Abstract
The global environmental crisis challenges the sustainable management of marine resources, with declining fish stocks threatening both livelihoods and biodiversity. In multi-species fisheries, non-selective fishing gear often results in the capture of non-target species (bycatch), including those subjects to catch limits under the European Union’s landing obligation. This regulation introduced the concept of ‘choke species’: species whose quota is reached but that continue to be caught incidentally, forcing fishing to stop—even if other quotas are still available. In the Bay of Biscay mixed demersal fishery, such technical interactions are linked to combination of targeted species - such as sole (Solea solea), megrim (Lepidorhombus whiffiagonis) and white anglerfish (Lophius piscatorius) - and less targeted or non-target species such as thornback and cuckoo rays (Raja clavata, Leucoraja naevus). To inform these complex interactions, we provide datasets for these five key demersal species including: (1) high-resolution spatial distribution maps of juvenile and adult stages; (2) comprehensive life-history parameter summaries incorporating uncertainty; and (3) structured data designed for integration into spatially explicit models.
Similar content being viewed by others
Background & Summary
The sustained consumer demand and rising prices have led to overcapitalization of fishing effort1, with certain fishing gears resulting in high multispecific catches2. This trend might have a significant impact on these species, potentially exceeding the effects of global climate change3. Fisheries catching multiple species, regardless of whether these species are targeted or incidental, are defined as mixed fisheries4. In this context, non-target species, known as bycatch species, are unintentionally caught during fishing operations, raising ecological, economic, and conservation concerns. Bycatch often results from the use of non-selective fishing gear or from fishing in regions with a diversity of marine life5. Bycatch species may include commercially valuable species of varying economic importance, as well as other marine organisms, some of which may be protected5. In addition to the waste of natural resources, unreported bycatch and discards result in high unrecorded mortality rates for affected stocks, with cascading effects on the entire marine ecosystem1,6. To limit overfishing and conserve ecosystems, catch quotas by species and maximum fishing effort quotas have been implemented7. In the European Union, the landings obligation introduced by the Common Fisheries Policy (Regulation (EU) No 1380/2013) requires fishers to report and retain all catches of species subject to catch limits, including those caught unintentionally8. In fact, if a species is caught in quantities that reach its set quota, it can ‘choke’ the fishing operation by triggering a ban on that species4. Thus, a species that can stop the entire catch is commonly referred to as a choke species4. To optimize their income, fishermen should avoid catching these “choke species,” because reaching the quota for these species would shorten the fishing season. These issues are particularly acute in European mixed demersal fisheries, such as those operating in the Bay of Biscay, where different species, both targeted and non-targeted, coexist and are often caught together with non-selective gear. This region provides a relevant case study to explore the ecological and regulatory challenges associated with bycatch and the implementation of the landing obligation.
The Bay of Biscay, located in the temperate North-east Atlantic Ocean, is characterized by high biodiversity, including around 700 fish species—approximately 20 of which are commercially important - and 42 marine mammal species, representing approximately 35% of global diversity9. This ecologically rich area is subject to multiple human pressures, including fisheries, pollution, shipping, and increasingly, the impacts of global climate change9. The region falls under the Oslo-Paris Convention (OSPAR) with several coastal protected areas, although offshore protection remains limited. Its environmental status under the Marine Strategy Framework Directive is generally assessed as good, though specific indicators highlight areas needing improvement and further research9. The Bay of Biscay’s mixed demersal fisheries primarily involve bottom trawling, gillnetting, and longlining. France and Spain operate the majority of these fleets, with smaller contributions from Belgium, the United Kingdom, and Ireland. The most commonly used gear is bottom otter trawls, which target a variety of species including hake (Merluccius merluccius), anglerfish (Lophius spp.), sole (Solea solea), and megrim (Lepidorhombus whiffiagonis). Rays and skates are regularly caught as bycatch in these trawl fisheries, although some species of ray, such as the thornback ray (Raja clavata), are only partially exploited commercially. Gillnets and longlines mainly target sole, hake, with anglerfish and pollack (Pollachius pollachius) often caught as bycatch10.
The Management measures and ACCessOry species in demersal fisheries in the Bay of Biscay (MACCO) project addresses specific issues related to choke species in mixed fisheries (https://www.macco.fr/). It focuses on the mixed demersal fisheries in the Bay of Biscay and the assessment of the regional multi-annual management plan for all exploited species11. The Bay of Biscay (ICES division 8.abd) is a productive ecosystem where many commercially important species share common habitats during part or all of their life cycle11. It also contains vulnerable benthic and functional habitats that may be subject to conservation measures11,12. The MACCO project aims to facilitate the implementation of management measures through an ecosystem-based approach. The multispecies nature of these fisheries presents a major challenge to sustainable management. A previous study provided a description of the Bay of Biscay demersal fishery with the ISIS-Fish platform, synthesizing available information of three key commercial species: hake, sole, and Norway lobster (Nephrops norvegicus)11.
The present study extends the biological knowledge included in previous dataset11 by focusing on additional species that may act as choke species, and providing an update concerning one of its main target species. Species with high tonnage and/or economic value were selected based on a preliminary analysis13,14: white anglerfish (Lophius piscatorius), megrim, and thornback and cuckoo ray (Leucoraja naevus). Megrim is one of the eight demersal TAC species with the highest landings in the Bay of Biscay and is primarily targeted by trawlers, like anglerfish and hake10. Anglerfish, both white and black (Lophius budegassa) species, have high economic value in European fisheries15 and play a key predatory role in the ecosystem16. However, distinguishing their spatial distributions remains challenging, with current evidence suggesting panmixia from the southern Bay of Biscay to northern waters17. Skates such as thornback ray and cuckoo ray are regularly caught by trawlers and gillnets, though rarely targeted, and are thus generally considered bycatch. Some species, like thornback ray, have partial commercial value10. Skates and rays are also commonly caught in the sole fishery18. Among them, the Thornback ray (is one of the most commercially important species in the Bay of Biscay, sub area 8)19. Listed as Near Threatened by the IUCN, it follows a K-strategy, with low reproductive output and slow growth, making it vulnerable to fishing pressure20. The cuckoo ray, also included in the study, has become the dominant species, while others such as the thornback ray, and the spotted ray (Raja montagui) have declined, suggesting a pattern of overexploitation21.
The aim of this study is to compile and generate new knowledge on the spatial and temporal dynamics of the life cycles of megrim, white anglerfish, Thornback ray, Cuckoo ray, and sole in the Bay of Biscay. For the common sole, we revisit and update existing information that had not previously been spatially analysed11. The group of four species – excluding the sole- includes both targeted species, such as megrim and white anglerfish, and non-target species, such as thornback ray and cuckoo ray, which are regularly caught as bycatch and may act as choke species in mixed fisheries. By synthesizing life history traits, spatial structures, and ecological processes, we produced a comprehensive and reusable dataset designed to support a wide range of spatio-temporal analyses within the framework of ecosystem-based fisheries management. While the dataset was integrated into an ISIS-Fish22,23 dataset in order to demonstrate its potential use in modelling. We stress on the fact that its structure and content is compatible with several modelling frameworks, and allows for a broader use by modellers or anyone interested in these species’ ecology.
-
1.
The first approach, which addresses the scientific gap in spatial distribution information for these species, involves the analysis of model-derived data estimated from landings and/or surveys to assess the distribution patterns of each population according to the quality of available information. This methodology provides insight into global distributions, as well as distribution maps at annual and monthly time steps, according to different abundance levels per stage (juveniles and adults). These maps have been incorporated into the ISIS-Fish dataset to simulate the monthly spatial distribution of all species.
-
2.
The second approach, referred to life history parameter input, involves a comprehensive literature review aimed at compiling an ecological description of the species and documenting the full range of life history parameter values for each species. These components work together to clarify species life histories, identify the range of variability, and select the most relevant life history parameter values for inclusion in the ISIS-Fish dataset.
Each of these two steps represents both the results of this study and a fundamental part of the biological component of the ISIS-FISH dataset of the mixed demersal fisheries of the Bay of Biscay. This includes ecological descriptions detailing the ecology of the species, tables of reference values for life history parameters, and the data, analyses, and scripts used to generate our species distributions.
Methods
Spatial mapping
The availability of accurate spatio-temporal data on the distribution of commercial and bycatch species remains a major constraint to the effective implementation of spatialized fisheries management14,24. This limitation is particularly marked in the context of ecosystem approaches and spatialized models used to assess the impacts of management measures such as fishing quotas and time-area restrictions14,24. In order to address this crucial need, a comprehensive set of high-resolution maps explicitly representing the spatial distribution of adults and juveniles of the species studied has been produced and documented, on a monthly and annual scale, to facilitate their direct integration into operational spatial and ecosystem modelling approaches.
Data sources
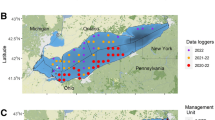
To generate spatial distribution maps for the studied species, we compiled data from four main sources (Fig. 1). A summary of the spatial scale, temporal coverage, number of maps, and data availability for each species is provided in Tables 1, 2.
-
Model outputs from two previous study25,26. These spatial predictions are derived from a spatial hierarchical model combining logbook and Vessel Monitoring System (VMS) data with scientific survey data - EVHOE27 and ORHAGO28 - collected between 2008 and 2018. This method corrects for preferential sampling by commercial fleets and uses a regular 0.5° × 1° grid to predict population densities, which are then clustered into adult population zones at a finer 1/16 ICES rectangle resolution. Monthly, yearly and combined temporal layers were produced. It is important to note that cuckoo ray was not included in this model. Additionally, for adult distribution maps, it was not possible to distinguish between the white anglerfish and the black anglerfish. However, we considered reasonable to attribute these data to the white anglerfish, unlike in the juvenile distribution maps where species could be differentiated (see Tables 1, 2).
-
EVHOE scientific bottom trawl survey27,29. This long-term scientific survey, coordinated by the International Bottom Trawl Survey Working Group (IBTSWG), covers the Bay of Biscay and the Celtic Sea. The dataset used in this study spans from 1987 to 2020 and provides geo-referenced haul data (latitude/longitude). For the purposes of this study, these data were aggregated onto a 1/16 ICES statistical rectangle grid to delineate juvenile zones and complement ObsMer records.
-
ObsMer programme30.the French on-board observer programme (ObsMer) provides commercial and discard data, stratified by fishing method (gear, season and vessel size). The dataset spans from 2003 to 2021. These data were used to map the distributions of both juveniles and adults for species not fully covered by other sources (notably cuckoo ray), and to validate patterns observed in EVHOE and model-derived data.
-
ICES stock assessment working groups. Abundance estimates and biomass indices were retrieved from the latest stock assessment outputs for sole, megrim, white anglerfish, thornback ray and cuckoo ray31. These were used to scale relative spatial patterns and derive initial conditions for spatial distribution where applicable.
Mapping methodology
To generate spatially explicit distribution maps for each life stage, we defined population zones for five species in the Bay of Biscay, distinguishing between juveniles and adults. Mapping was carried out at a resolution of 1/16 ICES statistical rectangles, with monthly and annually time steps. The resulting maps and shapefiles are available for each species and life stage in the associated repository (see Tables 1, 2).
Adults’ zones: Spatial clustering and biomass density gradients
Adult population zones were derived from spatial distribution revealed by hierarchical models25,26, combining fishery-dependent data (e.g. logbooks and VMS) with fishery-independent survey data (e.g. EVHOE and ORHAGO). These models produce monthly species density maps with a resolution of 0.5° latitude by 1° longitude. We then interpolated these maps to match the spatial resolution of 1/16 ICES rectangles used throughout the dataset.
To cluster spatial rectangles with « similar » abundance values in the distribution of adults, we applied a classification algorithm with spatial contiguity constraints32. This resulted in three abundance zones: low, medium and high. These classes were defined based on the 33rd and 66th percentiles of a species-specific latent abundance index (Sx), computed separately for each species and month. Within each zone, the average Sx was used to compute zone-specific weights (normalised to sum to one), providing a robust empirical basis for subsequent integration steps (see Tables 1, 2).
Juveniles’ zones: presence/absence of catch
Life stage classification (adults and juveniles) was determined using species-specific length-at-maturity (L₅₀) thresholds, derived from maturity stage and length data collected during scientific surveys, supplemented by values reported in published literature. Individuals smaller than L₅₀ were classified as juveniles, while those equal to or above L₅₀ were classified as adults. The L₅₀ values used are shown below: megrim: 23 cm; white anglerfish: 40.6 cm; thornback ray: 70 cm; cuckoo ray: 58 cm (see species sheets in the dataset).
Juvenile zones were delineated using presence-absence and count data from EVHOE scientific surveys (coordinated by ICES IBTSWG, 1987–2020)27,29 and the ObsMer programme30, both of which were projected onto the same 1/16 ICES spatial grid (ObsMer data were further stratified by métier; Fig. 1, Tables 1, 2). Where both EVHOE and Obsmer data were available, the spatial distribution was harmonised to improve coverage of juveniles’ map distribution.
Species-specific parameters of spatial population dynamics
The effective implementation of a fisheries management plan traditionally relies on a set of biological key parameters for species and population life cycle, including growth, natural mortality, sexual maturity, stock–recruitment relationships and spatial migration33,34. Standardised and well-documented values for these parameters are essential to ensure their integration into mechanistic models, enabling consistent simulation of demographic and spatial dynamics across exploited populations. In this study, we have compiled, reviewed and structured these key parameters for each species, with the aim of facilitating their direct reuse in widely used modelling frameworks, such as ISIS-Fish22,23, Ecopath with ecosim35,36, Atlantis37 and Osmose38,39, for example. ISIS-Fish is a particularly relevant tool in this regard, as it offers a structured framework to synthesize the standardized parameters compiled here, enabling their integration into a coherent modelling environment (see technical validation section). This facilitates operational re-use of the dataset for simulating population dynamics and supporting fisheries management planning in the Bay of Biscay.
Growth of individuals
Of the various growth models available, we have chosen to use the Von Bertalanffy growth function, which describes a logistic growth curve for size at age a, based on a growth rate K and a theoretical age t0 when size is zero (Eq. 1):
This relationship between the age (in months) and the length (in cm) gives a monthly average length for each age group (instead of a constant length over the year).
The key used to assign an average weight to each size class is often referred to as the size-weight relationship. This relationship provides a method for estimating the average weigh (w) of individuals within a given size class (L) based on empirical data collected from sampled individuals. It can be expressed at the group level (model structured by age or size) or at the population level (unstructured population). In this study, we consider the following allometric relationship (Eq. 2):
with w weight (in kg), L length (in cm), a and b parameters of the relationship.
In population dynamics, a stock can be organised according to size or age, or both (known as size-age structure). Alternatively, it may remain unstructured and be represented using a global population model. In the present dataset, all species except the two skate species (thornback and cuckoo ray) are modelled using an age-structured approach. The skate species follow an unstructured, global population model. This structure was chosen to align with species-specific life history traits and data availability, and it serves as a practical example of data integration within the ISIS-Fish modelling environment.
Maturity
First, the spatial distribution of species is distinguished between juveniles and adults (see map section). Second, maturity can be modelled in ISIS-Fish using a maturity ogive, which represents the proportion of mature individuals within each age or size class of a population. The maturity ogive typically illustrates the cumulative proportion of individuals that have reached sexual maturity at each age or size.
Reproduction & Recruitment
For sole, anglerfish and megrim populations, recruitment values estimated by the Working group for the Bay of Biscay and the Iberian Waters Ecoregion (WGBIE) for years 2015–202231, as well as mean recruitment over these years are provided. To take into account the fact that for anglerfish and megrim the stock’s distribution area exceeds the Bay of Biscay, we applied a correction factor (of pBoB_anglerfish = 0.15, pBoB_megrim = 0.31) based on the proportion of total landings corresponding to the Bay of Biscay. When modelling the population dynamics, it is also common to use stock-recruitment relationship to represent this process. This is especially useful when performing projections over medium to large time-scales (e.g. in order to find equilibrium reference points such as MSY) for which the use of average recruitment is not appropriate. We have then fitted at the scale of each stock the three most common stock-recruitment relationships, namely Beverton & Holt (Eq. 3), Ricker (Eq. 4), and the hockey stick (Eq. 5) and provided for each species the curve and associated parameters that fitted the best to stock assessment estimations. In the ISIS-Fish dataset provided as illustration, recruitment for these species can be simulated either by the average recruitment over 2015–2022 (default) or by the stock-recruitment relationship provided. In the latter case, we also take into account the fact that only a proportion of the stock was present in the Bay of Biscay by applying the correction coefficients to SSB in the model when computing recruitment.
The stock-recruitment relationships fitted are given by:
where a and b are two positive parameters, and SSBTot is the total spawning stock biomass for the whole stock. It can be obtained from the SSB in the Bay of Biscay by: SSBTot = SSBBoB/pBoB.
For these species, we provide parameters of the discrete logistic model with a yearly time-step (Eq. 6):
with Bt the biomass at time t, r the growth rate, and K the carrying capacity (in tons).
For cuckoo ray, which was modelled using a logistic population growth model, a correction factor (pBoB_cuckoo = 0.229) was applied to the carrying capacity parameter K, based on the proportion of total landings corresponding to the Bay of Biscay.
Natural mortality
Natural mortality refers to the number of deaths within a population that occur due to factors other than fishing activities (e.g. deaths caused by predation, disease, old age, or other natural factors). It is usually considered to be a constant rate and is often reported for the entire population or for specific size-age classes within the population. For example, a value of natural mortality M = 0.2 would indicate a constant natural mortality rate of 20% per unit of time (e.g. year) for the specified population or size-age class.
Spatial distribution over population zones
Population zones are designed to provide insight into species distribution according to their density and life stage (e.g. juveniles and adults; see Fig. 3, Tables 1, 2). These zones can be conceptualized and represented as clusters of spatial cells with similar abundance levels. For this dataset, zones were defined at a spatial resolution of 1/16 of an ICES statistical rectangle, allowing for fine-scale spatial representation, useful for representing the spatial distribution of a spatially discrete model (Tables 1, 2). As the information is spatially explicit, users are free to customise the maps by adding any other information they consider relevant, such as overlapping bathymetry.
To achieve a realistic representation of spatial dynamics in the simulations, the relative abundance of individuals across adult zones (with low, medium and high abundances) was derived for each species, based on the average monthly Sx values obtained from the spatial models (see ‘Mapping methodology’). These proportions serve two distinct but complementary purposes. Firstly, they are dynamically applied at each time step to redistribute adult individuals across spatial zones, thereby maintaining a consistent and ecologically plausible spatial structure throughout the simulation. Secondly, the same zone-specific weights are applied when juveniles reach maturity and transition into the adult zones. This recruitment allocation assumes that adult zones with higher observed densities, reflected by larger Sx values, represent more suitable habitats and are thus more likely to receive new individuals. These weights were normalised to sum to one for each species and are used consistently across the modelled time period to preserve the spatial patterns observed in the underlying data. Note: cuckoo ray is defined by a single global zone with no spatial structure (see section “Spatial mapping products”).
-
Megrim: 0.009 (low), 0.141 (medium), 0.85 (high).
-
White anglerfish: 0.025 (Low), 0.22 (Medium) and 0.755 (High).
-
Thornback ray: 0.007 (low), 0.086 (medium) and 0.907 (high).
-
Sole: 0.0421 (low), 0.2893 (medium), 0.6686 (high).
Abundance estimation
Initial abundance values represent the number of individuals within specific size or age categories in each population zone at the beginning of the studied period (2015–2022), required to run simulations of population dynamics. Typically, the values for each population or population*zone are key inputs derived from stock assessment models used by ICES and spatial density distributions.
As all the stocks except sole in the study extend beyond the Bay of Biscay, we applied biomass correction factors to align the data with the ICES divisions (8.abd). These adjustments were based on average landing proportions.
-
Megrim: The assessed stock spans divisions 7.b–k, 8.a–b and 8.d; France’s average landing share in divisions 8.a–b–d from 2010 to 2020 was 31.05%. Biomass estimates were therefore multiplied by 0.310540.
-
White anglerfish: The stock spans subarea 7 and divisions 8.a–b–d. Landings from divisions 8.a–b–d represent 15.2% of the total41.
-
Thornback ray: Abundance estimates were derived from Bayesian model outputs21. With an average individual weight of 3 kg, an initial biomass of 2,800 tonnes correspondent to ~933,333 individuals in the Bay of Biscay.
-
Cuckoo ray: For the stock rnj.27.678abd, the total abundance was estimated at 14,860,759 individuals based on ICES survey-derived biomass estimates42. To approximate the number of individuals within the Bay of Biscay (divisions 8.a–b), we applied a biomass-to-index conversion ratio of 0.2293, resulting in an estimated ~3,407,639 individuals for this region.
All abundance values were then scaled using the geometric mean of the recruitment rate derived from ICES stock assessment data (2010–2020), in line with standard practices for data-limited models. For sole, the spatial distributions were updated relative to those published previously11. Using new estimates25,26, the zones in which adults are distributed were redefined to better capture spatial heterogeneity in densities, thus improving the allocation of abundance across low, medium and high abundance zones.
Data Records
Repository overview and folder structure
The dataset is hosted on the SEANOE repository43 (https://doi.org/10.17882/102675) and is organized into three main folders (Fig. 2):
-
species_sheet/: For each species, this folder contains a summary document (DOCX) describing ecological traits and population dynamics, along with a spreadsheet (XLSX) compiling reviewed life history parameters from the literature and surveys (see section above for more details).
-
adult_map/ and juvenile_map/: These folders include HTML reports for each mapping product, accompanied by the associated raw.rds files and shapefiles (.shp, .shx, .dbf, .prj), fully documented in Table A.1 (see next section for more details).
-
R_Scripts_species_distribution/: A zipped folder containing all scripts used to generate distribution maps, also available on GitLab: https://gitlab.ifremer.fr/macco. This.zip folder (r script) and repository (SEANOE data) contain all the scripts necessary for the generation of species distribution maps.
Spatial mapping products
A total of 267 spatial distribution maps were produced to describe the monthly and annual dynamics of five demersal species in the Bay of Biscay, with separate outputs for juvenile and adult stages (Tables 1, 2). These maps capture both presence-absence information for juveniles and clustered abundance levels (low, medium, high) for adults (Fig. 3).
Distribution map of Megrim (a) for adult and (b) for juvenile), Common sole (c) for adult and (d) for juvenile), Anglerfish (e) for adult and (f) for juvenile), Cuckoo ray (g) for adult and (h) for juvenile), - Thornback ray (i) for adult and (j) for juvenile). The distribution can be by level (low, medium, high) for adults (except for Thornback ray) and by presence for adult Thornback ray and by presence for all juveniles.
Each mapping product is accompanied by an annotated HTML report and available shapefiles, allowing for direct visualization, re-use, and integration into spatial modelling workflows. Juvenile distributions are represented using both presence/absence layers and count-based summaries at a quarterly resolution, while adult distributions are modelled using monthly and yearly abundance gradients derived from hierarchical clustering procedures.
Figure 3 illustrates a representative selection of maps by species and life stage. Tables 1, 2 provides a comprehensive inventory of the available products, detailing the spatial resolution, temporal scale, data source, and shapefile availability for each species and maturity class.
Species sheet summaries & population dynamics parameter compilation
Each species is documented in a standardized ecological profile called “species sheet”, designed to support demographic modelling and ecosystem-based fisheries management (Fig. 2). These documents are divided into three sections:
-
1.
Ecological Summary: This section includes information on depth distribution, feeding behaviour, seasonal patterns, and known migratory traits, as well as spatial and temporal uncertainties in the life cycle.
-
2.
Life History Traits: Compilation of key demographic parameters such as growth curves (e.g Von Bertalanffy), maturity ogives, natural mortality, and size-weight relationships, disaggregated by sex when data permit.
-
3.
Model Parametrization: Detailed listing of the values used in mechanistic models, including stock-recruitment parameters, seasonal structuring, and spatial allocation coefficients (see Table 3).
Table 3 Overview of Biological Parameters Used in the ISIS-Fish Dataset for Spatio-Temporal Population Modelling.
Please note that all biological and ecological information is thoroughly referenced in the species sheets and summarised in the parameter table provided within the dataset.
ISIS-Fish folder organization
The folder titled ‘ISIS-Fish files’ includes four structured subdirectories: region, simulation, recruitment_initial_abundance, and technical_validation. Together, these folders provide all necessary components to initialize, populate, and technically validate an ISIS-Fish model focused on demersal species in the Bay of Biscay (see Fig. 4).
-
Region and simulation folders contain zipped.zip datasets for direct use with ISIS-Fish. The region folder includes all biological parameters and species configurations; the simulation folder includes the output from a reference simulation using these inputs.
-
Recruitment and abundance estimates (used as intial abundance) includes the.Rproj project and four subfolders:
-
historical_recruitment compiles recruitment time series (e.g., 2015–2018) derived from ICES advice for megrim, anglerfish, and sole.
-
initial_abundance provides pre-calculated biomass values per species between 2015 and 2022.
-
input_data stores all supporting material (in.RDS or.RData format) from stock assessments and scientific advice.
-
model_setting_scripts contain the scripts (.Rmd) and rendered reports (.html) used to build initial biomass and fit stock-recruitment relationships.
Summary of the content of the four folders composing the ISIS -Fish files. The first and second folders contain the dataset and the ISIS fish simulation, while the third folder contains all the data and scripts needed to generate the biomass and recruitment inputs to the model. Finally, the fourth folder contains the technical validation of the model.
Reproducibility and scripts
All scripts used to generate the spatial distribution products are available in the GitLab repository (https://gitlab.ifremer.fr/macco/R_Scripts_species_distribution) and archived in the ‘R_Scripts_species_distribution-main’ folder of the SEANOE dataset. These scripts were developed using R (version 4.3.2)44, and rely on R Markdown (.Rmd) documents to automate the processing and visualization steps. HTML reports were generated using the rmarkdown package45. Spatial clustering was performed using the geoclust package32, and data wrangling and visualization were handled using the tidyverse suite46, including ggplot2 and geom_sf47 for map plotting and shapefile generation. For recruitment and abundance extraction, and for fitting stock-recruitment relationships, ICES Workgroup stock-objects were treated using libraries from the © FLR collection (https://flr-project.org/). Each script is accompanied by a dedicated RStudio project (.Rproj), allowing users to reproduce specific species-level outputs from raw data to maps. The SEANOE repository contains all the data used and produced by these scripts: input data in.rds format (e.g., latent variables for adults; ObsMer or EVHOE survey data for juveniles), shapefiles, annotated HTML map reports, and species summary sheets. CSV files referenced in the scripts are intermediate outputs or data stored in.rds format and are included in the repository as part of the reproducibility pipeline. The structure of the GitLab repository mirrors the structure of the dataset folders, and a readme file explains how to run each script using the associated project environment. This ensures full reproducibility of all cartographic outputs described in the manuscript.
Technical Validation
The aim of this data paper is to compile biological and spatial information on key demersal species in the Bay of Biscay for use in fisheries modelling. To demonstrate the coherence, completeness and interoperability of the compiled parameters, we modelled the entire life cycle of five species - megrim, sole, white anglerfish, Thornback ray and Cuckoo ray - in the ISIS-Fish modelling environment22,23. This modelling platform provides an ideal framework to operationalize complex biological data, as it explicitly links life history traits, spatial distributions, and demographic transitions at a monthly time step. An overview of how species-specific inputs (e.g. abundance, growth, migration, reproduction, recruitment) are integrated into ISIS-Fish model is shown in Fig. 5, illustrating the spatio-temporal logic of population dynamics as implemented in the model.
Description of the “species” component of ISIS-Fish in which each successive step in the modelling process is described, from initial abundance to the age transition matrix for growth description and for migration processes to reproduction and recruitment. Monthly time steps are involved and the spatial scale is a 1/16 cell of the statistical rectangle in this study.
Population structure and recruitment
The three age-structured species - megrim, white anglerfish and sole - display multiple age classes that evolve according to the expected demographic processes. Age transitions are clearly observed in January (months 12 and 24), when individuals progress from one age group to the next. For the two unstructured species (thornback and cuckoo ray), a single age class is modelled (by default class 0) as defined by their global population model formulation (Fig. 6).
Monthly abundance per age group for each species over a 25-months simulation. The number of age groups differs between species according to their life-history structure. A single age group is used for skates (bottom row), while three or more age classes are modelled for megrim, anglerfish and sole (top row).
The decreasing trend in abundance within each age class confirms that natural mortality is being applied correctly on a monthly basis. Furthermore, the recruitment patterns align with the definitions established for each species. For age-structured stocks, the age class 0 cohort emerges in January, reaching levels consistent with the historical average of recruitment (Table 3). Recruitment values for thornback and cuckoo ray are derived from fitted stock-recruitment relationships specific to each species (Fig. 6).
Spatial allocation across zones
Figure 7 shows the number of individuals in each zone per month for three representative age groups (0, 1 and 2). This clearly illustrates how individuals move from juvenile to adult zones throughout their life cycle. For megrim and white anglerfish, individuals are recruited into age class 0 (i.e. aged 1) and are found exclusively in the juvenile zones (see Fig. 7). At age class 1, they are redistributed into the three adult zones (low, medium and high) in proportion to the species-specific density weights defined in the previous section. This pattern of redistribution persists over time, maintaining the expected abundance gradient across zones and ensuring that high-density zones consistently host the largest share of individuals (Fig. 7). For sole, recruitment occurs directly into the adult zones at age 2 (In Isis-Fish, age class 0 corresponds to true age 2; see Table 3). Consequently, no juvenile zones are defined for this species. However, we can see that the abundance of the adult population is well distributed between the different adult zones (low, medium and high; see Fig. 7). Two different cases are observed for the ray species. Thornback ray is modelled using a global model with only one group class i.e. adults; however, spatial heterogeneity is retained by defining three adult zones (low, medium and high). In contrast, cuckoo ray is modelled using a single global zone with no spatial structure, as spatialization was not supported by the available data (Fig. 7). The abundance remains fixed within this unique zone, as expected. This species-specific analysis shows that the modelled dynamics accurately reflect the intended biological and spatial structures of each population and that all life-stage transitions and spatial allocations are handled correctly over time.
Monthly abundance per spatial zone for representative age classes (0, 1, and 2) across the five species. Juveniles are localized in the appropriate juvenile areas (for megrim and white anglerfish), while older individuals are redistributed into adult zones (for megrim, white anglerfish, sole and thornback ray) in accordance with the zone-specific proportions defined in the input dataset. Note that for cuckoo ray there no distinction between adult and juvenile area under its global model.
Usage Notes
The spatialized life history dataset provided here has been designed for flexible integration into a wide range of fisheries and ecosystem modelling platforms. While the dataset was structured to be compatible with a range of population and ecosystem models, the potential of the dataset is illustrated by integrating the parameters and distribution maps into the ISIS-Fish simulation environment. The ISIS-Fish simulation environment offers a monthly, spatially explicit population framework suitable for multispecies demersal fisheries. The dataset has been designed with adaptability in mind, allowing users to integrate new species, fleets, or spatial management rules as needed for broader ecosystem-based applications.
To facilitate re-use, we provide all input files, R scripts, and documentation necessary to replicate the integration of these data into ISIS-Fish. The installation and initialization steps are as follows:
-
1.
Prerequisites:
A recent version of Java is required at the date and with the ISIS-Fish version used for this study Java 17 is recommended.
-
2.
Download and Installation:
-
The version of ISIS-Fish used can be downloaded from the official repository: https://forge.codelutin.com/projects/isis-fish/files
-
Extract the archive isis-fish-4.4.8.1.zip to the desired location.
-
The extracted folder contains the software environment and a dataset structure required to launch and configure the model.
-
3.
Launching the Interface:
-
On Windows: double-click the isisfish.bat file.
-
On Linux or MacOS: use the command./isisfish.sh from the terminal within the software directory.
-
4.
Importing the Dataset:
-
Within the ISIS-Fish interface, open the first tab (top-left icon), select “Region” → “Import Region”, and browse to the.zip file containing the provided region model.
-
5.
Set recruitment option:
For the species concerned, if one wants to simulate the dynamics using a stock-recruitment function instead of the mean recruitment, open the region in the first tab, then select the species file (e.g. “Espèces/Solea_solea/Solea_solea”). Once in the species environment, select the “Reproduction” tab, change the parameter “useSR” from false to true and save.
-
6.
Running a Simulation:
Once the region is imported, it is possible to use it to simulate the population dynamics. The simulation parameters are set in the second tab (“simulation”) of the ISIS-Fish interface.
-
In the tab “Simulation/Parametres”, select the good regions, the duration of simulation and set a name to the simulation. Then select the species to be simulated and import initial abundances as.csv files (those provided in the repository).
-
In the tab “Simulation/Export des résultats” select the results of interest to be exported as.csv files, e.g. “Abundances” or “Biomasses”. Those files will be saved in the following directory: /isis-fish-4/isis-dataset/simulations/sim_name/resultExports
-
In the tab “Simulation/Choix de résultat”, select the following results to be saved (necessary to good functionning of the simulation):
-
“MatrixAbundance”
-
“MatrixAbundanceBeginMonth”
-
“MatrixBiomass”
-
“MatrixBiomassBeginMonth”
-
Additional documentation and user guides are available at https://isis-fish.org/v4/index.html.
Code availability
The custom R scripts used to process the data and generate the spatial layers can be found here: https://gitlab.ifremer.fr/macco/. These scripts are necessary for reproducing the spatial mapping procedures described in the ‘Methods’ section, and are not included in the published dataset.
References
Crowder, L. B. & Murawski, S. A. Fisheries Bycatch: Implications for Management. Fisheries (Bethesda) 23, (1998).
Stobutzki, I., Miller, M. & Brewer, D. Sustainability of fishery bycatch: A process for assessing highly diverse and numerous bycatch. Environ Conserv 28, (2001).
Worm, B. et al. Rebuilding Global Fisheries. Science (1979) 325, (2009).
Mortensen, L. O., Ulrich, C., Hansen, J. & Hald, R. Identifying choke species challenges for an individual demersal trawler in the North Sea, lessons from conversations and data analysis. Mar Policy 87, (2018).
Boyce, J. R. An economic analysis of the fisheries bycatch problem. J Environ Econ Manage 31, (1996).
Abbott, J. K. & Wilen, J. E. Regulation of fisheries bycatch with common-pool output quotas. J Environ Econ Manage 57, (2009).
Ulrich, C., Reeves, S. A., Vermard, Y., Holmes, S. J. & Vanhee, W. Reconciling single-species TACs in the North Sea demersal fisheries using the Fcube mixed-fisheries advice framework. ICES Journal of Marine Science 68, (2011).
Condie, H. M., Grant, A. & Catchpole, T. L. Incentivising selective fishing under a policy to ban discards; lessons from European and global fisheries. Mar Policy 45, (2014).
Borja, A. et al. Chapter 5: The Bay of Biscay. in World Seas: An Environmental Evaluation Volume I: Europe, the Americas and West Africa (2019).
ICES. Bay of Biscay and the Iberian Coast Ecoregion – Fisheries Overview. (2022).
Vigier, A., Bertignac, M. & Mahévas, S. Monthly spatial dynamics of the Bay of Biscay hake-sole-Norway lobster fishery: an ISIS-Fish dataset. Sci Data 9, 1–12 (2022).
Galparsoro, I. et al. Research priorities and roadmap for deep-sea ecology in the Bay of Biscay (Northeast Atlantic). Deep Sea Research Part I: Oceanographic Research Papers 208, 104324 (2024).
ICES. Stock Annex for Megrim (Lepidorhombus Whiffiagonis) in Divisions 8.c and 9.a (Cantabrian Sea and Atlantic Iberian Waters). https://www.ices.dk/sites/pub/Publication%20Reports/Forms/DispForm.aspx?ID=34429 (2018).
Rindorf, A. et al. The Identification of Measures to Protect By-Catch Species in Mixed-Fisheries Management Plans (ProByFish). (2021).
Landa, J. et al. Weight-Length Relationships, Weight Conversion Factors and Condition Factor Trends for Two Stocks of Black Anglerfish (Lophius Budegassa) in Southern Bay of Biscay, Galician Waters and Northern Atlantic Areas from a Decade. (2016)
Díaz, P., Santos, J., Velasco, F., Serrano, A. & Pérez, N. Anglerfish discard estimates and patterns in Spanish Northeast Atlantic trawl fisheries. ICES Journal of Marine Science 65, (2008).
Aguirre-Sarabia, I. et al. Evidence of stock connectivity, hybridization, and misidentification in white anglerfish supports the need of a genetics-informed fisheries management framework. Evol Appl 14, 2221–2230 (2021).
Wiegand, J., Hunter, E. & Dulvy, N. K. Are spatial closures better than size limits for halting the decline of the North Sea thornback ray, Raja clavata? Mar Freshw Res 62, 722–733 (2011).
ICES. Thornback Ray (Raja Clavata) in Subarea 8 (Bay of Biscay) (Advice for 2021 and 2022). https://doi.org/10.17895/ices.advice.5786 (2020).
Elliott, S. A. M., Carpentier, A., Feunteun, E. & Trancart, T. Distribution and life history trait models indicate vulnerability of skates. Prog Oceanogr 181, 102256 (2020).
Marandel, F., Lorance, P. & Trenkel, V. M. Determining long-term changes in a skate assemblage with aggregated landings and limited species data. Fish Manag Ecol 26, 365–373 (2019).
Pelletier, D. et al. Evaluation of the bioeconomic sustainability of multi-species multi-fleet fisheries under a wide range of policy options using ISIS-Fish. Ecol Modell 220, 1013–1033 (2009).
Mahévas, S. & Pelletier, D. ISIS-Fish, a generic and spatially explicit simulation tool for evaluating the impact of management measures on fisheries dynamics. Ecol Modell 171, 65–84 (2004).
Pelletier, D. & Mahévas, S. Spatially explicit fisheries simulation models for policy evaluation. Fish and Fisheries vol. 6 Preprint at https://doi.org/10.1111/j.1467-2979.2005.00199.x (2005).
Alglave, B. & Vermard, Y. Spatio-Temporal Distribution for 4 Demersal Species of the Bay of Biscay Based on VMS-Logbooks Data. SEANOE https://doi.org/10.17882/97799 (2023).
Alglave, B. et al. Combining scientific survey and commercial catch data to map fish distribution. ICES Journal of Marine Science 79, 1133–1149 (2022).
Laffargue, P. et al. Evhoe evaluation halieutique ouest de l’europe. (1987).
Lecomte, J. B. & Coupeau, Y. Orhago. (2003).
Laffargue, P. et al. EVHOE Bottom Trawl Survey Catch Data: Long Term Benthic Community Dataset for Fish and Invertebrates on the Continental Shelves of the Bay of Biscay and the Celtic Sea. (2025).
IFREMER SIH. ObsMer - Observation Des Captures En Mer. (2022).
ICES. Working group for the Bay of Biscay and the Iberian Waters Ecoregion (WGBIE). ICES Scientific Reports 3, 1101 (2021).
Chavent, M., Kuentz-Simonet, V., Labenne, A. & Saracco, J. ClustGeo: an R package for hierarchical clustering with spatial constraints. Comput Stat 33, 1799–1822 (2018).
Hilborn, R. & Walters, C. J. & others. Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Rev Fish Biol Fish 2, 177–178 (1992).
Walters, C. J. & Martell, S. J. Fisheries Ecology and Management. (Princeton University Press, 2004).
Christensen, V. & Pauly, D. ECOPATH II - a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol Modell 61, (1992).
Christensen, V. & Walters, C. J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol Modell 172, (2004).
Audzijonyte, A. et al. Atlantis: A spatially explicit end-to-end marine ecosystem model with dynamically integrated physics, ecology and socio-economic modules. Methods Ecol Evol 10, (2019).
Shin, Y. J. & Cury, P. Exploring fish community dynamics through size-dependent trophic interactions using a spatialized individual-based model. Aquat Living Resour 14, (2001).
Shin, Y. J. & Cury, P. Using an individual-based model of fish assemblages to study the response of size spectra to changes in fishing. Canadian Journal of Fisheries and Aquatic Sciences 61, (2004).
ICES. Megrim (Lepidorhombus whiffiagonis) in divisions 7.b-k, 8.a-b, and 8.d (west and southwest of Ireland, Bay of Biscay). In Report of the ICES Advisory Committee, 2021. ICES Advice 2021, meg.27.7bk8abd (2021).
ICES. White Anglerfish (Lophius Piscatorius) in Subarea 7 and Divisions 8.a–b and 8.d (Celtic Seas, Bay of Biscay). https://doi.org/10.17895/ices.advice.7792 (2021).
ICES. Cuckoo Ray (Leucoraja Naevus) in Subareas 6–7, and in Divisions 8.a–b and 8.d (Westof Scotland, Southern Celtic Seas, and Western English Channel, Bay of Biscay). https://doi.org/10.17895/ices.advice.5817 (2022).
Vajas, P. et al. ISIS-Fish Database: Life History and Spatial Distribution of Sole, Megrim, Anglerfish, Thornback ray, and Cuckoo ray Populations in the Bay of Biscay. SEANOE. https://doi.org/10.17882/102675 (2025).
R Core Team. R Core Team 2023 R: A language and environment for statistical computing. R foundation for statistical computing. https://www.R-project.org/. R Foundation for Statistical Computing (2023).
Baumer, B. & Udwin, D. R Markdown. Wiley Interdisciplinary Reviews: Computational Statistics vol. 7 Preprint at https://doi.org/10.1002/wics.1348 (2015).
Wickham, H. et al. Welcome to the Tidyverse. J Open Source Softw 4, (2019).
Wickham, H. Ggplot2 - Elegant Graphics for Data Analysis (2nd Edition). Journal of Statistical Software vol. 77 (2017).
Acknowledgements
The authors gratefully acknowledge France Filière Pêche for funding this research and all members of the MACCO project (www.macco.fr) for their support in this study. Special thanks are extended to Baptiste Alglave, Youen Vermard, and Pascal Lorance for their valuable assistance in species selection, biological analyses, and the distribution assessments of the species discussed in this manuscript. The authors also thank IFREMER’s (French Institute for the Exploitation of the Sea) Système d’Information Halieutique (SIH) for providing data access. We would also like to thank ‘Code Lutin’ for their technical support on ISIS-Fish. Finaly, we thank Xabier Irigoien (handling editor), Alireza Foroozani (associate editor), and two anonymous reviewers for their constructive feedback and valuable suggestions, which greatly improved the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
P.V. drafted the original manuscript. P.V., J.-B.C., A.R., A.B., P.L. and S.M. conducted the analyses. A.R., J.-B.L., A.B., P.L. and S.M. reviewed and edited the manuscript. S.M. led and supervised the research project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vajas, P., Ricouard, A., Lecomte, JB. et al. Description of the monthly spatial dynamics of commercial and bycatch demersal species in the Bay of Biscay. Sci Data 12, 1585 (2025). https://doi.org/10.1038/s41597-025-05869-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-05869-x