Abstract
Climate change significantly affects the dynamics of mountain ecosystems, impacting not only species distributions but also their phenological characteristics. Nevertheless, our comprehension of the effects of climate change on biodiversity is still limited, primarily due to insufficient historical data. The dramatic temperature variations over short distances make permanent monitoring plots along elevational gradients an ideal natural laboratory for investigating species’ responses to climate change. Drawing on field survey information gathered from 2018 to 2022, we have developed a bryophyte species-trait dataset that encompasses 16,920 trait measurements across four categories: taxonomy, distribution, resistance traits, and reproductive traits, derived from 549 species in Eastern China. The compilation of this bryophyte species-trait dataset offers valuable opportunities to deepen our understanding of the factors, constraints, and effects associated with biodiversity variability, while also providing richer insights into predicting species distributions and implementing targeted in-situ conservation strategies.
Similar content being viewed by others
Background & Summary
Climate change exerts extensive effects on both biodiversity and the functioning of mountain ecosystems, influencing not just the distribution of species1,2 but also their phenological patterns3,4. As traits may be influenced by environmental factors, evolutionary background, and plasticity5,6, employing trait-based methods provides significant opportunities to enhance our understanding of the drivers, limitations, and repercussions of variability in biodiversity and ecosystem responses to climate change. Given that functional traits are effective tools to document the differences among individuals regarding their capabilities to thrive, reproduce, and perform in diverse environmental contexts, these traits can deepen our mechanistic insights into how species respond to and function amidst climate change, thereby establishing a connection between individual phenotypes and their surrounding environment7.
Mountains display significant differences in biodiversity along elevational gradients, largely attributable to their complex topographical features, climatic conditions, and geological past. By examining elevational gradients, researchers can deduce the climatic niches of various species and their potential distribution boundaries, which is particularly critical for understanding biological responses to climate change8,9. For example, an increase in temperature might trigger shifts in species ranges or elevate extinction risks for specialists thriving at high elevations10. These trends underscore the importance of studying elevation in forecasting changes in distributions and providing crucial insights for biodiversity conservation during the Anthropocene.
China stands out as one of the most biodiverse countries globally, especially within its vast subtropical zone. Covering more than 10 degrees of latitude across an area of 2.5 million km², this region harbors one of the world’s most extensive areas of evergreen broad-leaved forests, renowned for their unparalleled species richness and evolutionary significance11. This region contains vegetation types primarily established during the Tertiary period, along with a considerable number of relatively ancient genera and species12. Research has shown that the subtropical region of China acts as both a “museum” and a “cradle” for the emergence of woody plant diversity13. Within this subtropical area, the mountainous regions of Eastern China harbor an exceptionally high and unique diversity of species, resulting from the intricate interactions between mountain ranges, river systems, and climatic factors. These regions, which have served as significant refuges for paleovegetation and biota since both Tertiary and Quaternary eras, host many relict species dating back to the Tertiary, making them critical for biodiversity conservation efforts in China14,15. Nonetheless, this region is also one of the fastest-developing regions in China regarding economic growth and tourism, leading to increasing anthropogenic pressures that demand stricter biodiversity conservation measures.
Bryophytes, which include liverworts, mosses, and hornworts, consist of approximately 16,000 species, making them the second largest group of living terrestrial plants, only after angiosperms16. They play crucial roles in various ecosystem functions, such as improving the physical properties of soil17, facilitating nutrient biogeochemical cycling18, and retaining water19. Even though they are ecologically significant, bryophytes are infrequently taken into account in biodiversity assessments when compared to vascular plants20. On a global scale, the presence of a consistent latitudinal species richness gradient has sparked controversy for a long time, mainly due to the absence of an up-to-date synthesis of global species distribution patterns21.
In order to assess the effects of climate change on mountain biodiversity, a long-term initiative called BEST (Biodiversity along Elevational Gradients: Shifts and Transitions) Network (https://BEST-mountains.org) was launched in 2017. This project, which encompasses collaboration among over 20 research teams, led to the establishment of more than 300 permanent plots at various elevations across a total of 18 mountains located between 19.07°N and 43.37°N, ranging from 166 m to 3,835 m.
Utilizing the BEST Network, we are undertaking long-term observations to examine the alterations in the geographic distribution and abundance of bryophytes in response to climate changes. This paper presents a comprehensive dataset of bryophyte diversity and traits, which is collected from elevational gradients in four subtropical mountain ecosystems located in Eastern China. To compile a thorough inventory of bryophytes, we implemented both plot sampling (PS) and floristic habitat sampling (FHS) as described by Ilić et al.22. The plant traits of bryophyte communities were primarily gathered from the taxonomic revisions and regional bryofloras.
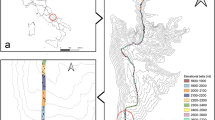
The dataset contains information on bryophyte species in four nature reserves in Eastern China within the range from 114.29°E–119.27°E and from 25.38°N–31.20°N (Fig. 1; Table 1), comprising Anhui Province, Zhejiang Province, Jiangxi Province and Fujian Province. The resulting dataset contains 549 bryophytes belonging to 195 genera, 71 families, accounting for 16% of Chinese bryophyte diversity. The dataset includes 16,920 trait measurements across 549 taxa, marking the first regional-scale bryophyte trait dataset for China. This dataset is released for noncommercial use only and is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0). All publications that use this dataset should appropriately cite the dataset and this paper.
The dataset can be utilized to: (i) identify regional hotspots of bryophyte diversity to inform the delineation of priority conservation areas; (ii) assess how species are distributed from regional species pools to local communities and evaluate the factors influencing this distribution; (iii) evaluate the endemism and conservation status of species at a regional scale; and (iv) serve as a fundamental baseline for assessing how bryophyte diversity and its elevational distribution respond to climate change and anthropogenic disturbances.
In conclusion, this dataset represents a significant regional dataset on elevational gradients of bryophyte species richness sourced from field investigations. The continuous monitoring project, which focuses on changes in the richness and distribution of bryophytes along various elevational and latitudinal gradients, will enhance our understanding of how climate-related factors affect the shifting ranges of these organisms over time.
Methods
Research site selection
The establishment of field plots and associated surveys for this study were carried out under the framework of the BEST Network. This network represents a long-term monitoring initiative aimed at investigating the dynamics of multi-taxa biodiversity in the context of climate change and land-use impacts in China. Through the BEST Network, permanent biodiversity monitoring plots have been established across 18 mountains situated in the tropical and subtropical regions of China. These plots are critical for conducting comprehensive surveys across various taxa, including plants, birds, insects, and soil fauna. The overarching aim of these efforts is to elucidate biodiversity patterns and identify the drivers behind their distribution with respect to elevation (as referenced on the BEST Network website: https://BEST-mountains.org).
In this study, we selected 67 permanent plots across four subtropical mountain ecosystems: Mt. Dabie in Anhui, Mt. Tianmu in Zhejiang, Mt. Guan in Jiangxi, and Mt. Daiyun in Fujian, spanning an elevation range from 281 m to 1,600 m (Fig. 1). Through a combination of field reconnaissance, expertise from local scholars, and remote sensing information, we chose sites characterized by representative zonal vegetation, minimal human disturbance, and uninterrupted elevational gradients. Employing a closed traverse approach, areas of plantations or secondary growth were intentionally excluded, plots measuring 20 m × 20 m or 20 m × 30 m were set up in mature forest zones, ensuring roughly 100 m elevation intervals between adjacent plots. To ensure a consistent approach in our research, we have decided to use a uniform plot size of 20 m × 20 m when examining the diversity of bryophytes. This standardized measurement allows for a systematic comparison across different sampling sites and facilitates the collection of reliable data on bryophyte populations. In light of the complexity of the terrain and the structure of the forests, 1-2 parallel plots were flexibly established at each elevation. A particularly noteworthy aspect of our study can be found in Mt. Tianmu, where 37 plots were set up along the elevational gradient. This particular arrangement was intended to investigate the effects of sampling effort on species diversity, thereby contributing to a deeper understanding of biodiversity in relation to elevational fluctuations.
Sampling strategy
All bryophyte species in each plot were surveyed between April 2018 and August 2022. To obtain data on species richness in a plot as complete as possible, both plot sampling (PS) and floristic habitat sampling (FHS) methods22 were used in the field work (Fig. 2). For detailed methodology regarding the sampling methods and strategies employed in this study, please refer to Dai et al.23.
Schematic diagram of sampling methodology (adjusted to cite Wang et al.71).
It is widely recognized that each sampling method has its unique strengths and weaknesses24. The PS method is particularly adept at identifying common species and evaluating their distribution and frequency; however, it often overlooks rare species25. Conversely, the FHS method uses the entire mesohabitat as its fundamental sampling unit, providing the flexibility to consider the variability of microhabitats within a mesohabitat, which notably enhances the chance of discovering rare species26. In this study, while the FHS method recorded a higher average species richness per plot when compared to the PS method, the PS method proved effective in detecting smaller bryophyte species, especially those in the Lejeuneaceae family. In fact, employing a combined strategy utilizing both sampling methods has shown to be advantageous in increasing species discoveries. In contrast, had we depended solely on the FHS method or the PS method, we would have missed approximately 26% to 29% of newly documented species27.
Species identification and taxonomic standardization
The specimens that were gathered have been deposited in the Biological History Museum East China Normal University (HSNU). Both morphological and anatomical examinations were carried out utilizing an Olympus SZX7 stereomicroscope in conjunction with an Olympus BX43 light microscope. A variety of publications and literature relating to the taxonomy and flora of Chinese bryophytes28,29,30,31 served as references for specimen identification. For those specimens that posed taxonomic challenges, expertise was sought from specialists across various taxonomic groups within China, ensuring that all identifications were made with a high degree of accuracy and reliability.
Dataset collection methods
Taxonomy
The standardization of species names is primarily based on The Bryophyte Nomenclator (https://www.bryonames.org/), while the Catalogue of Life China (http://sp2000.org.cn/) is also used as supplementary references. This multi-source approach enables robust validation of nomenclature and taxonomy. Additionally, for species with disputed taxonomic status, several specialized taxonomic monographs and dissertations have been referenced to ensure the reliability of the species list (e.g., Zhao & Liu32, Wang & Jia33 and Wang et al.34).
Distribution
The latitude, longitude, and elevation information for all sampling plots were obtained from the BEST Network. During the field investigation, the rough taxonomic classification corresponding to each specimen, as well as the host plant code number, and substrate type were recorded.
Functional traits
The extraction of bryophyte functional traits followed a sequential workflow (Fig. 3): Initially, primary reliance was placed on taxonomic revisions, such as A Taxonomic Study of the Family Bryaceae (Sensu Lato, Bryopsida) in China32, A Monograph of the Genus Ulota s.l. (Orthotrichaceae, Moss)33 and Taxonomic revision of Lejeuneaceae subfamily Ptychanthoideae (Marchantiophyta) in China34. For taxa that have not been revised, Flora Bryophytorum Sinicorum35,36,37,38,39,40,41,42,43,44 served as the core reference, supplemented by regional bryofloras, including Flora Yunnanica (Tomus 17) Bryophyta: Hepaticae, Anthocerotae28 and Bryophytes of Yachang-Liverworts and Hornworts45. Subsequently, traits that remained unresolved were extracted from the Bryophytes of Europe Traits (BET) dataset46. Persisting data gaps for some species were addressed by inferring phylogenetically conserved traits—including growth form, sex and spore surface ornamentation—from higher taxonomic ranks (family or genus), utilizing the Guide to the Bryophytes of Tropical America47 and related taxonomic treatments. In the case of some highly diverse families (such as Brachytheciaceae and Pottiaceae), the forementioned trait information may still exhibit instability at the family or genus level; therefore, these data are treated as missing values.
Data Records
The dataset is available from Figshare repository48 (https://doi.org/10.6084/m9.figshare.29826515.v5). Data from 8,112 specimens across four sampling sites were organized into four categories: taxonomy, distribution, resistance traits, and reproductive traits (Table 2). The taxonomic information in the bryophyte species-trait dataset includes species name, order, family and genus, revealing 549 bryophyte species in Eastern China, distributed across 21 orders, 71 families, and 195 genera. Among these, there are 158 liverworts, classified into 7 orders, 24 families, and 42 genera. Additionally, there are 391 mosses categorized into 14 orders, 47 families, and 153 genera.
The distribution information in the bryophyte species-trait dataset encompasses geographical distribution, elevational distribution and substrate. Geographical distribution data document the presence/absence of bryophytes across four sampling sites. Elevational distribution data, which cover an elevation gradient from 281 m to 1,600 m, were compiled by segmenting the range into 200 m elevation intervals and recording species occurrences within each elevational band. Substrate data indicate the presence/absence of bryophytes across various substrate types.
The functional traits of the dataset can be classified into two categories: resistance traits and reproductive traits. Notably, bryophytes are poikilohydric plants, which makes them particularly sensitive to variations in environmental moisture. Consequently, their resistance traits are predominantly associated with water. In this study, we selected and extracted five water-related resistance traits: growth form, leaf ornamentation, midrib extension, stem paraphyllia/pseudo-paraphyllia and hyaline hair point/hyaline margin, primarily for the following reasons: (i) the various growth forms exhibited by bryophyte gametophytes indicate different approaches to water conduction, retention, and gas exchange, which are vital for photosynthesis49. Leafy liverworts and pleurocarpous mosses thrive in humid and shaded locations, whereas thallus liverworts and acrocarpous mosses have evolved distinct mechanisms for enduring drought conditions50; (ii) leaf ornamentation is essential for the processes of water absorption and transportation. In leaves that are papillose or mammillose, tiny protrusions on the cell surfaces enhance water uptake by forming capillary channels and facilitate water movement, functioning like capillaries without hindering gaseous exchanges, thus promoting the rewetting of leaves more effectively51,52,53; (iii) midrib of a leaf can either extend throughout the entire length of the leaf, which is referred to as a percurrent midrib, or surpass it, known as an excurrent midrib, which plays a crucial role in providing structural support, particularly during periods of desiccation. Research has also shown that this structural feature can facilitates water transport within the plant51,52,54; (iv) stem paraphyllia or pseudoparaphyllia are reduced leaflike appendages on the stem or branch of some mosses, which is also associated with environmental water utilization, interception, and retention55; and (v) hyaline hair point refers to the transparent, often shiny apex found at the end of an elongated leaf blade. Species possessing hyaline hair points can reduce water loss by adjusting the orientation of their hair point during dry conditions49,56. Furthermore, a hyaline hair point aids in minimizing water loss also by shielding leaves from solar radiation49,57,58,59 and capturing dew49,51,60.
Reproductive traits play a crucial role in the establishment and maintenance of bryophyte communities. Sierra et al.61 utilized reproductive traits as indicators to predict the formation time of epiphyllous liverwort communities. In our study, we selected seven reproductive traits: vegetative reproduction, sex, maximum seta length, peristome, minimum spore size, maximum spore size, and spore ornamentation. We hypothesize that bryophytes possessing vegetative reproduction, elongated setae, small and mammillose/papillose spores, as well as distinctly peristome structures, are more conducive to dispersal and propagation29,50,62.
Data Overview
Families and genera with a number of species greater than or equal to 5 are defined as dominant families and dominant genera, while those with fewer than 5 species are combined into “Others” (Fig. 4). There are 30 dominant bryophyte families and 25 dominant genera in Eastern China. The most diverse families in bryophyte species-trait dataset are Hypnaceae (50 species, 9.11%) and Lejeuneaceae (42 species, 7.65%), followed by Brachytheciaceae (34 species, 6.19%), Dicranaceae (31 species, 5.65%), and Thuidiaceae (28 species, 5.10%). At the genus level, Frullania (21 species, 3.83%), Porella (20 species, 3.64%), Entodon (19 species, 3.46%) and Fissidens (17 species, 3.10%) emerge as the most speciose genera, followed by Brachythecium and Plagiothecium (13 species each, 2.36%), Plagiomnium (12 species, 2.19%).
Based on a comparative analysis of bryophyte diversity across four sampling sites, Mt. Tianmu exhibits the highest species richness with 387 documented bryophyte species, while Mt. Daiyun displays the lowest species richness, with 107 recorded bryophyte species (Fig. 5).
Across all substrate types, arboreal substrates, including tree bases and tree trunks, supported the highest bryophyte species richness, with 341 species (62.11%) and 326 species (59.38% of total regional bryophytes) respectively. This was followed by rock covered with a thin layer of soil, (174 species, 31.69%), decaying wood (171 species, 31.15%), rock surfaces (152 species, 27.69%), and soil (98 species, 17.85%). In sharp contrast, branch-dwelling and epiphyllous habitats exhibited minimal species representation, with only 54 species (9.84%) and 14 species (2.55%), respectively (Fig. 6).
Bryophytes of various growth forms exhibited distinct substrate preferences (Fig. 6). Acrocarpous mosses were relatively uniformly distributed across all habitats, with the exception of branch-dwelling and epiphyllous substrates. In contrast, pleurocarpous mosses and leafy liverworts demonstrated a pronounced preference for arboreal habitats, including tree trunks and tree bases. Thallus liverworts maintained broad distribution patterns, being absent only from epiphyllous habitats while occurring uniformly across other substrates.
Technical Validation
In order to evaluate how sampling effort influences bryophyte species richness, we purposefully selected 12 plots from a total of 37 located in Mt. Tianmu during the summer of 2020. These plots were strategically chosen at intervals of 100 m in elevation. Within each sampling plot, we randomly selected 10 trees that possessed a diameter at breast height (DBH) greater than 15 cm. The surveys conducted aimed to assess bryophyte diversity by examining subplots at four different heights (0.3 m, 1.1 m, 1.5 m, and 1.8 m above the ground) on both the northern and southern sides of each tree. The findings indicated that sampling exclusively at heights of 0.3 m and 1.5 m (which involved two subplots per tree) accounted for 75% of the overall species diversity found when sampling all four heights and aspects (eight subplots per tree)63. This indicates that concentrating sampling efforts at the 0.3 m and 1.5 m heights can yield substantial coverage of species diversity, particularly in situations where time and resources may be constrained.
In order to ensure a thorough and systematic approach to sampling both liverworts and mosses, all field collections were conducted collaboratively by experts who specialize in each respective group. The specimens that were collected were then identified by leading domestic experts in the fields of liverwort and moss identification. To aid in the identification of particularly challenging specimens, molecular phylogenetic techniques were employed. Additionally, to uphold consistency throughout the sampling and identification processes, the same team members were responsible for both the fieldwork and the laboratory identification of the specimens. This approach not only promoted uniformity in methodology but also enhanced the reliability of the findings.
Usage Notes
This study established a bryophyte species-trait dataset to ensure data accessibility and interdisciplinary integration. For data collection, the research team conducted PS surveys at 0.3 m, 1.1 m, 1.5 m, and 1.8 m heights along north-south transects in 12 of 37 permanent plots in Mt. Tianmu, thereby developing a standardized protocol for bryophyte surveys in Eastern China63. It should be noted that methodological inconsistencies in sampling may introduce observational biases in regional biodiversity assessments. Furthermore, bryophytes’ high climatic sensitivity drives their elevational/latitudinal range shifts in response to global warming. Our four-year investigation on bryophyte diversity in Eastern China lead to the following conclusions: sampling time differences may affect the comparison of diversity among different sites; the extension of the study period may exacerbate the differences in diversity and species composition among sites; and interannual variations in wet and dry seasons may drive dynamic changes in species composition.
Furthermore, it is important to emphasize that, consistent with other bryophyte databases such as BET46, BRYOATT64 and BryForTrait65, the dataset used in this study mainly depends on previously published literature and existing datasets, instead of being gathered from direct measurements taken in the field for trait values. While this approach is valid, data from these sources generally leans more towards qualitative aspects than quantitative ones. Additionally, this method of directly extracting trait data from literature frequently fails to account for intraspecific variation. Comparisons of functional traits among species often presume that trait variation within a species is minimal. Nevertheless, intraspecific trait variation is recognized to account for a significant portion of functional trait variation within communities66,67 and can even exceed interspecific trait variation along comparable environmental gradients68. Considering that intraspecific variation might be crucial for the survival of bryophytes in the face of climate change69,70, it is essential to prioritize the understanding of which functional traits exhibit plasticity and to identify any potential trade-offs associated with high levels of plasticity. Therefore, it is recommended that subsequent studies, when using this data for diversity pattern comparisons or analyzing community assembly processes from regional to local scales, should fully consider these limiting factors.
Code availability
No specific code was employed for the generation and analysis of the data presented.
Data availability
Bryophyte species-trait dataset is available from the Figshare repository. The direct link is: https://doi.org/10.6084/m9.figshare.29826515.v5.
References
Cahill, A. E. et al. Causes of warm-edge range limits: systematic review, proximate factors and implications for climate change. J. Biogeogr. 41, 429–442 (2014).
Steinbauer, M. J. et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234 (2018).
Wolkovich, E. M. et al. Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am. J. Bot. 100, 1407–1421 (2013).
Vitasse, Y., Signarbieux, C. & Fu, Y. H. Global warming leads to more uniform spring phenology across elevations. P. Natl. Acad. Sci. USA. 115, 1004–1008 (2018).
Violle, C. et al. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252 (2012).
Messier, J. et al. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography 40, 685–697 (2017).
Vandvik, V. et al. Plant traits and vegetation data from climate warming experiments along an 1100 m elevation gradient in Gongga Mountains, China. Sci. Data 7, 189 (2020).
Lomolino, M. V. Elevation gradients of species-density: historical and prospective views. Global Ecol. Biogeogr. 10, 3–13 (2001).
Rahbek, C. et al. Humboldt’s Enigma: What Causes Global Patterns of Mountain Biodiversity? Science 365, 1108–1113 (2019).
La Sorte, F. A. & Jetz, W. Projected range contractions of montane biodiversity under global warming. P. Roy. Soc. B-Biol. Sci. 277, 3401–3410 (2010).
Song, Y. C. Evergreen broad-leaved forests in China. Classification-Ecology-Conservation (Science Press, 2013).
Li, X. W. Floristic statistics and analyses of seed plants from China. Acta Bot. Yunnan. 18, 363–384 (1996).
Lu, L. M. et al. Evolutionary history of the angiosperm flora of China. Nature 554, 234–238 (2018).
Qiu, Y. X., Fu, C. X. & Comes, H. P. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 59, 225–244 (2011).
Tang, C. Q. et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 9, 4488 (2018).
Christenhusz, M. J. & Byng, J. W. The number of known plants species in the world and its annual increase. Phytotaxa 261, 201–217 (2016).
Soudzilovskaia, N. A., Van Bodegom, P. M. & Cornelissen, J. H. C. Dominant bryophyte control over high-latitude soil temperature fluctuations predicted by heat transfer traits, field moisture regime and laws of thermal insulation. Funct. Ecol. 27, 1442–1454 (2013).
Frego, K. A. Bryophytes as potential indicators of forest integrity. Forest Ecol. Manag. 242, 65–75 (2007).
Beringer, J. et al. The representation of arctic soils in the land surface model: the importance of mosses. J. Climate 14, 3324–3335 (2001).
Ah-Peng, C. et al. Bryophyte diversity and distribution along an altitudinal gradient on a lava flow in La Réunion. Divers. Distrib. 13, 654–662 (2007).
Patiño, J. & Vanderpoorten, A. Bryophyte biogeography. Crit. Rev. Plant Sci. 37, 175–209 (2018).
Ilić, M. et al. Field sampling methods for investigating forest-floor bryophytes: Microcoenose vs. random sampling. Arch. Biol. Sci. 70, 589–598 (2018).
Dai, Z. et al. Effects of microclimates on species richness of epiphytic and non-epiphytic bryophytes along a subtropical elevational gradient in China. J. Biogeogr. 52, e15134 (2025).
Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 77, 350–363 (1996).
Vanderpoorten, A. et al. The ghosts of Gondwana and Laurasia in modern liverwort distributions. Biol. Rev. Camb. Philos. Soc. 85, 471–487 (2010).
Newmaster, S. G. et al. The ones we left behind: Comparing plot sampling and floristic habitat sampling for estimating bryophyte diversity. Divers. Distrib. 11, 57–72 (2005).
Yao, X. et al. What determines the probability of discovering a species? A Study of the completeness of bryophyte inventories in Tianmushan National Nature Reserve (Zhejiang, China). Ecol. Evol. 14, e70593 (2024).
Gao, Q. & Cao, T. Flora of Yunnanica (Tomus 17) (Science Press, 2000).
Zhu, R. L. & So, M. L. Epiphyllous Liverworts of China (J. Cramer, 2001).
Li, X. J. Flora of Yunnan (Tomus 18) (Sicence Press, 2002).
Li, X. J. Flora of Yunnan (Tomus 19) (Sicence Press, 2005).
Zhao, J. C. & Liu, Y. Y. A Taxonomic Study of the Family Bryaceae (Sensu Lato, Bryopsida) in China (Hebei Science and Technology Press, 2021).
Wang, Q. H. & Jia, Y. A Monograph of the Genus Ulota s.l. (Orthotrichaceae, Moss) (Science Press, 2023).
Wang, J., Zhu, R. L. & Gradstein, S. R. Taxonomic revision of Lejeuneaceae subfamily Ptychanthoideae (Marchantiophyta) in China (J. Cramer, 2016).
Gao, Q. Flora Bryophytarum Sinicorum Vol. 1 (Science Press, 1994).
Gao, Q. Flora Bryophytarum Sinicorum Vol. 2 (Science Press, 1996).
Gao, Q. Flora Bryophytarum Sinicorum Vol. 9 (Science Press, 2003).
Gao, Q. & Wu, Y. H. Flora Bryophytarum Sinicorum Vol. 10 (Science Press, 2008).
Hu, R. L. & Wang, Y. F. Flora Bryophytarum Sinicorum Vol. 7 (Science Press, 2005).
Li, X. J. Flora Bryophytarum Sinicorum Vol. 3 (Science Press, 2000).
Li, X. J. Flora Bryophytarum Sinicorum Vol. 4 (Science Press, 2006).
Wu, P. C. Flora Bryophytarum Sinicorum Vol. 6 (Science Press, 2002).
Wu, P. C. & Jia, Y. Flora Bryophytarum Sinicorum Vol. 8 (Science Press, 2004).
Wu, P. C. & Jia, Y. Flora Bryophytarum Sinicorum Vol. 5 (Science Press, 2011).
Wei, Y. M. Bryophytes of Yachang-Liverworts and Hornworts (Shandong Science and Technology Press, 2024).
van Zuijlen, K. et al. Bryophytes of Europe Traits (BET) data set: A fundamental tool for ecological studies. J. Veg. Sci. 34, e13179 (2023).
Gradstein, S. R., Churchill, S. P. & Salazar-Allen, N. Guide to the Bryophytes of Tropical America (The New York Botanical Garden Press, 2001).
Wang, Y. R. et al. Bryophyte species-trait dataset in Eastern China. figshare https://doi.org/10.6084/m9.figshare.29826515.v5 (2025).
Ah-Peng, C. et al. Functional diversity of subalpine bryophyte communities in an oceanic island (La Runion). Arct. Antarct. Alp. Res. 46, 841–851 (2014).
Vanderpoorten, A. & Goffinet, B. Introduction to Bryophytes (Cambridge University Press, 2010).
Henriques, D. S. G. et al. Functional diversity and composition of bryophyte water-related traits in Azorean native vegetation. Plant Ecol. Divers. 10, 127–137 (2017).
Guerra, J., Martínez-Sánchez, J. J. & Ros, R. M. On the degree of adaptation of the moss flora and vegetation in gypsiferous zones of the south-east Iberian Peninsula. J. Bryol. 17, 133–142 (1992).
Vitt, D. H., Crandall-Stotler, B. & Wood, A. J. in Plant Ecology and Evolution in Harsh Environments (eds. Rajakaruna, N., Boyd, R. S. & Harris, T. B.) 267–295 (Nova Science, 2012).
Waite, M. & Sack, L. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol. 185, 156–172 (2010).
Zhang, Y. C., He, L. & Guo, S. L. Research on plant functional diversity and its application in bryophytes. J. Shanghai Norm. Univ. (Nat. Sci.) 49, 9–17 (2020).
Proctor, M. C. F. in Bryophyte Biology 2nd edn (eds. Goffinet, B. & Shaw, J.) Ch. 6 (Cambridge University Press, 2008).
Zhang, Y. M. & Wang, X. Q. Study on the Microbiotic Crusts in Junggar Desert (Science Press, 2008).
Zheng, Y. P. et al. Advances on ecological studies of algae and mosses in biological soil crust. Chin. Bull. Bot. 44, 371–378 (2009a).
Zheng, Y. P. et al. Morphological and structural adaptation and characteristics of protonemal development of Syntrichia caninervis in the mosses crust layer. J. Desert Res. 29, 878–884 (2009b).
Watson, W. Xerophytic adaptations of bryophytes in relation to habitat. New Phytol. 13, 149–169 (1914).
Sierra, A. M. et al. Reproductive traits as predictors of assembly chronosequence patterns in epiphyllous bryophyte metacommunities. J. Ecol. 107, 875–886 (2019).
Patiño, J. et al. Baker’s law and the island syndromes in bryophytes. J. Ecol. 101, 1245–1255 (2013).
Chen, X. et al. Bryophytes diversity of Tianmushan National Nature Reserve, Zhejiang Province. Biodiversity Science 31, 22649 (2023).
Hill, M. O. et al. BRYOATT: attributes of British and Irish mosses, liverworts and hornworts. (Centre for Ecology and Hydrology, Saxon Print Group, 2007).
Bernhardt‐Römermann, M., Poschlod, P. & Hentschel, J. BryForTrait - a life‐history trait database of forest bryophytes. J. Veg. Sci. 29, 798–800 (2018).
Messier, J., McGill, B. J. & Lechowicz, M. J. How do traits vary across ecological scales? A case for trait‐based ecology. Ecol. Lett. 13, 838–848 (2010).
Fajardo, A. & Siefert, A. Intraspecifific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 99, 1024–1030 (2018).
Anderegg, L. D. L. et al. Aridity drives coordinated trait shifts but not decreased trait variance across the geographic range of eight Australian trees. New Phytol. 229, 1375–1387 (2021).
Reynolds, L. A. & McLetchie, D. N. Short distances between extreme microhabitats do not result in ecotypes in Syntrichia caninervis. J. Bryol. 33, 148–153 (2011).
Beltrán‐Sanz, N. et al. Physiological plasticity as a strategy to cope with harsh climatic conditions: ecophysiological meta‐analysis of the cosmopolitan moss Ceratodon purpureus in the Southern Hemisphere. Plants 12, 499 (2023).
Wang, J., Zhang, J. & Ma, W. Z. Tracking the range shifts of bryophytes to climate and land-use changes in mountains. Bryol. Times 159, 65–68 (2024).
Acknowledgements
We are grateful to the BEST (Biodiversity along Elevational gradients: Shifts and Transitions) Network, the Tianmushan National Nature Reserve of Zhejiang, the Tianma National Nature Reserve of Anhui, the Guanshan National Nature Reserve of Jiangxi and Daiyunshan National Nature Reserve of Fujian, China. We would like to thank Luyan Tang, Shichen Xing, Xing Chen, and Xuan Lü for field assistance. We specially thank Xiaorui Wang from Shijiazhuang University (Hebei Province), Yongying Liu from Jiaozuo Normal College (Henan Province), and Dongping Zhao from Inner Mongolia University (Inner Mongolia) for their assistance in bryophyte species identification. This work was supported by the National Natural Science Foundation of China (nos. 32070228), and the Innovation Program of Shanghai Municipal Education Commission (2023ZKZD36).
Author information
Authors and Affiliations
Contributions
J.Z. and J.W. secured funding and organized the fieldwork. J.W., Y.R.W. and X.Y. drafted the paper; X.Y., P.Z., R.P.S., Z.D., X.C., S.W.T., K.S., Z.S.H., Z.C.Z., Q.W., J.Z. and J.W. engaged in field investigation; Y.F.W., M.L., X.Y., S.W.T. and J.W. participate in species identification; Y.R.W., Y.T.Y., E.D.W., H.Y.Z., Y.L.X. and E.Q.L. compiled dataset information; Y.R.W. and X.Y. made the figures and tables; all authors contributed to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Yr., Yao, X., Zheng, P. et al. Elevational distribution patterns of bryophytes in Eastern China - A comprehensive species-trait dataset. Sci Data 12, 2001 (2025). https://doi.org/10.1038/s41597-025-06247-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-06247-3