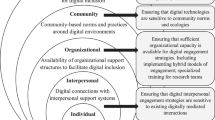
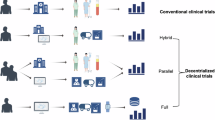
Abstract
Decentralized clinical trials involving the use of digital tools to facilitate remote research are gaining momentum. Rapid advancements in digital technologies have supported the adoption of these trials. These innovations facilitate virtual interactions between clinical trial teams and participants by making it easier to collect, transfer and store electronic data. While some studies have demonstrated the potential for these approaches to reduce barriers to clinical trial participation, they are associated with several challenges that may create or worsen existing health inequalities and limit the generalizability of trial results. Here we review the potential for digitally enabled and decentralized clinical trials to enhance clinical trial participation in an equitable manner. We describe the key barriers individuals from underserved groups may face, and provide recommendations to promote equity, diversity and inclusion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
US Food and Drug Administration. The evolving role of decentralized clinical trials and digital health technologies. FDA https://www.fda.gov/drugs/news-events-human-drugs/evolving-role-decentralized-clinical-trials-and-digital-health-technologies/ (accessed 25 September 2024).
Shen, R. et al. iinternet versus noninternet participation in a decentralized clinical trial: lessons from the ADAPTABLE study. J. Am. Heart Assoc. 12, e027899 (2023).
Retzer, A. et al. A toolkit for capturing a representative and equitable sample in health research. Nat. Med. 29, 3259–3267 (2023).
Adesoye, T., Katz, M. H. G. & Offodile, A. C. 2nd Meeting trial participants where they are: decentralized clinical trials as a patient-centered paradigm for enhancing accrual and diversity in surgical and multidisciplinary trials in oncology. JCO Oncol. Pract. 19, 317–321 (2023).
Nassif, M. et al. Recruitment strategies of a decentralized randomized placebo controlled clinical trial: the canagliflozin impact on health status, quality of life and functional status in heart failure (CHIEF-HF) trial. J. Card. Fail. 29, 863–869 (2023).
Vayena, E., Blasimme, A. & Sugarman, J. Decentralised clinical trials: ethical opportunities and challenges. Lancet Digit. Health 5, e390–e394 (2023).
Al, M., Levison, S., Berdel, W. E. & Andersen, D. Z. Decentralised elements in clinical trials: recommendations from the european medicines regulatory network. Lancet 401, 1339 (2023).
Hanley, D. F. Jr. et al. Decentralized clinical trials in the trial innovation network: value, strategies, and lessons learned. J. Clin. Transl. Sci. 7, e170 (2023).
Inan, O. T. et al. Digitizing clinical trials. NPJ Digit. Med. 3, 101 (2020).
National Academies of Sciences, Engineering & Medicine. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups (The National Academies Press, 2022).
Calvert, M. J. et al. Patient reported outcome assessment must be inclusive and equitable. Nat. Med. 28, 1120–1124 (2022).
Goodson, N. et al. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit. Med. 5, 58 (2022).
Aiyegbusi, O. L. et al. Digitally enabled decentralised research: opportunities to improve the efficiency of clinical trials and observational studies. BMJ Evid. Based Med. 28, 328–331 (2023).
de Las Heras, B. et al. Role of decentralized clinical trials in cancer drug development: results from a survey of oncologists and patients. Digit Health 8, 20552076221099997 (2022).
Weber, D. & Nøhr, C. Decentralized clinical trials: potentials for equity in digital health. Stud. Health Technol. Inform. 304, 91–95 (2023).
Dahne, J. & Hawk, L. W. Jr. Health equity and decentralized trials. JAMA 329, 2013–2014 (2023).
Krumholz, H. M. et al. The PAX LC Trial: a decentralized, phase 2, randomized, double-blind study of nirmatrelvir/ritonavir compared with placebo/ritonavir for long COVID. Am. J. Med. S0002-9343, 00271-7 (2024).
Van Norman, G. A. Decentralized clinical trials: the future of medical product development?. JACC Basic Transl. Sci. 6, 384–387 (2021).
Gill, S. K. et al. Consumer wearable devices for evaluation of heart rate control using digoxin versus beta-blockers: the RATE-AF randomized trial. Nat. Med. 30, 2030–2036 (2024).
Rosa, C., Marsch, L. A., Winstanley, E. L., Brunner, M. & Campbell, A. N. C. Using digital technologies in clinical trials: current and future applications. Contemp. Clin. Trials 100, 106219 (2021).
Oracle. The accelerated evolution of clinical trials in a pandemic environment. https://go.oracle.com/researchacceleratedtrials?elqCampaignId=257896/ (accessed 25 September 2024).
Cooper, L. & Jose, N. Despite negative perceptions of clinical trial conduct during the coronavirus disease 2019 (COVID-19) pandemic, are decentralized clinical trial methods here to stay? Ann. Transl. Med 11, 159 (2023).
McDermott, M. M. & Newman, A. B. Remote research and clinical trial integrity during and after the coronavirus pandemic. JAMA 325, 1935–1936 (2021).
Heads of Medicines Agencies, European Commission & European Medicines Agency. Recommendation paper on decentralised elements in clinical trials. European Commission https://health.ec.europa.eu/system/files/2023-03/mp_decentralised-elements_clinical-trials_rec_en.pdf (13 December 2022).
US Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials—guidance for industry. FDA https://www.fda.gov/media/157635/download (April 2022).
Dawson, S. et al. Trial Forge Guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups—practical guidance to support better practice. Trials 23, 672 (2022).
Clinical Trials Transformation Initiative. CTTI recommendations: decentralized clinical trials. https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_DCT_Recs.pdf (September 2018).
The Health Research Authority & Medicines & Healthcare products Regulatory Agency. Increasing the diversity of people taking part in research. NHS Health Research Authority https://www.hra.nhs.uk/planning-and-improving-research/best-practice/increasing-diversity-people-taking-part-research/ (accessed 25 September 2024).
Betcheva, L., Kim, J. Y., Erhun, F., Oraiopoulos, N. & Getz, K. Applying systems thinking to inform decentralized clinical trial planning and deployment. Ther. Innov. Regul. Sci. 57, 1081–1098 (2023).
de Jong, A. J. et al. Opportunities and challenges for decentralized clinical trials: European regulators’ perspective. Clin. Pharmacol. Ther. 112, 344–352 (2022).
Coran, P. et al. Advancing the use of mobile technologies in clinical trials: recommendations from the clinical trials transformation initiative. Digit Biomark. 3, 145–154 (2019).
Garcia, A. et al. Lessons learned in the Apple Heart Study and implications for the data management of future digital clinical trials. J. Biopharm. Stat. 32, 496–510 (2022).
Turakhia, M. P. et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am. Heart J. 207, 66–75 (2019).
Perez, M. V. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 381, 1909–1917 (2019).
Petrini, C., Mannelli, C., Riva, L., Gainotti, S. & Gussoni, G. Decentralized clinical trials (DCTs): a few ethical considerations. Front. Public Health 10, 1081150 (2022).
Garjani, A. et al. Decentralised clinical trials in multiple sclerosis research. Mult. Scler. 29, 317–325 (2023).
Park, J., Huh, K. Y., Chung, W. K. & Yu, K. S. The landscape of decentralized clinical trials (DCTs): focusing on the FDA and EMA guidance. Transl. Clin. Pharm. 32, 41–51 (2024).
Ghadessi, M. et al. Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet J. Rare Dis. 18, 79 (2023).
Doherty, G. J., Goksu, M. & de Paula, B. H. R. Rethinking cancer clinical trials for COVID-19 and beyond. Nat. Cancer 1, 568–572 (2020).
Wang, X. et al. Systematic approach to outcome assessment from coded electronic healthcare records in the DaRe2THINK NHS-embedded randomized trial. Eur. Heart J. Digit. Health 3, 426–436 (2022).
Joseph, J. et al. Pragmatic evaluation of events and benefits of lipid lowering in older adults (PREVENTABLE): trial design and rationale. J. Am. Geriatr. Soc. 71, 1701–1713 (2023).
Zawada, S. J., Ruff, K. C., Sklar, T. & Demaerschalk, B. M. Towards a conceptual framework for addressing state-level barriers to decentralized clinical trials in the US. J. Clin. Transl. Sci. 7, e162 (2023).
Bodicoat, D. H. et al. Promoting inclusion in clinical trials—a rapid review of the literature and recommendations for action. Trials 22, 880 (2021).
Dawson, S., Campbell, S. M., Giles, S. J., Morris, R. L. & Cheraghi-Sohi, S. Black and minority ethnic group involvement in health and social care research: a systematic review. Health Expect. 21, 3–22 (2018).
van Rijssel, T. I. et al. Ethics review of decentralized clinical trials (DCTs): results of a mock ethics review. Drug Discov. Today 27, 103326 (2022).
Fu, S., Gerber, D. E. & Beg, M. S. Decentralized clinical trials in oncology: are we ready for a virtual-first paradigm? J. Clin. Oncol. 41, 181–185 (2023).
Singh, P., Burden, A. M., Natanegara, F. & Beckman, R. A. Design and execution of sustainable decentralized clinical trials. Clin. Pharmacol. Ther. 114, 802–809 (2023).
Makri, A. Bridging the digital divide in health care. Lancet Digit. Health 1, e204–e205 (2019).
Ali, Z. et al. Exploring decentralized glucose and behaviometric monitoring of persons with type 2 diabetes in the setting of a clinical trial. J. Diabetes Sci. Technol. 17, 117–124 (2023).
Hall, C. L. et al. Opportunities and challenges of delivering digital clinical trials: lessons learned from a randomised controlled trial of an online behavioural intervention for children and young people. Trials 21, 1011 (2020).
Thakur, S. & Lahiry, S. Digital clinical trial: a new norm in clinical research. Perspect. Clin. Res. 12, 184–188 (2021).
Sachs, B. C. et al. The PRagmatic EValuation of evENTs And Benefits of Lipid-lowering in oldEr adults (PREVENTABLE) trial: Study design and procedures for cognitive assessment and adjudication. Alzheimer’s Dement. 17, e054022 (2021).
Dockendorf, M. F. et al. Digitally enabled, patient-centric clinical trials: shifting the drug development paradigm. Clin. Transl. Sci. 14, 445–459 (2021).
Sommer, C. et al. Building clinical trials around patients: evaluation and comparison of decentralized and conventional site models in patients with low back pain. Contemp. Clin. Trials Commun. 11, 120–126 (2018).
Coert, R. M. H., Timmis, J. K., Boorsma, A. & Pasman, W. J. Stakeholder perspectives on barriers and facilitators for the adoption of virtual clinical trials: qualitative study. J. Med. Internet Res. 23, e26813 (2021).
Tjeertes, J. et al. Enabling endpoint development for interventional clinical trials in individuals with Angelman syndrome: a prospective, longitudinal, observational clinical study (FREESIAS). J. Neurodev. Disord. 15, 22 (2023).
Cafaro, T. et al. Remote and semi-automated methods to conduct a decentralized randomized clinical trial. J. Clin. Transl. Sci. 7, e153 (2023).
Routen, A., Lekas, H. M., Harrison, J. & Khunti, K. Intersectionality in health equity research. BMJ 383, 2953 (2023).
Watson, N. L., Mull, K. E., Heffner, J. L., McClure, J. B. & Bricker, J. B. Participant recruitment and retention in remote ehealth intervention trials: methods and lessons learned from a large randomized controlled trial of two web-based smoking interventions. J. Med. Internet Res. 20, e10351 (2018).
Clark, L. T. et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr. Probl. Cardiol. 44, 148–172 (2019).
Ganesananthan, S., Rajkumar, C. A., Foley, M., Francis, D. & Al-Lamee, R. Remote digital smart device follow-up in prospective clinical trials: early insights from ORBITA-2, ORBITA-COSMIC and ORBITA-STAR. Eur. Heart J. Suppl. 24, H32–H42 (2022).
McKenna, K. C. et al. Investigator experiences using mobile technologies in clinical research: qualitative descriptive study. JMIR MHealth UHealth 9, e19242 (2021).
Moore, J., Goodson, N., Wicks, P. & Reites, J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J. Rare Dis. 17, 240 (2022).
Sarraju, A. et al. Pandemic-proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP). NPJ Digit. Med. 5, 80 (2022).
Pennestrì, F., Banfi, G. & Tomaiuolo, R. Remote decentralized clinical trials: a new opportunity for laboratory medicine. Clin. Chem. Lab. Med. 61, 1388–1394 (2023).
Coyle, J. et al. A secondary qualitative analysis of stakeholder views about participant recruitment, retention, and adherence in decentralised clinical trials (DCTs). Trials 23, 614 (2022).
Coyle, J. et al. Learning from remote decentralised clinical trial experiences: a qualitative analysis of interviews with trial personnel, patient representatives and other stakeholders. Br. J. Clin. Pharm. 88, 1031–1042 (2022).
NIHR Applied Research Collaboration East Midlands. Equality Impact Assessment (EqIA) Toolkit. https://arc-em.nihr.ac.uk/clahrcs-store/equality-impact-assessment-eqia-toolkit (accessed 25 September 2024).
Blatch-Jones, A. et al. Using digital tools in the recruitment and retention in randomised controlled trials: survey of UK Clinical Trial Units and a qualitative study. Trials 21, 304 (2020).
US Food and Drug Administration. Accessibility guidance and checklists. FDA https://www.fda.gov/about-fda/accessibility-fda/accessibility-guidance-and-checklists (accessed 25 September 2024).
DeCormier Plosky, W., Pluviose-Philip, M. J. & Bierer, B. E. Accessibility by Design (AbD): a toolkit for inclusion of people with disablities in clinical research. MRCT https://mrctcenter.org/diversity-in-clinical-research/tools/abd_toolkit/ (accessed 25 September 2024).
Ramsey, T. M. et al. Recruitment strategies and challenges in a large intervention trial: systolic blood pressure intervention trial. Clin. Trials 13, 319–330 (2016).
Apostolaros, M. et al. Legal, regulatory, and practical issues to consider when adopting decentralized clinical trials: recommendations from the clinical trials transformation initiative. Ther. Innov. Regul. Sci. 54, 779–787 (2020).
Dorsey, E. R., Kluger, B. & Lipset, C. H. The new normal in clinical trials: decentralized studies. Ann. Neurol. 88, 863–866 (2020).
All of Us Research Program Investigators; Denny, J. C. et al. The ‘All of Us’ Research Program. New Engl. J. Med. 381, 668–676 (2019).
Alsdurf, B. Digital vs. decentralized trials: what’s the difference & how do I meet implementation challenges? Clinical Leader https://www.clinicalleader.com/doc/digital-vs-decentralized-trials-what-s-the-difference-how-do-i-meet-implementation-challenges-0001/ (18 November 2021).
Ridge, D. et al. Imposter participants’ in online qualitative research, a new and increasing threat to data integrity? Health Expect. 26, 941–944 (2023).
Health and Care Research Wales, the National Institute for Health and Care Research (NIHR) & Chief Scientist Office. Guidance for recognising and addressing ineligible public involvement in health and care research. Health and Care Research Wales https://healthandcareresearchwales.org/sites/default/files/2024-07/Ineligible_Public_Involvement_paper_v1.pdf (accessed 25 September 2024).
Hirsch, I. B. et al. Incorporating site-less clinical trials into drug development: a framework for action. Clin. Ther. 39, 1064–1076 (2017).
Thomas, K. A. & Kidziński, Ł. Artificial intelligence can improve patients’ experience in decentralized clinical trials. Nat. Med. 28, 2462–2463 (2022).
Abdelazeem, B. et al. The effectiveness of incentives for research participation: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 17, e0267534 (2022).
Vellinga, A. et al. What do patients value as incentives for participation in clinical trials? A pilot discrete choice experiment. Res. Ethics 16, 1–12 (2020).
Griffith Fillipo, I. R. et al. Participant retention in a fully remote trial of digital psychotherapy: Comparison of incentive types. Front. Digit. Health 4, 963741 (2022).
Sridhara, R. et al. Cancer clinical trials beyond pandemic: report of an american statistical association biopharmaceutical section open forum discussion. Stat. Biopharm. Res. 15, 444–449 (2023).
Mowlem, F. D., Tenaerts, P., Gwaltney, C. & Oakley-Girvan, I. Regulatory acceptance of patient-reported outcome (PRO) data from bring-your-own-device (BYOD) solutions to support medical product labeling claims: let’s share the success stories to move the industry forward. Ther. Innov. Regul. Sci. 56, 531–535 (2022).
Al-Kaisey, A. M. et al. Accuracy of wrist-worn heart rate monitors for rate control assessment in atrial fibrillation. Int. J. Cardiol. 300, 161–164 (2020).
Maass, K. F. et al. Leveraging patient-centric sampling for clinical drug development and decentralized clinical trials: promise to reality. Clin. Transl. Sci. 15, 2785–2795 (2022).
Manyazewal, T. et al. Patient-reported usability and satisfaction with electronic medication event reminder and monitor device for tuberculosis: a multicentre, randomised controlled trial. EClinicalMedicine 56, 101820 (2023).
Browne, S. H. et al. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: a randomized controlled trial. PLoS Med. 16, e1002891 (2019).
Ramnath, S. et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob. Health 3, e001018 (2018).
Wei, X. et al. Effectiveness of a comprehensive package based on electronic medication monitors at improving treatment outcomes among tuberculosis patients in Tibet: a multicentre randomised controlled trial. Lancet 403, 913–923 (2024).
Myers, T. L. et al. Recruitment for remote decentralized studies in parkinson’s disease. J. Parkinsons Dis. 12, 371–380 (2022).
Goodday, S. M. et al. An alternative to the light touch digital health remote study: the stress and recovery in frontline COVID-19 health care workers study. JMIR Form. Res. 5, e32165 (2021).
Leroy, V. et al. Digital health technologies and Alzheimer’s disease clinical trials: might decentralized clinical trials increase participation by people with cognitive impairment? Alzheimers Res. Ther. 15, 87 (2023).
Haenel, E., Elash, C. A., Garner, K., Turner, M. & Kern, S. Flexible approaches to eCOA administration in clinical trials: the site perspective. Contemp. Clin. Trials Commun. 37, 101241 (2024).
Johnson, E. & Marsh, L. Clinical research nurse utilisation and role in the conduct of decentralised clinical trials: a literature review. J. Res. Nurs. 28, 214–226 (2023).
Kotecha, D. et al. CODE-EHR best-practice framework for the use of structured electronic health-care records in clinical research. Lancet Digit. Health 4, e757–e764 (2022).
Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26, 1364–1374 (2020).
Khozin, S. & Coravos, A. Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin. Pharmacol. Ther. 106, 25–27 (2019).
Suman, A. et al. A cross-sectional survey on the early impact of COVID-19 on the uptake of decentralised trial methods in the conduct of clinical trials. Trials 23, 856 (2022).
Ranganathan, P. & Pramesh, C. S. Virtual oncology research-different models and lessons learned. Curr. Opin. Support Palliat. Care 16, 117–122 (2022).
DiMasi, J. A. et al. Assessing the financial value of decentralized clinical trials. Ther. Innov. Regul. Sci. 57, 209–219 (2023).
Anguera, J. A., Jordan, J. T., Castaneda, D., Gazzaley, A. & Areán, P. A. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innov. 2, 14–21 (2016).
Acknowledgements
We thank M. K. ElZarrad, director of the Office of Medical Policy at the Center for Drug Evaluation and Research of the US Food and Drug Administration (FDA), who presented the FDA’s perspectives on DCTs at the workshop and provided valuable suggestions during the drafting of the manuscript. The views expressed in this publication are those of the authors and may not represent the views of the National Institute for Health and Care Research (NIHR), Medicines and Healthcare products Regulatory Agency, US FDA and Health Canada. This work was funded by Merck Healthcare. The funder, Merck, and the study sponsor, University of Birmingham, had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication. Several authors are employees of the University of Birmingham. However, beyond the declared author contributions, the sponsor had no additional role. B.M.-O.’s involvement in this piece of work is in a personal capacity and is not as a representative of the Health Research Authority.
Author information
Authors and Affiliations
Contributions
Concept and design, and acquisition, and data analysis: O.L.A., M.J.C., S.C.R. and P.K. O.L.A. and M.J.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Interpretation of data: all authors. Drafting of the manuscript: O.L.A. and M.J.C. Critical revision of the manuscript for intellectual content: all authors.
Corresponding author
Ethics declarations
Competing interests
O.L.A. receives funding from the NIHR Birmingham Biomedical Research Centre (BRC), NIHR Blood and Transplant Research Unit (BTRU) in Precision Transplant and Cellular Therapeutics, NIHR Applied Research Collaboration (ARC) West Midlands, UK Research and Innovation (UKRI), LifeArc, Health Foundation, Merck, Gilead, Anthony Nolan, Sarcoma UK and GSK. O.L.A. declares personal fees from Gilead Sciences, Merck and GSK outside the submitted work. S.C.R. receives funding from UK SPINE and Merck, and declares personal fees from Merck. M.J.C. is director of the Birmingham Health Partners Centre for Regulatory Science and Innovation, director of the Centre for the Centre for Patient Reported Outcomes Research and an NIHR Senior Investigator. M.J.C. receives funding from the NIHR, UKRI, NIHR Birmingham BRC, the NIHR Surgical Reconstruction and Microbiology Research Centre, NIHR ARC West Midlands, LifeArc, UK SPINE, European Regional Development Fund—Demand Hub and Health Data Research UK at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Innovate UK (part of UKRI), Macmillan Cancer Support, UCB Pharma, Janssen, GSK and Gilead. M.C. has received personal fees from Astellas, Aparito, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK, Pfizer, the Patient-Centered Outcomes Research Institute and Vertex outside the submitted work. In addition, a family member owns shares in GSK. N.A. is an NIHR Senior Research Leader and receives funding from the NIHR, NIHR ARC West Midlands and NIHR Birmingham BRC. S.E.H. receives funding from the NIHR, NIHR BTRU in Precision Transplant and Cellular Therapeutics, NIHR Birmingham BRC, NIHR (ARC) West Midlands, UKRI and UK SPINE. S.E.H. declares personal fees from Cochlear, Pfizer, Rinri Therapeutics, Astra Zeneca, Aparito and CIS Oncology outside the submitted work. K.K. has acted as a consultant, speaker or received grants for investigator-initiated studies for Astra Zeneca, Bayer, Novo Nordisk, Sanofi-Aventis, Servier, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Pfizer, Roche, Daiichi Sankyo, Applied Therapeutics, Embecta and Nestle Health Science. K.K. is supported by the NIHR Applied Research Collaboration East Midlands (ARC EM), the NIHR Leicester BRC and the British Heart Foundation (BHF) Centre of Excellence. D.K. reports grants from the NIHR (CDF-2015-08-074 RATE-AF, NIHR130280 DaRe2THINK, NIHR132974 D2T-NeuroVascular, NIHR203326 Biomedical Research Centre), the BHF (PG/17/55/33087, AA/18/2/34218 and FS/CDRF/21/21032), the EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074), EU Horizon and UKRI (HYPERMARKER 101095480), UK National Health Service—Data for R&D-Subnational Secure Data Environment programme, UK Department for Business, Energy & Industrial Strategy Regulators Pioneer Fund, the Cook & Wolstenholme Charitable Trust and the European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre and BHF/University of Birmingham Accelerator Award (STEEER-AF); in addition, D.K. has received research grants and advisory board fees from Bayer, Amomed and Protherics Medicines Development; all are outside the submitted work. C.M. receives funding from NIHR Surgical Reconstruction and Microbiology Research Centre, UKRI, NIHR, NIHR BTRU in Precision Transplant and Cellular Therapeutics and declares personal fees from Aparito outside the submitted work. A.D. is deputy director of the Birmingham Health Partners Centre for Regulatory Science and Innovation and is an NIHR Senior Investigator. A.D. receives funding from the NIHR, UKRI and NIHR Birmingham BRC. J.M. is chief editor at Nature Medicine and has excused himself from the peer review and editorial process of this article. P.M. is director of the Clinical Practice Research Datalink, a UK government real-world data research service that also provides clinical trial recruitment services. R.H. receives funding from the Leverhulme Trust.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aiyegbusi, O.L., Cruz Rivera, S., Kamudoni, P. et al. Recommendations to promote equity, diversity and inclusion in decentralized clinical trials. Nat Med 30, 3075–3084 (2024). https://doi.org/10.1038/s41591-024-03323-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03323-w
This article is cited by
-
Assessing reporting completeness and characteristics in decentralized randomized controlled trials: a scoping review protocol
Systematic Reviews (2025)
-
Mixed methods study of views and experience of non-hospitalised individuals with long COVID of using pacing interventions
Scientific Reports (2025)
-
Advancing equitable access to innovation in breast cancer
npj Breast Cancer (2025)
-
Correlates of patient-centered discussions on colorectal cancer screening: results from the health information national trends survey (HINTS)
Cancer Causes & Control (2025)