progenitor TFH cells are similar to stem-like populations of CD8 T and Th17, which sustain long-term responses28,29. Whether TFR cells undergo similar development is unknown.
Here we used longitudinal sampling, fate mapping and subset deletion to fully elucidate the dynamics and signals for TFR development and stability. We find TFR cells require progressive development through progenitor, early effector and late effector stages to generate functional TFR cells in both mice and humans. Moreover, we find that only late effector TFR cells have inherent instability and form ExTFR cells, a process controlled by Tcf7/TCF-1. Moreover, ExTFR cells persist longer than effector TFR and can be redeemed through re-expression of FoxP3. FoxP3 instability is heterogeneous and acquisition of effector TFH programs in TFR cells precedes instability. Using inducible deletion strategies, we further find that TFH cells extrinsically redirect late effector stage TFR cells from a default Prdm1/Blimp-1-expressing, to Bcl6-expressing, late effector state. Together these data reveal how progressive development generates effector TFR cells and how intrinsic and extrinsic mechanisms control humoral immunity.
Results
TFR cells expand clonally throughout lymphoid organs after immunization
TFR cells undergo clonal expansion, which is poorly understood12,21. We isolated the superior and inferior regions of a spleen 10 days after immunization (Extended Data Fig. 1a). These regions had similar frequencies of total follicular T cells (CD4+CXCR5+PD-1+), TFH cells (CD4+CXCR5+PD-1+FoxP3−), TFR cells (CD4+CXCR5+PD-1+FoxP3+) and PD-1hi TFR cells (CD4+CXCR5+PD-1hiFoxP3+) (Fig. 1a). Moreover, GC B cells (CD19+CD38−GL7+) were similar (Fig. 1b). To assess whether TFR cells expand clonally we performed T cell receptor (TCR) sequencing (TCR-seq) on sorted TFR cells from the superior and inferior regions. When we compared all TFR TCR sequences we found ~1,000 TFR clones per mouse with ~40% of these being expanded (Fig. 1c). Within expanded clones, most were minimally expanded, although some clones appeared more than ten times (Fig. 1d). To assess whether TFR cells can move between distinct regions of the spleen during expansion we compared TCR sequences and found the same TFR clones in both the superior and inferior regions (Fig. 1e–g and Extended Data Fig. 1b). Of all expanded TFR clones, ~10% had evidence of ‘traveling’ from one region of the spleen to another (Fig. 1f). In some cases, clones had a higher proportion in one of the regions, possibly where they first developed (Fig. 1g). Together these data indicate that TFR cells undergo clonal expansion and can migrate to neighboring regions of the same lymphoid organ.
a, Representative gating strategy and quantification of total TFR cells or PD-1hi TFR cells (n = 5). b, Representative gating strategy and quantification of CD19+GL7+CD38− GC B cells (n = 5). c, Clonality of total TFR cells from spleens of mice with superior and inferior regions combined. Singleton clones are grouped and shown in black. d, Distribution of expanded TFR clones as in c. e, Identification of ‘traveling clones’ defined as clones found in both the superior and inferior regions of the same spleen. Data from n = 3 are shown. The top ten most frequent clones from each mouse are indicated by color. f, Left: percentage of traveling clones within indicated populations (n = 3). Right: amino acid sequences of each highly expanded clone for mouse 2. g, Left: relative abundance of expanded clones in each region of the same spleen. Right: amino acid sequence for each clone. Data shown in a, b and f are presented as mean ± s.e.m. Statistical analyses were performed using unpaired two-tailed Student’s t-test.
Effector TFR cells require progressive differentiation from stem-like progenitor to effector TFR cells
We next studied the dynamics of TFR differentiation while incorporating assessment of FoxP3 stability. Foxp3CreERT2GFPRosa26LoxSTOPLoxTdtomato mice (cells previously FoxP3+ are Tdtomato+, cells currently FoxP3+ are GFP+) were vaccinated and assessed on day 24 (during GC contraction) (Extended Data Fig. 2a). Around 30% of TFR cells lost FoxP3 to become ExTFR cells (Fig. 2a). We next assessed TFR cell developmental dynamics incorporating transcriptional programming (differentiation state), FoxP3 history (stability and ExTFR (ref. 25)) and TCR sequence (clonal dynamics) in a longitudinal sampling setting. We performed a survival hemisplenectomy surgery (used in T cell studies23,30) in which the inferior region of the spleen was removed surgically at day 10 after immunization (initial TFR response) and the superior region profiled 2 weeks later (GC contraction) (Fig. 2b). We sorted TFR cells (CD4+CXCR5+GFP+Tdtomato+) at days 10 and 24, and ExTFR (CD4+CXCR5+GFP−Tdtomato+) on day 24, and performed single-cell RNA sequencing (scRNA-seq)/TCR-seq. All TFR cells together segregated into ten distinct clusters in uniform manifold approximation and projection (UMAP) space (Fig. 2c). Using published TFR cell genes, we separated clusters into three distinct differentiation stages: progenitor, early effector and late effector (Fig. 2c–e). The progenitor TFR cell stage included a single cluster ‘Prog’ that expressed Sell/CD62L and S1pr1, which was notable since other studies used Sell to label naive T cells23. The early effector stage included four clusters; a circulating-like cluster (expressing Klf2, Crip1, Vim, Cxcr3 and S100a6), an interferon (IFN)-responsive cluster (expressing Ifit1, Isg15 and Ifit3), an early effector 1 cluster (expressing Lars1 and Klf2) and an early effector 2 cluster (expressing Izumo1r and Il2ra). The late effector stage contained five clusters including CD25+ effector and TNFR+ effector clusters (expressing Il2ra, Tnfrsf9/4-1BB, Tnfrsf4/OX40, Cd69), late effector 1 and 2 (both expressing Pdcd1, Maf, Tigit, Tox2 and Sostdc1) and memory (expressing Ikzf2, Camk1d, Jarid2 and Lars2). Pseudotime analysis demonstrated progressive development from progenitor to early effector to late effector stages with three terminal outcomes; CD25+ effector, late effector 2 or memory TFR cells (Fig. 2d). In addition, a fourth terminal outcome of progenitor to Circulating TFR differentiation was found. Stages could be separated based on Pdcd1/PD-1 and Il2ra/CD25 expression with progenitor TFR being Il2ra+Pdcd1low, early effector TFR being Il2ra+Pdcd1+ and late effector being Il2ra−Pdcd1hi (Fig. 2e,f and Extended Data Fig. 2b,c). Network analysis revealed Bcl6 as a transcription factor driving stage transitions (Extended Data Fig. 2d). To confirm progenitor potential, we adoptively transferred progenitor TFR cells and found these differentiated into Effector TFR cells with enhanced expansion (Extended Data Fig. 2e). Moreover, progenitor TFR cells that were activated in vitro had suppressive capacity (Extended Data Fig. 2f).
a, Left: gating strategy for TFR and ExTFR cells. Right: frequency of ExTFR cells and PD-1+CD25− TFR cells at indicated timepoints postimmunization (n = 6). D10: day 10; D24: day 24. b, Schematic of longitudinal sampling of spleens by survival hemisplenectomy surgery to assess temporal dynamics of TFR differentiation over time in immunized and tamoxifen-treated Foxp3CreERT2Rosa26LoxSTOPLoxTdTomato mice. c, Left: annotated UMAP of TFR cells from experiments in b including description of TFR substages. Right: grouping of TFR cells into broader progenitor, early effector and late effector stages. d, Left: Monocle3 pseudotime analysis of clusters with root node set as progenitor cluster. Only day 24 TFR data are shown. Right: expression of indicated genes with pseudotime indicated. e, Gene expression of indicated genes in TFR cells from indicated stage/substage. f, Feature plots showing S1pr1, Il2ra, Pdcd1, Bcl6 and Prdm1 expression on UMAP projections as in c. g, Left: UMAP projections of day 10 and day 24 TFR cells separately. Right: proportion of each developmental substage in day 10 and day 24 TFR cells. h, Pie charts showing TCR clonality of day 10 TFR and day 24 TFR cells for indicated mice. TCR clones were assigned based on identical CDR3 amino acid sequences. Black indicates grouped singleton clones. Expanded clones shared between day 10 and day 24 are marked red and clones not shared are shown in gray. Data shown in a are presented as mean ± s.e.m. Statistical analyses were performed using unpaired two-tailed Student’s t-test. Illustrations in b created using BioRender.com.
TFR cells at day 10 had more progenitor and early effector cells, whereas day 24 TFR had increased late effector TFR cells (Fig. 2g and Extended Data Fig. 2g). Using TCR sequences, we found almost all expanded TFR clones at day 10 were also found expanded at day 24 (Fig. 2h). The substantial overlap of clones from day 10 and day 24 suggests expanded TFR clones originate from progressive differentiation. Together, these data indicate that TFR cells undergo progressive differentiation, that it occurs over the course of weeks, and that these clones can travel to distinct regions of the spleen during the differentiation process.
Human lymph node TFR cells undergo progressive differentiation, but only early effector TFR can be found in the circulation
To confirm progressive differentiation of TFR in humans, we assessed TFR cells from lymph nodes (LNs) and blood. Total follicular T cells (live+CD4+CXCR5+CD19−) from iliac LNs and similarly gated cells from the blood of another two participants were profiled (Fig. 3a). TFR cells were separated from TFH cells using FOXP3 expression (Extended Data Fig. 3a,b). TFR cells formed five distinct clusters (Fig. 3b). Cluster C4 was found only in blood. Clusters C1/C2 and C3, enriched in both LN and blood, were consistent with progenitor/early effector TFR and early-to-late transitioning cells, respectively (using gene modules from Fig. 2) (Fig. 3c). Cluster C5 was the only late effector cluster and highly expressed PDCD1/PD-1 and TOX (Fig. 3c,d). Although most blood TFR were in cluster C4, some were also found in clusters C1, C2 and C3. However, no blood TFR cells were found in cluster C5. This trend was consistent for each donor (Extended Data Fig. 3c). The early effector clusters C1–C3 expressed KLF2, which promotes egress of T cells into the circulation31, whereas the late effector cluster C5 lacked expression of circulating genes (SELL/CD62L, S1PR1 and CCR7) (Extended Data Fig. 3d).
a, Schematic of experiment to assess human LN and blood TFR cells. Total CD4+CXCR5+CD19− cells were sorted from LN (n = 3) or blood (n = 2) and sequenced. TFR cells were extracted from dataset by identifying FOXP3+ clusters and analyzed further. b, UMAP clustering of TFR cells, including by tissue origin. c, Mean module scores of TFR developmental stage gene modules (derived from Fig. 2) in indicated human clusters. Each datapoint represents the mean module score of cells from each participant per cluster: Prog, progenitor TFR; Early, early effector TFR (encompassing early effector 1, early effector 2 and IFN-responsive TFR); Late, late effector TFR gene set (encompassing CD25+ effector TFR, TNFR+ effector TFR, late effector 1 TFR, late effector 2 TFR and memory TFR); Circulating, circulating TFR gene set. Statistical analyses were performed using one-way ANOVA for group comparisons. Violin plots display the median and quartile values of the three datapoints as dashed and dotted lines, respectively. d, Dotplots showing differentially expressed gene markers in clusters C1–C5. e, Schematic of longitudinal profiling by scRNA-seq of human blood TFR cells after vaccination. f, Top: UMAP plots illustrating the compositional changes of six clusters (B0–B5) over the indicated timepoints. Bottom: relative proportion of clusters at each timepoint for all eight subjects. g, Module scores for indicated TFR developmental stage gene modules for clusters B2 and B4 (n = 8 for B2, n = 7 for B4, respectively). Data are presented as mean values of each subject. h, Top: differentially expressed genes between clusters B2/B4 versus all other clusters. Bottom: gene set enrichment analysis enriched pathways in clusters B2/B4 versus all other clusters. Statistical analyses were performed using two-sided Wilcoxon rank sum test. Genes that showed log fold changes >0.1 and adjusted values < 0.05 are shown in red. mu, mouse. Illustrations in a and e created using BioRender.com.
To confirm appearance of early effector TFR cells in blood after vaccination we analyzed a scRNA-seq dataset of human CXCR5+CD4+ cells32 (Fig. 3e). A cluster expressing FOXP3 was further interrogated as TFR cells (Extended Data Fig. 3e,f). Total TFR cells formed five distinct clusters (Fig. 3f and Extended Data Fig. 3g). Clusters B2/B4 were less abundant at baseline but increased after vaccination before being attenuated at day 14 (Fig. 3f). People with B2 and B4 responses had a higher overall TFR:TFH ratio (Extended Data Fig. 3h). The vaccine-elicited clusters B2/B4 were enriched for early effector TFR gene modules as well as programs for cellular activation, metabolic flux and motility (Fig. 3g,h). Genes expressed in B2/B4 included: CXCR4 (expressed on activated TFH33), CD69, ID3 (which controls TFR programs34) and RGS1 (required for TFH differentiation35). Together, these data indicate that human TFR cells undergo progressive differentiation within LNs and that a small proportion of early effector TFR cells (but not late effector TFR cells) gain access to the blood and maintain programs for at least a short amount of time, where they are indicators of TFR responses to vaccination.
ExTFR cells originate from unstable late effector TFR stages but maintain transcriptional programming
Next, we assessed the developmental origins of ExTFR cells and found these were contained within annotated TFR clusters, but with over-representation in circulating and late effector (late effector 1/2, TNFR+ effector and memory) stages (Fig. 4a,b). ExTFR cells maintained transcriptional programs including expression of Pdcd1/PD-1, Bcl6 and Icos (Fig. 4c and Extended Data Fig. 4a,b). However, ExTFR cells did have altered expression of some genes within each cluster, notably attenuation of Foxp3, Il2ra (directly regulated by FoxP336) and Cd74 (a Treg stabilizing factor37) (Fig. 4d). Moreover, the T cell stemness and TFH differentiation gene Tcf738,39, the TCR signaling gene Themis, the GC TFH and exhaustion regulator Tox240, and the costimulatory molecule Cd40lg were upregulated in ExTFR cells compared to TFR in most clusters.
a, Left: schematic of experiment. Right: UMAP of day 24 TFR and day 24 ExTFR with developmental stage annotation. Proportions of each TFR substage in day 24 TFR and day ExTFR are shown. b, Expression of Foxp3 in TFR and ExTFR cells in UMAP space from a. c, Feature plots showing expression of indicated genes in UMAP space for day 24 TFR and ExTFR cells. d, Differentially expressed genes between d24 TFR and ExTFR for each TFR substage. min.pct = 0.1 logfc.threshold = 1, two-sided Wilcoxon rank sum test. e, Pie charts indicating clonality based on TCR sequence. Black indicates grouped singleton clones. Blue clones indicate expanded clones shared between day 24 TFR and ExTFR. Each slice is shaded with a different hue to distinguish individual clones, with no particular implications behind the specific hues. Gray clones are expanded clones but are not shared between day 24 TFR and ExTFR. f, UMAP plots showing expanded clones (found more than five times) for each group in UMAP space. g, UMAP plots showing clones shared between day 10 TFR cells and day 24 TFR and/or ExTFR. h, Assessment of clonal expansion for clones shared between day 24 TFR and ExTFR. Log10 values of the number of each clonotype found in day 24 TFR and day 24 ExTFR are plotted on the x and y axes respectively. Clonotypes also found at day 10 are indicated in red. Illustration in a created using BioRender.com.
To assess potential clonal origins we compared the TCR sequences. Most expanded clones in TFR were shared with ExTFR cells, suggesting that ExTFR originate from expanded TFR cells (Fig. 4e). Clonal expansion was similar between TFR and ExTFR within each stage, with the exception of Circulating stage cells, which were more expanded in ExTFR (Fig. 4f,g). Moreover, all of the most expanded clones in TFR and ExTFR cells were from clones also found at day 10 (Fig. 4h). Together, these data suggest that ExTFR cells arise from clonally expanded late effector TFR cells, and largely maintain developmental stage transcriptional signatures.
We next assessed whether the ExTFR state is terminal or if ExTFR cells could be ‘redeemed’ through reacquisition of FoxP3. In vitro cultures of ExTFR cells showed some FoxP3 re-expression indicating redemption—a process that was augmented in presence of TFH cells (Extended Data Fig. 4c–f). Furthermore, ExTFR cells persisted longer than late effector TFR cells and underwent less apoptosis, indicating superior fitness (Extended Data Fig. 4g–i). The superior longevity combined with redemption capabilities indicate ExTFR may resupply late effector TFR cells over time.
Acquisition of effector TFH cell transcriptional programs in TFR cells precedes instability
We next assessed transcriptional predictors of TFR cell instability by stratifying clones based on stability. We designated TFR clones as ‘Stable’ if the clonal frequency was higher in TFR versus the ExTFR compartments at day 24 (Fig. 5a). Likewise, ‘Unstable’ clones had fewer cells in TFR versus ExTFR. Both Stable and Unstable clones were found in each mouse, the latter of which were found in late effector stages (Fig. 5b,c and Extended Data Fig. 5a).
a, Schematic illustrating classification of expanded traveling clones as either ‘Stable’ or ‘Unstable’ on the basis of relative abundance in TFR versus ExTFR compartment at day 24. b, Number of clone occurrences at each timepoint for Stable and Unstable clones. c, UMAP plots corresponding to Fig. 4a that show stable and unstable clones as well as distribution of clones at each development substage. d, Volcano plot showing differentially expressed genes in d24 TFR cells for Stable and Unstable clones. Statistical analyses were performed using Wilcoxon rank sum test. Genes that showed log fold changes >0.1 and adjusted P values < 0.05 are indicated. Dashed line indicates P < 0.05 and log2 fold change >1. e, GSEA of a ‘TFHFull versus TFHProg’ program (derived from ref. 27) in Stable and Unstable clones at the day 24 TFR compartment. FDR, false discovery rate. f, GSEA of a GOBP_REGULATION_OF_CELL_CYCLE gene set in Stable and Unstable clones at the day 24 TFR compartment. g, Developmental substage distribution for representative Stable and Unstable clones. Number in the center of each plot indicates number of cells in the distribution. h, Left: module score for an Unstable gene module (derived from d) in human LN TFR clusters from Fig. 3b). Right: overlapping differentially expressed genes between Unstable TFR clones and human LN cluster 5. Illustrations in a created using BioRender.com.
To uncover factors preceding and contributing to stability, we assessed differentially expressed genes between stable and unstable clones, but only when cells still expressed FoxP3 in the TFR stage. Unstable TFR clones had increased expression of effector TFH genes (Sostdc1, Ascl2, Tox2, Il21, Pou2af1, Plekho1 and Gzmk) and stable clones had increased expression of negative regulators of TFH (Il2ra, Bach2, Klf2, Il7r) (Fig. 5d). To determine whether unstable clones had enrichment for effector TFH genes, we performed gene set enrichment analysis (GSEA) utilizing gene sets for effector TFH (effector versus progenitor TFH27, Bcl6-expressing TFH41). We found unstable TFR clones had enrichment of both effector TFH gene modules, suggesting unstable TFR clones obtain an effector-like TFH program before downregulating FoxP3 (Fig. 5e). Moreover, unstable TFR clones were also enriched for genes in cell division and cell cycle, suggesting enhanced activation (Fig. 5f).
We further assessed how TFR cells transition between TFR and ExTFR cells on an individual clone basis. Both stable and unstable clones were dominated by late effector TFR cells at day 24 and largely maintained a similar distribution of developmental stages when they transitioned to ExTFR cells (Fig. 5g and Extended Data Fig. 5b). Two distinct clones illustrated divergent fates as ExTFR cells. Stable clonotype 9 was found in late effector clusters as TFR cells, but had reduced conversion to ExTFR. In contrast, unstable clonotype 3 expanded as ExTFR even though these cells had similar distribution in the late effector stage. Furthermore, we identified a stable clone (clonotype 31) that did not advance to the most terminal late effector 2 stage either as TFR or ExTFR. Both stable and unstable clones had evidence of ExTFR cells in circulating stages even though circulating cells were not found in ancestor TFR cells.
We also assessed our human dataset to assess which LN TFR cells had evidence of instability utilizing an ‘unstable TFR gene module’ predicting descendent instability. We found that late effector-like cluster C5 from the human LN dataset had the most enrichment (Fig. 5h). When we assessed shared genes between mouse unstable TFR cells and human cluster C5 TFR cells we found a number of effector TFH genes such as PDCD1/PD-1, TOX2, BCL6, ICOS, BATF, CTLA4 and CD40LG, suggesting late effector TFR cells in human LN have evidence of instability. Together, these data indicate that acquisition of effector TFH cell programs in late effector TFR cells precedes and predicts descendent instability and that when TFR cells lose FoxP3 to become ExTFR, they reside in similar developmental stages.
TFH cells extrinsically redirect late effector TFR cell development to generate Bcl6-expressing cells
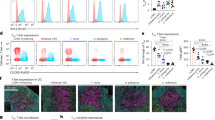
Next we sought to understand how TFH cells control TFR cell differentiation. We required a system in which TFH cells could be acutely deleted without affecting TFR cells. We used F/Ex-DTR mice which delete effector TFH cells without eliminating TFR27,42 (Extended Data Fig. 6a). We vaccinated F/Ex-DTR (Il21CreCxcr5LoxSTOPLoxDTRRosa26LoxSTOPLoxYFP) or control (Il21CreCxcr5wtRosa26LoxSTOPLoxYFP) mice then administered diphtheria toxin (DT) on days 7 and 9 to delete TFH before collection on day 10 (Fig. 6a). Deleting TFH cells acutely minimally affected GC B cells so effects on TFR could be studied (Extended Data Fig. 6b). TFR cells (CD4+CXCR5+PD-1+GITR+) were sorted from control or TFH-deleted mice and scRNA-seq/TCR-seq performed. TFR cells separated into eight distinct clusters, which were annotated for TFR cell developmental stages (Fig. 6b,c). Milo neighborhood analysis indicated over-representation of C4 (early effector 2/memory stage) and C5 (Prdm1hi/late effector 1/Circulating stage), as well as under-representation of C7 (Bcl6hi/late effector 2 stage) in TFR cells from TFH-deleted mice (Fig. 6d and Extended Data Fig. 6c). We further confirmed that C5 and C7 were consistent with late effector 1 and 2 stages, respectively, due to mutually exclusive expression of Bcl6/Prdm1 (Fig. 6e and Extended Data Fig. 6d,e). Together, these data indicate that the default developmental pathway in TFR cells is from progenitor > early effector > Blimp-1+ late effector stage 1 > CD25+ or TNFR+ effector stages. However, when TFH cells are present, TFR are redirected to a late effector 2/Bcl6hi TFR stage. To determine whether Blimp-1 was required to limit C7 genes, we performed bulk RNA-seq on TFR cells (CD4+ICOS+CXCR5+FoxP3-YFP+) from control (Foxp3CrePrdm1wt) or Blimp-1cKO (Foxp3CrePrdm1floxed) mice. C5 genes (Snx9, Il2ra, Ccr2, Arl5a, Ccr5, Gbp6 and Vim) had reduced expression in Blimp-1-deleted TFR cells (Fig. 6f and Extended Data Fig. 6f). Likewise, C7 genes were upregulated in Blimp-1-deleted TFR cells, indicating Blimp-1 limits late effector 2 stage differentiation.
a, Schematic of experiment to assess acute roles of effector TFH cells in TFR differentiation. F/Ex-DTR (Il21CreCxcr5LoxSTOPLoxDTR) or control (Il21CreCxcr5WT) mice were immunized with NP-OVA and DT was given starting at day 7 and organs collected on day 10 for TFR cells scRNA-seq/TCR-seq analysis. b, UMAP plots showing eight clusters (1–8) of TFR cells in F/Ex-DTR or control mice. c, Enrichment of TFR developmental substage gene modules in UMAP space. The colored dots represent cells exhibiting gene set activity, with the color gradient indicating the corresponding AUC scores as calculated by the AUCell R package. Gene sets were generated from data in Fig. 3. d, Left: results of differential abundance testing using MILO neighborhood analysis. Each dot or cell neighborhood that shows significant differential abundance between Ctrl and F/Ex-DTR are marked red (down in F/Ex-DTR) or blue (up in F/Ex-DTR) using an FDR cutoff of 0.1. Right: proportion of clonal expansion for each cluster based on TCR-seq. e, Left: differentially expressed genes between C7 and C5 from d. Vertical lines represent log2 fold changes of 1 and −1, whereas the horizontal line indicates a threshold log10 P value of 3. Statistical analyses were performed using two-sided Wilcoxon rank sum test. Genes that showed log fold changes >0.5 and adjusted P values < 0.05 were plotted. Right: feature plots indicating Bcl6 and Prdm1 expression in UMAP space. f, Top: schematic of transcriptional analysis of TFR cells from Blimp-1 deleted (ΔBlimp-1; Foxp3CrePrdm1fl/fl) or control (Foxp3CrePrdm1wt) mice. Bottom: heatmap showing expression of genes highly expressed in either C5 or C7 in indicated TFR cells. Genes marked with asterisks have an adjusted P value (Padj) < 0.1. g, Left: schematic of experiment to assess ExTFR generation after effector TFH deletion. Il21LoxDTRLoxFoxp3CreERT2GFPRosaLoxSTOPLoxTdTomato (TFH-deleted) mice or Il21wtFoxp3CreERT2GFPRosaLoxSTOPLoxTdTomato (control) mice were given DT to delete TFH cells and TFR/ExTFR assessed. Right: percentages of TFH and ExTFR cells, and frequencies of circulatory and late effector 2-like cells within TFR and ExTFR populations in TFH-deleted mice (n = 7, Control; n = 6, TFH-deleted). h, WT mice were immunized with NP-OVA and on day 24 spleens were analyzed. TFR cells (CD4+FoxP3+CXCR5+) were further subdivided into progenitor (CD62L+PD-1−), early effector (CD62L−CD25+), late effector (CD62L−CD25−PD-1+) or CD25+ effector (CD62L−CD25+PD-1+). Tcf1 expression was then assessed on each population. Left: histogram of TCF-1 expression with B cells as a negative control is shown. Right: percentage of TCF-1 positive population of each TFR stage is shown (n = 7). i, In vitro stability assay with Tcf7 deletion. TFR cells from control (Foxp3ERT2CreTcf7wt) or TregΔTcf7 (Foxp3ERT2CreTcf7floxed) tamoxifen-treated and immunized mice were cultured with B cells (n = 6 wells of culture). ExTFR, FoxP3 MFI and B cell GL7 expression were quantified. Data were replicated across two independent experiments. Data shown in g, h and i are presented as mean ± s.e.m. Statistical analyses were performed using unpaired two-tailed Student’s t-test. Illustrations in a, f, g and i created using BioRender.com.
Next we determined whether TFH cells control TFR cell stability. We required a strategy to delete TFH cells inducibly while allowing FoxP3 fate mapping. We crossed an Il21LoxDTRLox strain43 to FoxP3 fate mapping alleles. In this way, effector TFH, which produce IL-21, will be eliminated with DT and TFR cells assessed for FoxP3 stability. In addition, since the DTR cassette is flanked by LoxP sites, any cell that has expressed FoxP3 (TFR/ExTFR) will be insensitive to deletion regardless of IL-21 expression. We immunized IL21DTR (Il21LoxDTRLoxFoxp3CreERT2GFPRosa26LoxSTOPLoxTdtomato) or control Il21wtFoxp3CreERT2GFPRosa26LoxSTOPLoxTdtomato) mice and deleted TFH with DT, and assessed ExTFR. ExTFR were higher in TFH-deleted versus nondeleted mice, suggesting that TFH cells promote TFR stability (Fig. 6g). Further profiling revealed Circulating-like TFR (CXCR3+PD-1−) were enhanced, whereas the late effector 2 population (CXCR3−PD-1+) were attenuated upon TFH deletion (Extended Data Fig. 6g).
To further investigate mechanisms of TFR stability, we focused on C7 genes also expressed in ExTFR, including Tcf7 (Fig. 6e,f). In TFR cells, Tcf7 was differentially expressed in C7 compared to C5 cells (Fig. 6e and Extended Data Fig. 6h). At the protein level, TCF-1 (encoded by Tcf7) was highest in progenitor TFR cells, but was still expressed in effector stages (Fig. 6h). To assess the role of TCF-1 in TFR stability we vaccinated Foxp3ERT2CreTcf7fl/fl mice and administered tamoxifen to delete Tcf7. TregΔTcf7 mice had less total TFR, as reported39 (Extended Data Fig. 6i). We cultured TFR from control or TregΔTcf7 mice with B cells and assessed conversion to ExTFR cells. Tcf7-deficient TFR cells were more stable compared to Tcf7-sufficient TFR cells, indicating Tcf7 promotes instability (Fig. 6i). Taken together, TFH cells redirect progressive differentiation of TFR cells away from a default Blimp-1+ late effector 1 pathway toward a Bcl6-dominated late effector 2 pathway without inducing instability (Extended Data Fig. 6j). In this manner, the GC reaction contains negative feedback loops in which increased TFH cells redirect sequential TFR cell differentiation toward late effector TFR cells to limit humoral immunity (Extended Data Fig. 6k).
Discussion
TFR cells potently regulate the GC reaction in health and disease, yet factors controlling TFR cell differentiation and stability are poorly understood. Here we uncovered three main developmental stages in TFR cells; progenitor, early effector and late effector stages, with substages contained within each main stage. The stem-like progenitor stage in TFR cells is similar to the progenitor stage in TFH cells27, with both having expression of Sell/CD62L and can rapidly differentiate and expand in vivo. The current prevailing paradigm suggests that TFR cells invade GCs only late during immune responses, and regulation of GCs is dictated by de novo recruitment of TFR cells. However, studies supporting this paradigm utilized Sell expression to identify naive T cells23. Our work suggests this strategy probably marked progenitor TFR cells as naive CD4 T cells. Our data support a paradigm in which TFR cells enter the GC reaction early as progenitor cells but require weeks for sequential development to occur to generate effector TFR cells. In support of this concept, we find little evidence of new TFR clones entering the GC reaction after a few weeks. This prolonged presence in lymphoid organs may allow the immune system several opportunities to regulate the TFR response.
We observed similar developmental stages in human LNs and peripheral blood after seasonal influenza vaccination. A recent finding similarly suggests heterogeneity in human tonsillar TFR cells through varying expression of ICOS and PD-144. Our study indicates human late effector TFR cells do not gain access to the blood, but some progenitor/early effector-like TFR cells can. These data support previous reports that human blood TFR cells are not fully developed19. Our data indicate that progenitor/early effector TFR cells peak in blood within a week after influenza vaccination. Therefore, identifying and analyzing progenitor/early effector TFR cells in the easily accessible blood may offer a window into lymphoid organ TFR cell responses during human studies. However, these cells seem to quickly lose their LN progenitor/early effector programming, probably to generate memory TFR cells, which mount quicker responses upon antigen exposure16.
Although ExTFR cells have been reported, the signals conferring instability are poorly studied25. We found that late effector TFR cells can downregulate FoxP3, whereas progenitor and early effector TFR are more stable. The transcriptional similarities between TFR and ExTFR cells in each stage were an unexpected finding. This observation underscores that the transcriptional program controlling TFR cell identity is complex and probably involves factors beyond FoxP3 and may be distinct for each stage. In a similar way, tissue-resident Treg cells can adapt in response to fluctuating signals within tissues45,46,47. Comparison of Unstable and Stable TFR clones revealed that the acquisition of TFH cell transcriptional programs preceded and predicted downstream TFR instability. Although previous work suggested ExTFR cells have distinct transcriptional programs as a bulk population25, our current single-cell data show that ExTFR cells largely maintain their transcriptional programs but are found in later developmental stages. Nevertheless, we did find some key genetic changes in ExTFR cells, including upregulation of Tox2, Il-21, Tcf7 and Cd40lg, as well as loss of Cd74. Due to these features, ExTFR may be identified as TFH cells in studies lacking FoxP3 fate mapping. Moreover, in limited settings some Treg may lose FoxP3 and undergo TFH differentiation; however, the lack of ExTFR in progenitor/early effector stages in our study suggest this is not a dominant pathway48,49. Although we cannot assess ExTFR cells in human lymphoid organs in vivo due to the lack of fate mapping strategies, our finding of enriched Unstable TFR gene modules in human LN late effector TFR cells suggests this process also occurs in humans. The superior persistence of ExTFR along with reduced cell death and ability to regain FoxP3 expression indicate this population may be an important source of TFR cells during prolonged GC reactions. Relatedly, reports of TFH cells upregulating FoxP3 during GC contraction50,51 may contain some ExTFR cells that re-acquire FoxP3. The possible contribution of Foxp3− Treg or ExTreg cells to ExTFR through further differentiation also remains uncertain52.
Our work also uncovers extrinsic factors that control TFR development. The accumulation of Blimp-1+ Effector TFR cells when TFH are acutely deleted suggests that development of TFR cells until the Blimp-1+ Effector stage is largely intrinsic, and later stages conditional on TFH cells. Specifically, TFH cells redirect TFR cells to a fully suppressive Bcl6+ late effector 2 TFR stage. This arrangement allows for the formation of partially developed TFR cells as a resource pool that can be converted quickly to suppressive effector TFR cells as TFH cells accumulate. The precise mechanisms by which TFH cells redirect late effector TFR cell differentiation and control stability are unclear, but Bcl6 is involved. Tcf7, a stemness marker in some T cells29,53, was highly expressed in ExTFR cells. Tcf7-deficient TFR cells had greater stability, suggesting Tcf7 promotes TFR instability. Paradoxically, TFH cells induce Tcf7 in TFR but also promote TFR stability. Therefore, we hypothesize TFH provide other stabilization factors to counteract Tcf7/TCF-1 effects. Taken together, these data indicate that TFR cells are a dynamic population that undergoes sequential development and that the immune system incorporates several intrinsic and extrinsic strategies to control TFR cells as a negative feedback loop to control humoral immunity.
Methods
Mice
Foxp3CreERT2GFP, Rosa26Lox-STOP-Lox-TdTomato and Tcf7fl/fl were from Jackson Laboratories. Il21Cre mice were a kind gift from U. Hoepken. Cxcr5LoxSTOPLoxDTR mice have been published previously5,11. Il21LoxDTRLox was made by CRISPR-based knock-in of a LoxP-IRES-DTR-LoxP cassette into the 3′ UTR of the Il21 gene43. Both male and female mice, aged 6–10 weeks, were included in the study. Mice were maintained on a 12-h/12-h dark/light cycle at 22 °C with 42% humidity and were fed a 5053 PicoLab rodent diet 20 (LabDiet). All animals were handled in accordance with the policies of the Brigham and Women’s Hospital Institutional Animal Care and Use Committee and the guidelines of the National Institutes of Health.
Immunizations and treatments
Mice were immunized with 100 μg NP-OVA (Biosearch Technologies) emulsified (1:1) in complete Freund adjuvant (CFA, Sigma-Aldrich) for subcutaneous immunization or emulsified in incomplete Freund adjuvant (IFA, Sigma-Aldrich) for intraperitoneal immunization. For deletion experiments, mice were administered 0.5 μg of diphtheria toxin intraperitoneally in PBS at indicated timepoints. In experiments with Foxp3 fate mapping, tamoxifen (2.5 mg per dose, Sigma-Aldrich) was injected intraperitoneally for 5 consecutive days before immunization to induce the activity of Cre recombinase.
Adaptive TCR sequencing
TFR cells (gated as CD4+CD19−CXCR5+FoxP3+) were sorted from superior and inferior regions of spleens from NP-OVA immunized mice at day 12 postimmunization. From the sorted TFR cells, genomic DNA was extracted according to the sample preparation guidelines provided for ImmunoSEQ assays (Adaptive Biotechnologies). TCR B regions were sequenced and TCRs with identical VDJ CDR3 amino acid sequences were considered clonotypes.
Human LNs and blood samples
LNs were collected from consenting patients before kidney transplantation. All patients had renal failure but had not received any immunosuppression. LN tissue was mashed through 70-µm strainers. Total follicular T cells, gated as CD4+CXCR5+, were sorted and subjected to scRNA-seq. Peripheral blood samples were collected from healthy people (Research Blood Components). For human blood analysis after influenza vaccination, data were reanalyzed from a published dataset32. In this dataset, blood was collected at indicated timepoints from influenza vaccine recipients between the ages of 18 and 85 years.
Mouse hemisplenectomy
Inferior parts of spleens from two NP-OVA immunized FoxP3FM mice (Foxp3ERT2Cre-eGFPRosa26Lox-STOP-Lox-TdTomato) were collected 10 days post-immunization with a survival hemisplenectomy, and the remaining superior parts of spleens were collected 24 days post-immunization from the matched mice for longitudinal assay. Briefly, a laparotomy was performed following anesthesia. The spleen was exposed by making a midline abdominal incision. The lieno-pancreatic vein and artery were ligated and disconnected close to the spleen with silk suture. The spleen was ligated twice around its mid-body with silk suture and cut off from the middle. The inferior part of the spleen was then removed, and the superior part was placed back into the peritoneum. The surgical site was kept sterile throughout the procedure. After the laparotomy, the mice were placed on a heating pad for recovery.
scRNA-seq of mouse TFR/ExTFR cells
Spleen or inguinal LN TFR cells (gated as DAPI−CD4+CD19−CXCR5+FoxP3FM-tdTomato+FoxP3-GFP+ and DAPI−CD4+CD19−CXCR5+GITR+ for the hemisplenectomy experiments in Fig. 2 and Fig. 4 and the F/Ex-DTR experiment in Fig. 6, respectively) were sorted. Before sorting, CD4+ T cells were enriched through magnetic selection (Miltenyi Biotec, mouse CD4(L3T4) microbeads). Enriched CD4+ T cells in single-cell suspensions were stained with fluorochrome-labeled cell surface antibodies as well as barcoded antibodies (Cell-Hashing antibody, TotalSeq-C, Biolegend) following the manufacturer’s protocol. Subsequently, TFR cells and/or ExTFR cells were sorted per gating strategies above using BD FACS Symphony S6. The sorted cells were loaded onto a single lane of a Chromium chip K (10x Genomics) and encapsulated in lipid droplets using the Single Cell 5′ kit V2 (10x Genomics) at the Brigham and Women’s Hospital Single Cell Genomics Core. cDNA and library generation were performed according to the manufacturer’s protocol. The gene expression (GEX) library and the V(D)J library were sequenced using Illumina Novaseq. Reads were processed with CellRanger, and quantification was performed using the STAR aligner against the mm10 transcriptome.
scRNA-seq of human TFR cells
From cryopreserved peripheral blood mononuclear cells and external iliac LN single-cell suspensions, CD4+ T cells were enriched magnetically according to the manufacturer’s instructions (Miltenyi Biotech). Samples were barcoded with TotalSeq-C antibodies from Biolegend. CD4+ CXCR5+ CD19− cells were sorted and loaded onto a single lane of a Chromium chip K (10x Genomics) and encapsulated in lipid droplets using the Single Cell 5′ kit V2 (10x Genomics) at the Brigham and Women’s Hospital Single Cell Genomics Core. Subsequently, cDNA synthesis and library preparation were executed as per the manufacturer’s instructions. The 5′ mRNA library was sequenced on an Illumina Novaseq. Postsequencing reads underwent processing using CellRanger, and quantified using the STAR aligner against the human transcriptome. CellRanger output data were imported into the R programming environment for analysis using the Seurat package.
scRNA-seq data processing
The raw data output from CellRanger were imported and analyzed using the Seurat v.5.0 in R 4.3.1. Initially, sample demultiplexing and doublet exclusion were conducted to ensure that only singlets were selected for downstream analysis. Additional quality control measures were applied based on unique molecular identifier counts, gene counts, log-transformed genes per unique molecular identifier and mitochondrial RNA content. Non-T cell contaminants were identified and excluded using the cell annotation tool SingleR (v.2.4.1)54, referencing the ImmGen database for mouse data and HumanCellAtlas for human data. For mouse hemisplenectomy data and human blood kinetics, data from different timepoints were preprocessed independently in separate Seurat objects then merged and integrated using the IntegrateLayers function, employing the anchor-based CCA (canonical correlation analysis) or Harmony integration method. Postintegration, the data were subjected to SCT normalization to generate a merged object, FindNeighbors, FindClusters and RunUMAP for visualization using the new dimensional reduction (integrated.cca or harmony). Afterwards, the layers of each timepoint were rejoined for further differential expression analyses. Monocle pseudotime analysis and Milo differential abundance analysis were performed as described55,56. Gene regulatory network inference was performed using R SCENIC (v.1.1.2) as described57.
Area under the curve analysis and gene set enrichment analysis
Area under the curve (AUC) analysis was utilized to identify corresponding TFR developmental stages of each cluster from F/Ex-DTR scRNA-seq data presented in Fig. 6. AUC scores were calculated to assess the enrichment of gene sets representing different TFR stages in a cluster using R AUCell (v.1.24.0)57. These gene sets were curated using the FindMarker function on mouse hemisplenectomy experiments, with parameters set to a log fold change threshold of 0.1 and a min.pct of 0.1. GSEA was performed to assess the gene signature of mouse Unstable TFR clones and blood human follicular T cells. For mouse Unstable TFR clones, of the in-house curated TFH/TFR-related gene modules and gene ontology categories (C5) and immunological conditions (C7) gene modules from the mSigDB (Molecular Signature Database), significantly enriched gene sets were selected based on the NES (normalized enrichment score). For human blood follicular T cells, the TFH versus Tcon and TFH versus TFR gene modules from previous work25 were used to elucidate the identity of C3.
TCR clonotype analysis
The 10x Genomics CellRanger VDJ output, comprising indexed FASTA files and contig annotations, was processed using the R package Platypus (v.3.6.0)58 to construct a VGM (V(D)J-GEX-Merged) project. This project generated a matrix of TCR clonotype information on a per-cell barcode basis. VGM projects for day 10 TFR, day 24 TFR and day 24 exTFR were merged into a single matrix, and public clonotypes were assigned across the three datasets using the VDJ_clonotype function. Clonotyping was based on CDR3 amino acid sequences. To address cells with atypical chain numbers, a single-chain-based hierarchical clonotyping approach was implemented. Cells with two VDJ chains were excluded, whereas those with only one chain (either VDJ or VJ) were incorporated into preexisting clones if they met the clonotyping criteria. Cells from the GEX library were annotated with their corresponding clonotypes based on their barcodes.
Flow cytometry
Single-cell suspensions were treated with antibody cocktails targeting surface markers for 30 min at 4 °C, typically using a 1:200 dilution unless specified otherwise: anti-CD4 (BD Horizon, cat. no. GK1.5), anti-CD19 (BD Horizon, cat. no. 1D3), anti-PD-1 (BioLegend, cat. no. RMP1-30), Biotin anti-CXCR5(BioLegend, cat. no. L138D7, 1:100), anti T cell and B cell activation antigen (BD Biosciences, cat. no. GL7), anti-CD62L (BioLegend, clone MEL-14), anti-CD25 (BioLegend, cat. no. PC61), anti-CD38 (BD OptiBuild, 90), anti-IgG1 (BioLegend, cat. no. A85-1, 1:100), anti-CD357 (BioLegend, cat. no. DTA-1), anti-Foxp3 (Invitrogen, cat. no. FJK16-s), anti-TCF-1 (BD Biosciences, cat. no. S33-966). For CXCR5 detection, cells were further stained with Streptavidin-BV421 (BioLegend, 1:300) for 20 min at 4 °C. Intracellular staining involved fixation with Foxp3 Fix/Perm buffer (eBiosciences) according to the manufacturer’s instructions, followed by incubation with intracellular antibody cocktail in permeabilization buffer for 30 min. For apoptosis analysis, Annexin V Apoptosis Detection Kit with 7-AAD (BioLegend) was used per manufacturer’s instructions. Analysis was performed using FlowJo v.4.0 software.
Statistical analyses
Statistical analyses were performed using GraphPad Prism v.9.0 or R v.4.3.1. Student’s two-tailed unpaired t-test was used for comparisons between two groups, while one-way analysis of variance (ANOVA) was used for comparisons involving more than two groups. For DEG analysis between clusters in scRNA-seq data, the Wilcoxon rank sum test was applied. For bulk RNA-seq analysis, DESeq2 was used with the default settings, employing the Wald test, and the Benjamini–Hochberg method was applied to adjust P values for multiple testing. The number of mice per group, the number of replicates per experiment, summary statistics and measures of dispersion are indicated in the legend of each figure.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data generated in this study have been deposited in the GEO database under GSE306818 and GSE307004. All data are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Sage, P. T. & Sharpe, A. H. The multifaceted functions of follicular regulatory T cells. Curr. Opin. Immunol. 67, 68–74 (2020).
Wing, J. B., Lim, E. L. & Sakaguchi, S. Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol. Rev. 296, 104–119 (2020).
Fonseca, V. R., Ribeiro, F. & Graca, L. T follicular regulatory (Tfr) cells: dissecting the complexity of Tfr-cell compartments. Immunol. Rev. 288, 112–127 (2019).
Sage, P. T. et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat. Immunol. 17, 1436–1446 (2016).
Clement, R. L. et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat. Immunol. 20, 1360–1371 (2019).
Botta, D. et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat. Immunol. 18, 1249–1260 (2017).
Fu, W. et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J. Exp. Med. 215, 815–825 (2018).
Eschweiler, S. et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nat. Immunol. 22, 1052–1063 (2021).
Noel, G. et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Invest. 131, e139905 (2021).
Lu, Y. et al. CD4+ follicular regulatory T cells optimize the influenza virus-specific B cell response. J. Exp. Med. 218, e20200547 (2021).
Cavazzoni, C. B. et al. Follicular T cells optimize the germinal center response to SARS-CoV-2 protein vaccination in mice. Cell Rep. 38, 110399 (2022).
Maceiras, A. R. et al. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat. Commun. 8, 15067 (2017).
Aloulou, M. et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat. Commun. 7, 10579 (2016).
Kumar, S. et al. Developmental bifurcation of human T follicular regulatory cells. Sci. Immunol. 6, eabd8411 (2021).
Sage, P. T., Francisco, L. M., Carman, C. V. & Sharpe, A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Sage, P. T., Alvarez, D., Godec, J., von Andrian, U. H. & Sharpe, A. H. Circulating T follicular regulatory and helper cells have memory-like properties. J. Clin. Invest. 124, 5191–5204 (2014).
Sage, P. T., Paterson, A. M., Lovitch, S. B. & Sharpe, A. H. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039 (2014).
Wing, J. B., Ise, W., Kurosaki, T. & Sakaguchi, S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41, 1013–1025 (2014).
Fonseca, V. R. et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci. Immunol. 2, eaan1487 (2017).
Jandl, C. et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat. Commun. 8, 14647 (2017).
Ritvo, P. G. et al. High-resolution repertoire analysis reveals a major bystander activation of Tfh and Tfr cells. Proc. Natl Acad. Sci. USA 115, 9604–9609 (2018).
Wing, J. B. et al. A distinct subpopulation of CD25(-) T-follicular regulatory cells localizes in the germinal centers. Proc. Natl Acad. Sci. USA 114, E6400–E6409 (2017).
Merkenschlager, J. et al. Continually recruited naive T cells contribute to the follicular helper and regulatory T cell pools in germinal centers. Nat. Commun. 14, 6944 (2023).
Panduro, M., Benoist, C. & Mathis, D. Tissue Tregs. Annu. Rev. Immunol. 34, 609–633 (2016).
Hou, S. et al. FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J. Exp. Med. 216, 605–620 (2019).
Feng, H. et al. A novel memory-like Tfh cell subset is precursor to effector Tfh cells in recall immune responses. J. Exp. Med. 221, e20221927 (2024).
Podesta, M. A. et al. Stepwise differentiation of follicular helper T cells reveals distinct developmental and functional states. Nat. Commun. 14, 7712 (2023).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Schnell, A. et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298 (2021).
Merkenschlager, J. et al. Dynamic regulation of T(FH) selection during the germinal centre reaction. Nature 591, 458–463 (2021).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Nelson, C. S. et al. The inflammaging microenvironment induces dysfunctional rewiring of Tfh cell differentiation. JCI Insight 10, e187271 (2025).
Estes, J. D. et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J. Immunol. 173, 6169–6178 (2004).
Miyazaki, M. et al. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat. Immunol. 15, 767–776 (2014).
Caballero-Franco, C. & Kissler, S. The autoimmunity-associated gene RGS1 affects the frequency of T follicular helper cells. Genes Immun. 17, 228–238 (2016).
Marson, A. et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445, 931–935 (2007).
Bonnin, E. et al. CD74 supports accumulation and function of regulatory T cells in tumors. Nat. Commun. 15, 3749 (2024).
Wu, T. et al. TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep. 12, 2099–2110 (2015).
Choi, Y. S. et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16, 980–990 (2015).
Xu, W. et al. The transcription factor Tox2 drives T follicular helper cell development via regulating chromatin accessibility. Immunity 51, 826–839 e825 (2019).
Liu, X. et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 209, 1841–1852 (2012).
Zhang, H. et al. IL-21-producing effector Tfh cells promote B cell alloimmunity in lymph nodes and kidney allografts. JCI Insight 8, e169793 (2023).
Chandrakar, P. et al. Progressively differentiated T(FH)13 cells are stabilized by JunB to mediate allergen germinal center responses. Nat. Immunol. 26, 473–483 (2025).
Ribeiro, F. et al. PD-1 and ICOS are coexpressed in T follicular helper cells but define three stages of maturation of T follicular regulatory cells. Sci. Adv. 11, eadt8901 (2025).
Cipolletta, D. et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Wang, Y., Su, M. A. & Wan, Y. Y. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35, 337–348 (2011).
Sjaastad, L. E., Owen, D. L., Tracy, S. I. & Farrar, M. A. Phenotypic and Functional Diversity in Regulatory T Cells. Front. Cell Dev. Biol. 9, 715901 (2021).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science 323, 1488–1492 (2009).
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Jacobsen, J. T. et al. Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science 373, eabe5146 (2021).
Le Coz, C. et al. Human T follicular helper clones seed the germinal center-resident regulatory pool. Sci. Immunol. 8, eade8162 (2023).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158, 749–763 (2014).
Chen, Z. et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51, 840–855 e845 (2019).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Dann, E., Henderson, N. C., Teichmann, S. A., Morgan, M. D. & Marioni, J. C. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 40, 245–253 (2022).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Cotet, T. S. et al. ePlatypus: an ecosystem for computational analysis of immunogenomics data. Bioinformatics 39, btad553 (2023).
Acknowledgements
We would like to thank U. Hoepken for kindly providing mice. We would like to thank the Brigham and Women’s Hospital Center for Cellular Profiling, the Beth Israel Deaconess Medical Center Flow Core, the Harvard Medical School Biopolymer facility and Harvard Medical School Flow Core for their support. This work was supported by grants from the NIH (R01AI153124 to P.T.S., R01AI158413 to P.T.S., U01AI165442 to W.A.M.), the São Paulo Research Foundation (grant number: 2022/07035-3 to P.L.R.) and TaiwanBio Therapeutics.
Author information
Authors and Affiliations
Contributions
P.T.S. and J.-M.L. conceptualized the project. J.-M.L., C.S.N. and M.A.P. developed bioinformatics. J.-M.L., P.L.R., H.Z., S.G.R., R.B.R., K.P.B., P.C. and M.G.G. performed the experiments. C.S.N., C.B., D.K., S.J.G., M.W.S. and J.S.B. provided resources. P.T.S. and J.-M.L. wrote the paper, which was reviewed and edited by all authors. P.T.S., A.H.S., W.A.M., M.C.H. and C.Y. provided technical support and funding.
Corresponding author
Ethics declarations
Competing interests
C.Y. is an employee of TaiwanBio Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Luis Graca and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: S. Houston, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Clonality of Tfr cells in superior and inferior regions of the spleen.
a) Schematic of sampling superior and inferior regions of the spleen after immunization. Mice were immunized with NP-OVA and the superior/inferior regions of spleens were analyzed on day 10. b) Pie charts depicting Tfr clonality in inferior and superior regions in each mouse (n = 3). TCR clones were identified based on identical CDR3 amino acid sequences. The number at the center of each pie chart indicates the total number of TCR clones detected. Black segments represent grouped singleton clones.
Extended Data Fig. 2 Additional analysis of Tfr stage progression over time.
a) Flow cytometry data from a kinetics experiment profiling Tfh and Tfr cells. Mice were administered NP-OVA adjuvanted with IFA intraperitoneally to induce follicular cells (D0, n = 4; D10, n = 3; D17, n = 5; D24, n = 5). Tfr cells were gated as CD4+CXCR5+Foxp3+, and Tfh cells were gated as CD4+Foxp3−CXCR5+PD-1+. P-value indicates results from one-way ANOVA. b) Identification of Tfr developmental stages by flow cytometry (n = 7). c) Differential gene expression analyses between indicated stage transitions. From the mouse hemisplenectomy scRNA-Seq data in Fig. 2, only the day 24 Tfr data was used for analyses using FindMarkers function of R Seurat package with the parameter setting of log2fc threshold = 0.5 and min.pct = 0.1. The dashed line indicates the threshold for a log2 fold change of 1 and an adjusted p-value of 0.05. d) Transcription factor regulatory network analysis. Day 24 Tfr and ExTfr cells from Fig. 4 were subject to SCENIC pipeline. The AUC scores of top 30 regulons (that is, transcription factor and its target genes) that have high variance of activities across all the cells are shown. e) Adoptive transfer of Progenitor/Effector Tfr cells. WT CD45.1 mice were vaccinated with NP-OVA and on day 10 spleens were used to sort Progenitor Tfr cells (CD4+CXCR5+GITR+CD62L+) and Effector Tfr cells (CD4+CXCR5+GITR+CD62L−PD-1+) (ProgTfr + Tfh, n = 2; Eff Tfr + Tfh, n = 3). Cells were transferred along with effector Tfh cells (CD4+CXCR5+GITR−PD-1+) into TCRa−/− mice that were vaccinated with NP-OVA on the same day. Lymph nodes were assessed on d7 post vaccination. f) In vitro suppression assay in which progenitor or effector Tfr cells (gated as above) were cultured with Tfh and B cells (n = 3 wells per experimental group). GL7 expression was assessed on B cells. Statistical analyses were performed using one-way ANOVA for group comparisons. g) UMAP cluster distribution of Tfr cells at day 10 or day 24 in Fig. 2g separating replicate mice.
Extended Data Fig. 3 Additional assessment of human Tfr cell developmental stages in lymph nodes and blood.
a) Gating strategy and representative FACS plots for sorting follicular T cells from human lymph nodes and blood. b) (left) UMAP plots showing thirteen clusters of total CD4+CXCR5+ cells. (right) Feature plot of FOXP3 expression. Clusters 2 and 11 were defined as FOXP3+ (n = 692 cells) and used for downstream analyses of lymph node/blood Tfr. c) Relative abundance of Tfr clusters C1-C5 in each donor for human lymph nodes and blood as in Fig. 3a-b. d) Expression of indicated genes in human LN Tfr clusters taken from Fig. 3b. e) (left) UMAP plots showing eight clusters of total CD4+CXCR5+ cells from human blood vaccination dataset32. (right) Feature plot of FOXP3 expression. Cluster 7 (n = 434 cells) and the Tfr Cluster 3 (n = 5,471 cells) identified as FOXP3+ are indicated. The Tfr cluster 3 was used for downstream analyses. f) GSEA showing enrichment of TfhvsTcon or TfhvsTfr gene modules25 in Cluster 7 versus Cluster 3. g) Dot plots showing gene markers for clusters B0-B5. h) (top) Representative flow cytometry plots of human blood Tfr cells gated as CD3+CD4+CXCR5+CD45RA−Foxp3+. (bottom) Relative percentages of total Tfr as a frequency of total CD4 + T cells or of total follicular T cells at indicated timepoints. Number indicates donor. The donors that showed the increase of vaccine-elicited clusters B2 and B4 are marked in dark red.
Extended Data Fig. 4 Additional assessment of ExTfr transcriptional programs and in vitro characteristics.
a) Heatmap showing key marker gene expression in developmental stages in Tfr and ExTfr cells. Marker genes identified using FindAllMarkers on day 24 Tfr data. Top 8 markers per stage (avg_log2FC > 2 and pct.1 > 0.2) displayed for side-by-side Tfr and ExTfr comparison. b) Pathway analysis of differentially expressed genes between Tfr and ExTfr in LateEffector or CD25 + /TNFR+Effector stages. DEGs with significance threshold 0.05 (Benjamini-Hochberg FDR) queried in gProfiler. c-f) FoxP3 reacquisition by ExTfr cells. Tfr/ExTfr cells from day 17 post NP-OVA vaccination were cultured. c) ExTfr with B cells plus anti-IgM (5 µg/mL) and anti-CD3/28 Dynabeads on day 4 (n = 3 wells). d) ExTfr with Tfh cells plus anti-CD3/28 Dynabeads on day 4 (n = 3 wells). e) ExTfr with B cells ± Tfh cells plus anti-CD3 (2 µg/mL) and anti-IgM (5 µg/mL) on day 6 (n = 3 wells). f) Tfr and ExTfr cultures with anti-CD3/28 Dynabeads (n = 4 ExTfr; n = 3 Tfr). Foxp3 mRNA measured by RT-PCR on day 4. g) In vitro Tfr instability. Tfr cultured with B cells anti-IgM (5 µg/mL), and varying anti-CD3/CD28 concentrations (0.2 n = 2; 2 n = 3; 2/0.5 n = 3; 2/5 n = 3) on day 4. (left) Representative Foxp3 expression in live CD4+MHC-II− cells. (right) Quantification. h) B cell suppression assay. Tfr (CD4+CXCR5+tdTomato+GFP+) and ExTfr (CD4+CXCR5+tdTomato+GFP−) sorted from NP-OVA immunized Foxp3-fatemapper mice; Tfh cells (CD4+Foxp3−CXCR5+ICOS+) from Foxp3-GFP mice. Cultures contained 30,000 Tfh, 50,000 B cells, and 15,000 Effector Tfr or ExTfr cells with anti-CD3 (2 μg/mL) and anti-IgM (5 μg/mL) (No Tfh/Tfr n = 3; No Tfr n = 3; Effector Tfr n = 3; ExTfr n = 3), day 6. i) Apoptosis analysis. 30,000 Progenitor Tfr (CD62L+ Tfr), Effector Tfr (CD62L−PD-1+ Tfr), or ExTfr (CD4+CXCR5+tdTomato+GFP−) from immunized Foxp3-fatemapper mice cultured with anti-CD3/CD28 Dynabeads (Prog Tfr n = 2; Effector Tfr n = 2; ExTfr n = 3). Annexin-V and 7-AAD assayed day 3. (left) Representative plots. (right) Quantification.
Extended Data Fig. 5 Relative proportions and clonal features of Stable and Unstable Tfr clones.
a) (top) Relative proportions of Stable and Unstable clones of all TCRs detected at day 24 encompassing Tfr and ExTfr. (bottom) Relative proportions of Stable and Unstable clones for traveling clones (found in both d10 and d24 datasets). b) Individual Tfr clones at distinct timepoints and in Tfr and ExTfr compartments. The highlighted cells in each plot denote the cells from each time point that share the same TCR sequence.
Extended Data Fig. 6 Additional analysis of Tfr cells in Tfh-deleted mice.
a) Characterization of F/Ex-DTR mice. Control (Il21CreRosaYFPCxcr5wt) or F/Ex-DTR (Il21CreRosaYFPCxcr5LoxSTOPLoxDTR) were immunized, given diphtheria toxin on days 7-9, and dLN and spleen assessed on d10 as in Fig. 6a (Ctrl n = 5, F/Ex-DTR n = 3). b) Flow cytometry showing Tfh deletion and GC B cell frequencies. Mice vaccinated with NP-OVA, followed by diphtheria toxin treatment on days 7 and 9, and lymph nodes collected on day 10 (Ctrl n = 5, F/Ex-DTR n = 11). Unpaired Student’s t-test for statistical analysis. c) Flow cytometry of Tfr cells from F/Ex-DTR mice as in Fig. 6a-d and Extended Data Fig. 6a (Ctrl n = 4, F/Ex-DTR n = 5). Gating strategy shown in Extended Data Fig. 2b was used. Statistical significances determined using two-tailed Mann-Whitney test. d) Expanded clones of Tfr cells shown as blue on UMAP plot from Fig. 6a-d. Expanded clones defined as TCR clones found more than twice. e) Violin plots showing area under curve (AUC) scores for Late Effector 1 (Late1) and Late Effector 2 (Late2) in clusters C1-C8. f) Volcano plot showing differentially expressed genes between Control and ΔBlimp-1 Tfr cells. Genes identified as significant are highlighted in light orange, while C5 and C7 marker genes are marked in red. Horizontal dotted line represents significance threshold at padj = 0.1, and vertical lines indicate fold change thresholds of 1.5. g) Flow cytometry of Tfr subsets in Tfh-deleted experiments as in Fig. 6i (Control n = 7; Tfh-deleted n = 6). h) Feature plot of Tcf7 from the scRNAseq data of F/Ex-DTR described in Fig. 6a. i) Foxp3ERT2CreTcf7floxed mice immunized with NP-OVA, and lymph nodes were taken at day 10 post immunization for Tfr profiling (+/+ n = 9; fl/+ n = 7; fl/fl n = 5). Unpaired Student’s t-test for statistical analysis. j) Illustration summarizing the role of Tfh cell in controlling Tfr cell developmental stage progression and stability. k) Schematic summary of progressive differentiation of Tfr cells as well as roles of Tfh cells or Tfh programs in controlling fate decisions. For a, b, g, and i, unpaired two-tailed Student’s t-test was used for statistical analysis. Illustrations in j and k created using BioRender.com.
Supplementary information
Source data
Source Data Figs. 1–6
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, JM., Raeder, P.L., Rashid, R.B. et al. Stability and progressive differentiation of TFR cells are intrinsically and extrinsically controlled by TFH programs. Nat Immunol (2026). https://doi.org/10.1038/s41590-025-02373-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41590-025-02373-7