Abstract
Continuous temperature monitoring with wearable devices may lead to earlier fever detection. This may improve the outcome of fever in neutropenia episodes in pediatric patients with cancer. This prospective two-center observational and proof-of-concept study recruited pediatric patients undergoing chemotherapy for cancer. Two different wearable devices (WDs), the CORE® and the Everion®, were worn for 14 days to continuously record multiple vital signs. Both WDs record core temperature and its quality score. Everion® records 17 further vital signs or health scores. All measurements resulted in 4945 (CORE®) and 5360 hours (Everion®) of data. Combined, 6’085’943 measurements of vital signs and health scores were recorded. In addition, non-WD data such as acceptability, side effects and discrete ear temperature measurements were collected. The described methods and resulting data can be reused to study acceptability, usability, compliance, and data quality of the CORE® and Everion® devices in pediatric patients. Additionally, these methods and data can be utilized to study vital signs in pediatric patients undergoing chemotherapy for cancer.
Similar content being viewed by others
Background & Summary
In children and adolescents undergoing chemotherapy for cancer, fever in neutropenia (FN) is the most frequent potentially lethal complication. Emergency hospitalization and empirical treatment with i.v. broad-spectrum antibiotics is the current standard of therapy1.
Continuous monitoring of fever can lead to earlier fever detection compared to the usual discrete fever measurements performed only for clinical reasons. Earlier detection of fever leads to earlier assessment and treatment and thus may reduce the risk of complications2,3,4.
Wearable devices (WDs) allow continuous monitoring of vital signs. They are already well established during sport activities or for personal health tracking, and are increasingly used for continuous measurements of patients’ vital signs5,6,7. In pediatric oncology patients, few studies and a case report with different WDs have assessed usability, acceptability and fever outcome8,9,10,11,12,13. These studies show the potential of this technology, but evidence currently remains low.
The data presented here has been collected during the prospective two-center observational study “Continuous timely monitoring of core temperature with two wearable devices in pediatric patients undergoing chemotherapy for cancer - a comparison study” (NCT04914702)14. Twenty pediatric patients aged ≥1 month and <18 years, undergoing chemotherapy for cancer, wore two different WDs (CORE®, Everion®) simultaneously for 14 days14.
The primary aim of this study was to compare the two different WDs regarding feasibility of continuous core temperature recording with timely data accessibility in children undergoing chemotherapy14.
At study entry, participants were instructed in WD and gateway handling, to wear the WDs during the entire 14-day study period as often as possible using size-specific elastic bands on the upper arm or leg, and to measure ear temperature at least twice daily plus when clinically indicated. Results of ear temperature measurements during hospitalization or outpatient visits were retrieved from patient charts14. Of the 20 patients included (16 from Bern, 4 from Basel), three patients later withdrew informed consent (IC) (one before study start, one on the first and one on the fourth day)14. Therefore, data were available for 243 of the 280 study days. The CORE® recorded core temperature and its quality score on 206 of 280 study days (4945 of 6720 hours). The Everion® recorded vital signs and health scores, plus data quality for some of them, on 223 study days (5360 hours). Taken together, the two WDs recorded data on 618’352 measurement points, corresponding to 6’085’943 measurements of vital signs and health scores, plus 2’226’467 quality scores.
Quality of core temperature data from the CORE® was at least sufficient during 4873 hours (73%) and arrived timely on the dashboard during 4213 hours (63%). Quality of Everion® core temperature data was at least sufficient during 5056 hours (75%) and arrived timely on the dashboard during 3556 hours (53%)14. Moreover, 650 discrete measurements of ear temperature were recorded and 19 patients answered the follow-up questionnaire. Non-WD data (patients’ basic characteristics, adverse events, discrete temperature measurements and patient contact details) were also collected14.
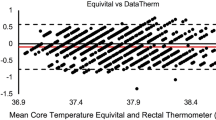
These data can be used to study the feasibility, acceptance, compliance and data quality of two WDs (CORE®, Everion®), to study various vital signs and signals in pediatric patients undergoing chemotherapy for cancer, and to compare the core temperatures measured by the two WDs with the corresponding discrete measurements (ear temperatures).
Methods
This methods section is an expanded version of the methods described in the analytical manuscript14. The goal of this manuscript is to provide well-documented and accessible data for further exploration and analysis.
Experimental design
The study, designed as a prospective, dual-center feasibility and proof-of-concept study, was performed in two Swiss university-based pediatric hematology/oncology centers, the Division of Pediatric Hematology/Oncology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Bern and the Department of Pediatric Oncology and Hematology, University Children’s Hospital Basel (UKBB), University of Basel, Basel.
Both Pediatric Hematology/Oncology Departments provide tertiary care for a population of approximately two, respectively one million inhabitants. Both departments contain an outpatient unit and an inpatient unit with nine beds, including one transplant unit in Bern and three transplant units in Basel. In both centers myeloablative chemotherapy followed by autologous stem cell transplantation (Bern) and or allogeneic stem cell transplantation (Basel) are performed.
Good clinical practice
The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice15,16. Approval was granted by the local Ethics Committee (Kantonale Ethikkommission Bern, BASEC-No.: 2021-00967) prior to patient recruitment and the trial has been registered at ClinicalTrials.gov (NCT04914702). Prior to study entry, written IC was obtained from the patients, if able to judge, and from their legal guardians. They all gave their non-mandatory IC for publication of coded study data.
Participation in this observational study did not influence any diagnostic nor therapeutic decisions14.
Patients and data acquisition
Sixty-five patients were screened for eligibility between September 2021 and March 202214. No specific screening procedures except verifying inclusion and exclusion criteria were needed. These criteria included age ≥1 month and <18 years at time of recruitment and treatment with myelosuppressive chemotherapy for any malignancy expected to last ≥1 month or with at least one cycle of myeloablative therapy prior to autologous/allogeneic hematopoietic stem cell transplantation at the time of recruitment14. Not meeting the inclusion criteria, local skin disease preventing them from wearing WD, or denied IC were exclusion criteria. Patients were assessed during a routine outpatient visit or during hospitalization14.
A total of 20 patients (median age 8 years; range 2 to 17 years; 4 patients <6 years) were recruited into the study as planned, 16 in Bern and 4 in Basel. All patients opted to wear both WDs in parallel. The individual study period was 14 days14. Of the 20 patients included, one withdrew IC before study start, one on study day 1 and one on study day 414. Patient characteristics, clinical data as well as the reported outcomes can be found in Table 1.
Basic patient characteristics were collected at study inclusion. The participants were equipped with one CORE®, two Everions®, one gateway, and a set of paper clinical research forms (CRFs). Participants were instructed on handling of the devices and study procedures14.
Non-WD data were collected on the CRFs. Patients measured their discrete ear temperature twice daily, plus when clinically indicated, and noted it on the provided CRFs14.
The research team performed daily data checks and participants received a daily feedback on data quality and wearing via either text message, e-mail, phone call or during an out/inpatient visit. Contacts, adverse events and problems were also recorded on the CRFs14.
The treating physicians did not have access to the recorded vital signs. Participants and their parents had access to some of their continuously updated data: Battery and transmission status (both devices), core temperature (CORE®) and heart rate (Everion®) at any time of the study via a private dashboard link. A follow-up interview was performed within three days after study end to assess acceptability, usability and side-effects of the WDs14.
WDs and gateway on study
Two different WDs were used: the CORE® by GreenTEG17 and the Everion® VSM-1 by Biovotion (now Biofourmis)18. The CORE® (firmware version 0.3.15), is a commercially available consumer WD. It measures skin temperature once per second, from which it calculates core temperature once per minute using an algorithm optimized in adults for arm positioning, and calculates a quality score (range, 1 to 4; sufficient quality, score ≥ 2) (Table 2).
The Everion® VSM-1 (firmware version 3.0.6) is a medical device class IIa. It records 18 vital sign signals and assigns a quality score (range, 0 to 100, good quality, score ≥ 50) to six of them (Table 2). Skin temperature is recorded once per second, and core temperature calculated once per minute, including its quality score. Additionally heart rate, heart rate variability, respiration rate and further vital signs plus calculated measures, most of them once per second, some with their quality scores are given. To reduce energy consumption, the Everion® was set to mode «green only», which precludes from measuring oxygen saturation. This measure had been found not to be reliably measured in a predecessor study12,13.
To compensate for the wider range of the Bluetooth connection of the CORE®, the gateway configuration setting “default minimum synchronization interval” was increased to 120 seconds for the CORE® versus 60 seconds for the Everion®.
The WDs had to be charged off-body (CORE®, daily for 30 minutes, Everions®, alternating during night/day for several hours). The gateway was rechargeable during function.
Both WDs transmitted data via Bluetooth® Low Energy (BLE) to a custom-built mobile gateway (software version 1.11.0), consisting of a Raspberry Pi 3B + microcomputer, an uninterruptible power supply UPS HAT, an E3372h-320 GSM dongle plus a BLED112 USB dongle programmed by Leitwert for the Bluetooth® connection to the Everion®18,19. The gateway forwarded the data via Wireless LAN or via cellular network to a password protected cloud-based dashboard without storing or processing any data. The dashboard was a GCP-ready server application hosted in Switzerland. Only the research team had access to the dashboard14.
Computational processing and data management
The entire data processing (transport and storage) was encrypted by Leitwert19. Using json scripts, the data was retrieved by the researchers from the dashboard as csv files and then imported into R20. Data recorded once per second was pseudo-randomly reduced to a per minute format by selecting every 60th line. After completion of data retrieval in a local research database, data were irreversibly deleted from the dashboard.
Non-WD data, both from participants and investigators, were collected on CRFs and stored in the REDCap electronic data capture tools21.
WD and non-WD data were transferred to Microsoft® Excel and prepared for sharing on Figshare for this Data Descriptor project: https://doi.org/10.6084/m9.figshare.2250734222.
Data Records
This study generated a single data record, containing totally 41 files. Eighteen files contain the recorded data from the CORE® WDs (file “CORE_data.patient_xx.csv”) and 19 files contain the recorded data from the Everion® WDs (file “Everion_data.patient_xx.csv”). For each patient one file per WD is available, where xx refers to a patient-specific random number. Explanation of the variable specification is provided in a separate “readme file”, one file per device (file “CORE_data_Readme.csv” and file “Everion_data_Readme.csv”).
Another file contains all non-WD data from both devices and all 20 patients on administration, daily CRFs, discrete measurements and follow-up interviews (file “NonWD_data_both_devices.all_patients.csv”). A corresponding “readme file” contains detailed information on variable specification (file “Readme_NonWD_data_both_devices.all_patients.csv”).
All these files are published on Figshare: https://doi.org/10.6084/m9.figshare.2250734222.
Technical Validation
Reduction of recruitment bias
Our study population corresponds to a representative group of pediatric cancer patients in terms of gender, age and diagnosis. This was achieved by screening and inviting all patients from the respective study centers to participate in the study, regardless of diagnosis, age and gender. However, patient recruitment stopped when the target number of 20 patients was reached. Distribution of age, gender and type of malignancy in patients screened, in patients refusing IC, in patients not asked for IC, and the 20 patients included in the study were comparable14.
Data collection and increasing data reliability
As part of the clinical study, data from study administration, daily CRFs and follow-up questionnaires were carefully extracted and entered into the REDCap database by two experienced members of the research team. Discrete measurements recorded during outpatient visits/hospitalization during the study period, or taken at home and noted on the specific CRFs, were retrieved from the medical records or the specific CRFs and also stored in REDCap21.
The WD raw data were downloaded from the dashboard as csv files using json scripts provided by Leitwert19 and then imported into R20.
Anonymization procedure
Prior to publication, the data were anonymized in accordance with Swiss legislation. The study start date for each patient was considered a potential identifier. Study Day 1 therefore replaced it for each patient. Further study days were then calculated from Study Day 1 onward and the real time of the day was expressed as a fraction of the day. Diagnosis, sex and age remain in this data set, as all patients, if able to judge, or their legal guardians gave non-mandatory IC for publication of these data14.
Usage Notes
As two WDs transmitted via one gateway, a data transmission bottleneck due to large data volumes occurred, which was detected at data analysis after the study. A retrospective calculation was performed to eliminate effects of this bottleneck for part of the analysis. Details are described in the main manuscript14. In this manuscript, we present transmission delay data without correction for this bottleneck.
Code availability
For downloading the WD raw data from the dashboard as csv files, json scripts provided by Leitwert were used19. These json scripts are not needed to access, reuse or process the here presented datasets. Non-WD data was extracted from REDCap21 as csv files. Both types of csv files were directly imported into R20, which was used for storage, quality control and analysis of all data. The R script used to process WD data is available on Figshare22. R code for correcting the transmission bottleneck mentioned above is available from the corresponding author upon reasonable request.
References
Lehrnbecher, T. et al. Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J. Clin. Oncol. 41, 1774–1785, https://doi.org/10.1200/JCO.22.02224 (2023).
Koenig, C. et al. Association of time to antibiotics and clinical outcomes in patients with fever and neutropenia during chemotherapy for cancer: a systematic review. Support. Care Cancer 28, 1369–1383, https://doi.org/10.1007/s00520-019-04961-4 (2019).
Rackoff, W. R., Gonin, R., Robinson, C., Kreissman, S. G. & Breitfeld, P. B. Predicting the risk of bacteremia in childen with fever and neutropenia. J. Clin. Oncol. 14, 919–924, https://doi.org/10.1200/JCO.1996.14.3.919 (1996).
Rosenman, M., Madsen, K., Hui, S. & Breitfeld, P. P. Modeling Administrative Outcomes in Fever and Neutropenia: Clinical Variables Significantly Influence Length of Stay and Hospital Charges. J. Pediatr. Hematol. Oncol. 24, 263–268, https://doi.org/10.1097/00043426-200205000-00009 (2002).
Reyzelman, A. M. et al. Continuous Temperature-Monitoring Socks for Home Use in Patients With Diabetes: Observational Study. J. Med. Internet Res. 20, e12460, https://doi.org/10.2196/12460 (2018).
Koshy, A. N. et al. Smart watches for heart rate assessment in atrial arrhythmias. Int. J. Cardiol. 266, 124–127, https://doi.org/10.1016/j.ijcard.2018.02.073 (2018).
Ajčević, M. et al. A Novel Non-Invasive Thermometer for Continuous Core Body Temperature: Comparison with Tympanic Temperature in an Acute Stroke Clinical Setting. Sensors 22, 4760, https://doi.org/10.3390/s22134760 (2022).
Vaughn, J. et al. Mobile Health Technology for Pediatric Symptom Monitoring: A Feasibility Study. Nurs. Res. 69, 142–148, https://doi.org/10.1097/nnr.0000000000000403 (2020).
Sampson, M. et al. Feasibility of continuous temperature monitoring in pediatric immunocompromised patients: A pilot study. Pediatr. Blood Cancer 66, e27723, https://doi.org/10.1002/pbc.27723 (2019).
Kakarmath, S. S. et al. Assessing the Usability of an Automated Continuous Temperature Monitoring Device (iThermonitor) in Pediatric Patients: Non-Randomized Pilot Study. JMIR Pediatr. Parent. 1, e10804, https://doi.org/10.2196/10804 (2018).
Nessle, C. N., Flora, C., Sandford, E., Choi, S. W. & Tewari, M. High-frequency temperature monitoring at home using a wearable device: A case series of early fever detection and antibiotic administration for febrile neutropenia with bacteremia. Pediatr. Blood Cancer 69, e29835, https://doi.org/10.1002/pbc.29835 (2022).
Haemmerli, M., Ammann, R. A., Roessler, J., Koenig, C. & Brack, E. Vital signs in pediatric oncology patients assessed by continuous recording with a wearable device, NCT04134429. Sci. Data 9, 89, https://doi.org/10.1038/s41597-022-01182-z (2022).
Koenig, C., Ammann, R. A., Kuehni, C. E., Roessler, J. & Brack, E. Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer-an operational feasibility study. Support. Care Cancer 29, 5283–5292, https://doi.org/10.1007/s00520-021-06099-8 (2021).
Koenig, C. et al. Continuous timely monitoring of core temperature with two wearable devices in pediatric patients undergoing chemotherapy for cancer - a comparison study. Support. Care Cancer 32, 188, https://doi.org/10.1007/s00520-024-08366-w (2024).
Ordinance on Clinical Trials in Human Research (ClinO).
World Medical Association. WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. The World Medical Association https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (2013).
greenTEG. CORE. https://shop.greenteg.com/core-body-temperature-monitor (2024).
Biovotion, A. G. Everion monitor. Vol. 2019 (Revealing medical grade data, 2018).
Leitwert. (2024).
R Core Team. R: A language and environment for statistical computing. Vienna, Austria. http://www.R-project.org/ (2014).
Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381, https://doi.org/10.1016/j.jbi.2008.08.010 (2009).
Farner, L., Koenig, C., Ammann, R. A. & Brack, E. BernBasel2021WDP data. figshare https://doi.org/10.6084/m9.figshare.22507342 (2024).
Acknowledgements
We thank all our patients and parents for participating in this study. We also thank. Mrs. A. Ammann-Meile, Mosnang, Switzerland, for the kind donation of the CORE® devices. This study was supported by a grant from the “Batzebär” foundation, by the “Abteilungsmittel für Forschung und Lehre” of the Division of Pediatric Hematology/Oncology in Bern, by an unrestricted SPOG (Swiss Paediatrics Oncology Group) Young Investigator Grant 2021 to C.K., and by the “Berner Stiftung für krebskranke Kinder und Jugendliche”.
Author information
Authors and Affiliations
Contributions
L.F. prepared data for publication, commented on the design of the database, drafted and wrote the manuscript. C.K. conceived and designed the study, recruited study participants, analyzed study data, interpreted the results, participated in writing the manuscript and approved the final submitted research manuscript. C.S. recruited study participants, participated in writing the manuscript and approved the final submitted research manuscript. J.R. participated in writing the manuscript and approved the final submitted research manuscript. J.W. recruited study participants, participated in writing the manuscript and approved the final submitted research manuscript. R.A.A. conceived and designed the study, analyzed study data, interpreted the results, participated in writing the manuscript and approved the final submitted research manuscript. E.B. conceived and designed the study, recruited study participants, interpreted the results, participated in writing the manuscript and approved the final submitted research manuscript.
Corresponding author
Ethics declarations
Competing interests
J.R. is meanwhile an employee of Novartis Pharma, Basel, Switzerland. E.B. has a consulting agreement with Bayer Healthcare Pharmaceuticals. There is no conflict of interest for all authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farner, L., König, C., Rössler, J. et al. Vital signs continuously monitored by two wearable devices in pediatric oncology patients, NCT04914702. Sci Data 12, 807 (2025). https://doi.org/10.1038/s41597-025-05081-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-05081-x