Abstract
Community paleoecology is a powerful approach for analyzing ecological communities during long-term climate shifts like the Pleistocene-Holocene transition, but it depends on accurate estimates of species co-occurrences. The Neotoma Paleoecology Database is an open paleodata resource that stores assemblage-level taxonomic, spatial, and temporal information for Quaternary fossil localities. However, its age estimates for many vertebrate fossil localities are based on uncalibrated radiocarbon dates, hindering comparisons with other paleoenvironmental proxies. In order to provide consistent and updated age inferences suitable for broad-scale paleoecological studies, we have reassessed the radiocarbon chronologies for all 14C-dated North American small mammal collections in Neotoma. Here we present the resulting database update, including 2074 radiocarbon dates newly added to Neotoma and new calibrated radiocarbon chronologies for 1553 fossil collections. The new chronologies cover more sites and include more dates than the chronologies previously available in Neotoma. They also provide fossil assemblage age estimates in calendar years, facilitating integration with other data sources. We anticipate that these updates will be useful for various applications in community paleoecology.
Similar content being viewed by others
Background
In order to address complex global ecological questions such as the drivers of past ecosystem change, paleoecological studies increasingly rely on approaches that synthesize data from multiple sites, and that combine multiple data sources and proxy types, such as stable isotopes, pollen counts, macrofossil occurrences, and climate models (e.g.1,2,3,4). Each proxy, site, and sedimentary archive has its own unique taphonomic pathway. These local- and proxy-scale differences in the fossil record mean that understanding change across spatial scales, a central focus of paleoecology, requires accurate estimates of the ages of fossil samples5,6,7,8,9,10. Aligning fossil data in time across multiple sites and proxies is challenging. A variety of radiometric dating techniques, biostratigraphic markers, tephras, or annual laminations (such as tree rings or varves) can be used to directly estimate sample age, each with their own challenges and biases. Typically, only a subset of samples are dated, then once sample ages are determined, researchers develop an appropriate chronology to infer ages of undated samples based on a range of age modeling methods6,11,12,13. New methods and improvements in both sample age estimation and age modeling continually improve approaches, and different methods will provide slightly different estimates of time6,12,13,14,15. Additionally, for chronologies based on radiocarbon dates14,16, the calibration curves used to translate radiometric age in radiocarbon years before present (14C BP) to age in calendar years before present (cal BP) are regularly updated as new information is generated that increases the accuracy and precision of the curves11,17. For example, five curves have been developed by the IntCal effort between 1998 and 202016,18,19,20,21,22. Thus, chronologies need to be continually updated and refined to facilitate accurate research inferences.
The challenge of updating chronologies is particularly acute in paleo-data resources that store proxy information from many different sites and studies. One such resource is the Neotoma Paleoecology Database (hereafter “Neotoma”), which is a composite database comprised of 41 constituent databases of biological proxy information, including vertebrate fossils, fossil pollen, ostracodes, diatoms, stable isotopes, and many other proxies23,24. Neotoma addresses the challenge of managing changing date calibrations and age interpretation by linking biological proxy data to one or more chronological inferences, which can be updated to incorporate new data, and which may be linked to the individual chronological data used to create them (e.g. radiometric ages, biostratigraphic or archaeological dates). Within Neotoma, sites may contain one or more collection units (collections), defined as a unit from a site from which a collection of fossils or other data have been made; a single site may contain multiple collection units. A collection unit is typically associated with at least two datasets: a dataset of the proxy data associated with samples from different analysis units (usually stratigraphic units) within the collection and a dataset of geochronological ages associated with samples from some or all of the analysis units within the collection. Direct geochronological ages are linked to the set of observations at a site through a set of chronological controls which form the foundation for an overall inferred chronology for the collection unit, including a sample age estimate for each analysis unit within the collection unit. The overall collection unit chronology, which merges geochronological information and any other types of chronological information such as biostratigraphic information, tephras, etc. with an age model, provides age estimates for the collection unit overall as well as for each analysis unit within the site24.
Each collection unit in Neotoma may be associated with multiple chronologies. One chronology is typically the original chronology developed by individual researchers as part of their work at a site, though additional chronologies may be associated with a collection unit as chronologies are revisited and revised as part of other research efforts5,25. Some chronologies may include uncalibrated radiocarbon ages, reported in 14C BP, and others may use the calibration curve that was most current at the time of publication. Many collection units have chronologies based only on non-geochronologic dates such as archaeological periods, tephra layers, or biostratigraphic indicators, which may increase uncertainty. Some collection units have no inferred chronology at all, or have chronologies with no reported uncertainty. Overall, there is considerable variation among sites in chronology estimation and quality, which limits potential for broad-scale paleoecological syntheses across space and time.
This challenge is particularly evident in the FAUNMAP database, one of the Neotoma constituent databases, which stores information on Pliocene through Holocene vertebrate fauna of North America26. FAUNMAP was a collaborative research effort in the 1990s that produced a standardized set of chronologies associated with vertebrate faunal localities, but where those chronologies were based on radiocarbon dates (Late Pleistocene and later), they were typically provided in 14C BP, or a combination of 14C BP and cal BP26,27. These inconsistencies limit the utility of Neotoma’s chronological information and, where the differences in technique are not clearly labeled, they may mislead researchers. Additionally, since the cessation of the original FAUNMAP effort, many new radiocarbon dates from existing sites have been published, and many new dated fossil localities have been added to the database. An update to the chronologies underlying the FAUNMAP constituent database is long overdue. We therefore undertook a revision of North American vertebrate radiocarbon chronologies in Neotoma with the aim of producing a consistent set of age estimates for Pleistocene to Holocene vertebrate fossil assemblages, suitable for use in broad-scale paleoecological studies.
We focus specifically on localities that include records of small mammals, defined here as mammals in the orders Rodentia, Lagomorpha, and Eulipotyphla, and that have at least one radiocarbon or other geochronological age estimate rather than only biostratigraphic or other age estimates. Most of the available geochronological ages are radiocarbon dates, which have a maximum age of ~55,000 14C BP16; we have therefore restricted the temporal range of our update to the Late Pleistocene to Holocene. We focus specifically on locality-level chronologies that provide age estimates for all samples recovered from a locality. While generating chronologies based on individual specimens is now standard for many single-species studies focused on, e.g., estimating the timing of extinction or population expansion, these approaches tend to focus on larger mammals with large amounts of fossil material available for dating28,29,30,31 and are not easily applied to individual localities. Many Quaternary localities contain abundant small mammal fossils, and generating individual dates for all taxa within an assemblage is often impractical; thus inferences of age for small mammals often rely on indirect dates and inferred chronologies for the assemblage (e.g.27,32,33). Small mammals are abundant, diverse, and pervasive in terrestrial ecosystems; their ubiquity and trophic importance makes them a good choice for paleoecological studies comparing large numbers of fossil assemblages. The improved chronologies resulting from this effort will be of use to future researchers studying many aspects of vertebrate assemblages across the Pleistocene to Holocene climatic transition.
Methods
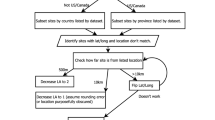
This data update consisted of two stages (Fig. 1):
-
1)
Updates to, and augmentation of, radiocarbon dates in Neotoma
-
2)
Estimation of new chronologies for vertebrate localities in Neotoma
Workflow diagram summarizing the main steps in updating and augmenting radiocarbon dates to sites in the Neotoma Paleoecology Database and generating updated chronologies. Stage 1 gathers data from multiple sources (including Neotoma), cleans data, resolves transcription errors and improves the data holdings within Neotoma. Because Neotoma provides additional collection, geographic and chronological metadata we ensure that data is submitted to Neotoma to ensure additional long term curation. Stage 2 uses the existing Neotoma APIs to download the full geochronological metadata for individual records. This allows the script to be run in the future, independently of Stage 1 as new records are added or as new statistical models are developed for chronology construction. Green boxes indicate steps that were done using R scripts; steps in blue boxes were done manually; and yellow boxes indicate steps that involved the upload of data into Neotoma.
Data acquisition
We undertook a data discovery and mobilization effort to first identify existing dated vertebrate localities in Neotoma, then update and augment these sites based on 1) the contents of the Canadian Archeological Radiocarbon Database (CARD) and 2) directly searching the literature for new ages from undated small mammal sites in Neotoma.
Data sources
Neotoma
The focus of our effort was on late Quaternary vertebrate localities within the Neotoma Paleoecology Database. Neotoma comprises multiple constituent databases, which typically focus on a particular proxy and/or region. Most of the data in this effort are curated within the FAUNMAP constituent database, but a subset of the sites are associated with the Alaskan Archeofaunas, PaleoVertebrates of Latin America, or other constituent databases. Relevant sites were selected from Neotoma according to the following procedure: We first downloaded a list of all sites with either vertebrate fossils or geochronological data within a polygon defining North America. From those sites, we then selected all collection units containing both vertebrate fauna and at least one geochronological date with a minimum (younger) 1-sigma age estimate (age minus error) less than 30,000 14C BP years ago. The list of remaining sites was filtered to include only those collection units whose vertebrate faunas contained fossil specimens identified to taxa belonging to the mammalian orders Rodentia, Lagomorpha, and Eulipotyphla. We then assembled the full list of geochronologic dates from these collection units. The workflow described above returns 5673 dates from Neotoma (as of 24 January 2025), 5411 of which were radiocarbon dates, from 1361 FAUNMAP collection units that contained small mammal fossils (Fig. 2). Complete R code for this procedure is contained in the project Github repository (see “Code availability”).
New dates added to Neotoma from literature and CARD database. (a) Map of all sites in this project. Blue = no new dates found; cream = some dates corrected or newly added; orange = all new dates (i.e. previously undated site). (b) Number of new dates added in this project for each modern ecoregion (EPA level 1 55) and time intervals. Time interval abbreviations: LGM (Last Glacial Maximum, 30,000 - 16,000 cal BP); DG (“deglacial”, 16,000 -11,700 cal BP); EH (Early Holocene, 11,700 - 6,000 cal BP); LH (Late Holocene, 6000 - 0 cal BP). (c) Proportional representation of EPA ecoregions and time intervals among all dates used in chronology construction in this project (left), compared to the same for new dates only (right).
Canadian Archeological Radiocarbon Database (CARD)/p3k14c. CARD34 is a database of over 120,000 radiocarbon dates from archaeological and geological sites around the world, although the focus has historically been on North American archaeology35,36. Lists of dates from CARD in Canada, the United States (US), and Mexico were downloaded, and the list of full references was requested from site maintainer (author A.M.). CARD is both a user-populated database and the result of regional research initiatives (e.g.36). Dates that were assigned to the US but not to a particular state were excluded. The two datasets of radiocarbon samples were first matched by whether the lab number was present in both CARD and Neotoma, and then by the uncalibrated (14C years) age and error values. If the age, error, and lab number were identical, the date was marked as correct and present in both databases. If the lab number and uncalibrated age and error values were not identical, the record was flagged for review. 408 such conflicting age or error values were checked against the original references before being added to the final table of dates to be uploaded (see “Data quality control” below.)
All remaining dates from CARD were matched against existing Neotoma sites where possible, using a variety of non-required or non-unique identifying information: machine numbers from the FAUNMAP project26,27, government-designated archeological site numbers (e.g. Smithsonian trinomial37 or Borden system number38), site names (using custom regular expressions to fuzzy-match names), and/or geographic proximity of coordinates (within a 50-meter radius of an existing Neotoma site). Each of these pieces of information might individually fail to identify a matching pair of sites for a variety of reasons, but in combination, they proved to be sufficient (SJG, VJPS, JLB et al., in prep.). The list of possible matches was then manually reviewed to identify which sites were genuinely present in both databases. We only added dates from CARD to Neotoma if they either matched an existing site in Neotoma with small mammal data, or if the date or site in CARD contained the names of taxa within Rodentia, Lagomorpha, and Eulipotyphla in the CARD fields taxa_dated or associated_taxa. In the latter case, we also added any new taxa to the Neotoma vertebrate fauna dataset separately.
Potential dates from identified shared sites were then checked against the original references. The abbreviated references recorded in the main CARD database were identified in the CARD reference list where possible, or using WorldCat or Google Scholar if necessary. 209 dates with unidentifiable or inaccessible references were dropped. Coding of raw 14C ages as “measured age” versus “normalized age” in CARD was inconsistent, so the date type was checked in the references and entered as described in the original publications. Based on the historical and stratigraphic context recorded in CARD or the original references, dates were assigned in Neotoma to either an existing analysis unit, a new analysis unit belonging to an existing collection unit, or a new collection unit at the site. In total, 1,187 dates were found and verified by this process.
Within CARD, publicly-available site locations of dates from the US were obfuscated to the county level in order to safeguard potentially sensitive locations. In order to avoid exposing these data through Neotoma, we dropped 1,098 dates that came from 139 sites whose locations were obfuscated in CARD to a lower precision than their location in Neotoma. We recognize that this is a compromise that means that some sites are spatially resolved more finely in Neotoma than in CARD; though we have not added new dates to these sites, the sites still exist in their original spatial resolution in Neotoma. Neotoma leadership (including author J.L.B.) is currently focused on identifying sites within Neotoma for which the current spatial resolution is not appropriate and implementing changes to the spatial precision of some sites. In the future, if site spatial precision is changed to a resolution that matches CARD, we will upload the newly identified dates and update their chronologies following the process outlined here.
Overall, we uploaded 89 dates from 16 collection units from CARD into Neotoma. The p3k14c database39, which incorporated CARD, was accessed using the p3k14c R package40 in order to check for additional dates. All relevant dates in p3k14c were either present in Neotoma already or were originally from CARD and therefore had been captured by the process detailed above.
Literature search
Additional dates were added from the literature based on a search using xDD41, the journal Radiocarbon, and Google Scholar. We focused our efforts on sites already in Neotoma for which focal taxon occurrences were recorded, but for which there were no geochronological dates. We compiled a list of potential sites, and then each of these site names was searched against xDD along with the dictionary terms “radiocarbon” and “North America”, using the “snippets” API tool. Each site name was also searched against the radiocarbon date lists published in Radiocarbon, which is not indexed in xDD but has open-access machine-readable PDFs available on the journal website. Remaining undated small mammal sites were individually searched in Google Scholar. All references returned by any of these search methods were checked manually for any dates not already present in Neotoma. These searches resulted in the addition of 1,985 radiocarbon dates for collection units already in Neotoma. Searches also yielded new small mammal records for 22 existing collection units, 36 new dated sites with small mammal fossils, and a number of corrections to previously recorded dates. The full process is documented in the code in the project Github repository.
Date validation and quality control
Neotoma supports the use of 19 different date types, including geochronologic, stratigraphic, and event-based date types. The final small mammal chronology dataset included 7695 uncalibrated radiocarbon dates, as well as 92 radiocarbon dates for which the uncalibrated date could not be found, 51 thermoluminescence, 34 uranium-series, 1 argon-argon, 4 optically stimulated luminescence, and 2 paleomagnetic dates. OxCal can incorporate date types other than uncalibrated radiocarbon into composite chronological models by coding them as calendar dates (C_Date). We added the non-radiocarbon dates into the OxCal age model as calendar dates for collection units containing both radiocarbon and non-radiocarbon dates. The resulting age models were computed for the other multi-date collections and interpreted as described in “Computing age estimates”. No new chronologies were computed for analysis units without any uncalibrated radiocarbon dates.
Error checking
Dates present in both CARD and Neotoma were checked against the original publication. Of these, 214 dates had incorrect or missing mean ages in Neotoma; 22 of these had additional metadata errors. All such records were corrected in Neotoma. Some dates were incorrect or incomplete in both databases when compared to the original publications; these were also corrected in Neotoma and submitted to CARD. 12 dates in Neotoma or CARD had asymmetric error values (e.g. “1200, −110/ + 70”). In all these cases, we located the original error values via literature search and confirmed that they were originally reported as uncalibrated values with asymmetric errors, and retained them in the database in this form. For 208 dates in CARD, either no original publication could be identified or the original publication was not available online or via the University of California library system, including dates for eight sites with no other identified dates. However, as all eight of these sites were from the Late Holocene, ≤6ka (for which data are available from many other sites), and the remainder of the untraceable dates were from already-dated sites, we did not pursue them further. We did not retain dates for which the original publication or the CARD notes indicated they were derived from human remains. Note that not all sites with vertebrate fauna data in Neotoma were checked, only those from collections that contained small mammal fossils and radiocarbon dates ≤30,000 14C BP; errors may remain in Neotoma collections older than 30 ka and/or not containing small mammals.
Revisiting the original publications as part of quality control revealed 58 dated small mammal fossil collections from new sites not previously included in Neotoma, as well as a number of small mammal fossils belonging to existing Neotoma collection units but not previously included in the taxon lists for those sites in Neotoma. These data were individually added to the database. Finally, two sites in our list, Lubbock Lake and Natural Trap Cave, have undergone significant recent stratigraphic revisions and redating, and have been omitted from this effort due to the complex revisions needed to their data structure in Neotoma.
Date types and qualities
Experimental determination of radiocarbon dates is known to be affected by the choice of original material, sample treatment, and dating method5,42,43. The effects of these factors on 14C date precision and accuracy have been documented in depth. In general, for the purpose of dating small mammal fossil sites, the ideal original material would be bone from a specimen of the particular species of interest; the optimal sample pretreatment for bone samples would be collagen extraction and XAD ultrafiltration44,45; and the modern standard for dating method is accelerator mass spectrometer (AMS) dating42,46,47. Unfortunately, only a handful of the dates in Neotoma are documented as meeting these standards. Of the 7695 radiocarbon dates in our final data set, 2579 have no dating method entered, and only 809 are labeled as AMS. The material dated mentions “bone” for 1950 dates and “collagen” or “gelatin” (presumably from bone) for 829. Filtering, purification, or extraction, including XAD, is mentioned in the metadata for only 72 dates; the vast majority of dates do not have metadata on sample pretreatment. Additionally, the 284 dates missing their original lab numbers are effectively untraceable and therefore must also be regarded as less reliable. If we restrict our data set to only AMS dates with known lab numbers from XAD-extracted bone collagen, we are left with a total of 18 dates and 7 dateable collections.
As our goal is to provide a large set of chronologies suitable for regional- or continental-scale analyses of species distributions or comparisons to other data sets, we have chosen to prioritize breadth over precision by including all dates accepted by the original authors in our chronology revision, including low-quality and/or poorly-documented dates. This includes dates with missing metadata and outdated methods, as well as those derived from materials such as plant charcoal48 or insect chitin49, which may tend to be older or younger than co-occurring small mammal fossils. The potential uses of a wide-ranging, homogeneous, but lower-precision set of chronologies are discussed further in the section “Usage notes”.
Computing new age estimates
In order to generate new chronologies, we started with a fresh date list by using the neotoma2 R package50 to download all the geochronological dates from collections with small mammal fossils in North America and current age estimates <30,000 14C BP, including both previous and newly-updated dates (total n = 8459). We then filtered the dataset to eliminate 183 radiocarbon dates that were single-sided (lower age limit only, e.g. “ >31,500”) or modern (age estimate ≤10 cal BP), 303 with missing or zero error values, 314 for which the materialdated or notes fields indicated that the age estimate had been rejected by the original author, and 9 for which the materialdated or notes fields indicated that the sample came from human remains (categories may overlap). This eliminated 28 collections lacking reliable or usable radiocarbon dates. We additionally dropped 32 collections that had no radiocarbon dates at all. The final data set used for age estimation consisted of 7695 radiocarbon dates and 92 other dates, allowing us to compute chronologies for 1551 collection units from 1382 sites.
We then used OxCal (v. 4.4)51 to compute an age model for each collection unit in the data set (Fig. 3a,b), using a script that automatically computed OxCal scripts (Fig. 3c), as follows:
-
1.
All geochronological dates in each analysis unit were grouped into a single phase using the Phase() function, to capture the time horizon over which depositional processes formed the analysis unit. Uncalibrated 14C dates, which make up the majority of the data set, were calibrated using R_Date(). Pre-calibrated or non-radiocarbon dates were incorporated using C_Date(), as discussed under “Date types” below. The phase for a single analysis unit could contain one or multiple dates.
-
2.
Two different age estimates were calculated for each analysis unit; one used a Date() call inside the phase and the other used two Boundary() calls outside the phase. All age distributions were estimated based on uniform prior distributions. The difference between these two types of age distribution model, and the rationale for calculating both, is discussed under “Dated analysis units” below.
-
3.
Each collection unit was modeled as a single Sequence() unit in order to constrain the Bayesian age estimates, with one phase for each analysis unit. If the analysis unit names or depths indicated a clear stratigraphic order, they were ordered from oldest to youngest, and the whole collection was modeled with Sequence(); otherwise, it was modeled as a phase with no ordering. (Note that the overall collection unit age boundaries were not separately modeled; see “Overall chronology age estimates” for details).
Example chronology. This collection unit belongs to the Neotoma site “Selby” from eastern Colorado (siteid: 3534) and can be viewed in Neotoma Explorer using that name (or the dataset landing pages data.neotomadb.org/4562 and data.neotomadb.org/9273). It consists of two analysis units with no recorded stratigraphic order, each containing two radiocarbon dates. (a) Calibrated radiocarbon dates and analysis unit age estimates, plotted on a vertical age axis. Red curves show the calibrated age distributions of the individual 14C dates; solid lines indicate medians and dotted lines indicate 5% and 95% quantiles. Green curves show the estimated sample age distribution for each of the two analysis units; solid lines indicate medians and dotted lines indicate 32% and 68% quantiles. Blue curves show the estimated upper and lower age boundaries for each analysis unit; solid lines indicate medians. (b) Collection-unit-level age boundaries for each of the two chronology types based on the age distributions in (a), plotted on the same vertical age axis and using the same colors. As indicated by the dotted lines connecting (b) to (a), the event chronology (in green) is bounded by the maximum and minimum 32%/68% quantiles of the event age distributions; the bounds chronology (in blue) is bounded by the maximum and minimum 50% quantiles of the boundary age distributions. (c) Automatically-generated OxCal code used to calculate the example chronology in Fig. 2.
Estimating sample ages for analysis units
Chronologies are reported in Neotoma as the individual control points on which the sample ages are based (Fig. 3a, red curves), the estimated age range of each analysis unit (‘sample age’) in the collection (Fig. 3a, blue and green curves), and the maximum and minimum age of the overall collection unit (Fig. 3b). Our procedure for estimation of sample ages consisted of first estimating the ages of analysis units with geochronologic dates, then combining those estimates with stratigraphic context to infer the ages of undated analysis units in the same collection. We developed two different chronologies for each collection unit, corresponding to two different methods for inferring the sample ages, which we named the ‘event’ and ‘bounds’ chronologies. The difference in methods between the two is detailed below.
Dated analysis units
For analysis units with at least one geochronologic date, the sample ages were directly calculated as quantiles of the age probability distributions generated by OxCal (Table 1).
The sample age estimate for each directly dated analysis unit in the ‘event’ chronology was based on the OxCal estimate of the age distribution for that analysis unit; it can be thought of as the expected value of a new date drawn from the same pool as all the dates currently in the analysis unit15,51. The age estimates for the ‘bounds’ chronology, by contrast, was based on the OxCal estimates of the age distribution for the minimum and maximum ages of the dated analysis unit, and therefore can be thought of as the estimated starting and ending dates for the deposition of the analysis unit. From a researcher’s perspective, there are advantages to each. In general, the bounds chronology provides a more conservative estimate of unit age for time-averaged deposits, and thus produces a more consistent result between collections with a single date and collections with more complex structures. The event chronology provides a statistically meaningful central estimate of collection age, but we found that it is more sensitive to the number of dates and stratigraphic units in the collection.
In choosing which quantiles to report from the different estimated age distributions, we have attempted to balance a variety of priorities: limiting sensitivity to the number of dates, and providing a usable but not misleading level of precision. However, we recognize that some arbitrariness is unavoidable in the choice of how to report discrete values from continuous distributions. For the bounds chronologies, highly outward-skewed distributions are characteristic of the boundary age estimates (see Fig. 3); the medians (50% quantiles) of the boundary age estimates provide a conservative estimate of the depositional window for the collection, but largely avoid this statistical artifact. The event age distributions, while typically not highly skewed, are often platykurtic, falling somewhere between normal and uniform; our choice to report the 32% and 68% quantiles is intended to dampen the sensitivity of this measure to sparse data and outliers.
Undated analysis units
For collection units containing some dated and some undated analysis units, the results were individually manually examined, and the age estimates for the dated samples (analysis units) were used to infer ages for the undated samples. Many analysis units correspond to stratigraphic units; stratigraphic relationships can therefore be used to constrain the possible ages of some of the undated samples.
The inferred sample age boundaries for the undated analysis units in each collection unit were assigned according to the following heuristics, graphically summarized in Fig. 4:
-
a.
If an undated analysis unit was a subset of a dated analysis unit (e.g., a single undated specimen from a dated stratum), it was assigned the age range of that dated unit.
-
b.
An undated analysis unit bounded above and below by dated analysis units was assigned a maximum (oldest) age from the top of the next dated unit below it and the minimum (youngest) age from the bottom of the next dated unit above it.
-
1.
If the age ranges of the two dated units overlapped, it was assumed that time-averaging had occurred, so the undated unit was instead assigned the full range of the dated analysis units above and below it.
-
c.
An undated analysis unit stratigraphically above all dated analysis units was assigned a maximum (older) age of the top of the next dated unit below it and a minimum age of 0, unless other biostratigraphic or archeological information allowed its minimum age to be further constrained.
-
d.
An undated analysis unit stratigraphically below all dated analysis units was assigned a minimum (younger) age of the bottom of the next dated unit above it and a maximum (older) age based on any biostratigraphic or archeological constraints recorded in existing chronologies or, if no information about this is available in Neotoma, in the original publication.
-
e.
If an undated analysis unit had no apparent stratigraphic relationship to the other units in the collection, it was assigned the full age range of all dated units in the collection, i.e. the same maximum and minimum age as the whole collection unit.
-
f.
No central age values were estimated for undated analysis units.
Summary of rules used to infer the ages of undated analysis units in a collection from ages of dated units. The column on the left marked “stratigraphy” shows the stratigraphic field relationships of all analysis units in the collection unit, as well as the presence of 14 C-dated specimens (red filled outlines). Blue-shaded analysis units indicate analysis units containing 14 C-dated specimens; orange shading indicates analysis units without direct dates, whose ages must therefore be inferred from stratigraphic relationships. The same shades are used for the corresponding analysis unit ages on the right side, marked “age ranges”. Calculated analysis unit age ranges (blue shaded) are based on 14 C-dated specimens, as described in the section “Dated analysis units”, and are connected to the corresponding strata with solid lines. Inferred analysis unit age ranges (orange shaded) are derived from calculated ages based on stratigraphic relationships, and are connected to the corresponding strata with dotted lines; lowercase letters in each analysis unit indicate which rule was used to infer the age of that unit: (a.) subset of dated layer; (b.) bounded above and below by dated layers; (b.1) bounded by overlapping dated layers; (c.) above all dated layers; (d.) below all dated layers. These heuristics are described in more detail in the section “Undated analysis units”.
These rules were used to assign inferred age ranges for a total of 926 undated analysis units belonging to 197 of the collection units.
Overall chronology age bounds
The age range for any one collection unit is defined as the oldest and youngest ages inferred for all analysis units within the chronology, including analysis units that extend to the surface (set to the standard 14C “year zero”, 1950 CE) or analysis units below 14C-dated units with maximum age estimates based on stratigraphic, biostratigraphic, or archeological data (i.e. orange-shaded analysis units in Fig. 4). Both dated and undated sample ages were used in estimating the collection unit maximum and minimum ages in the Neotoma table chronologies.
The decision to include inferred age ranges was based on the expectation that the primary use of collection unit age ranges is for API-based data filtering. When a user of either the neotoma2 R package or the Neotoma Explorer web interface requests sites falling within a certain age range, the API will filter the data using the collection unit age ranges in the default chronologies and return all sites with collection units that overlap the desired age range. Since users may be looking for information on fossils that pre- or post-date the range of directly dated units (in Fig. 4, the highest or lowest orange-shaded units), limiting the search to only directly dated units might exclude relevant results. We have therefore reported the maximum and minimum ages for the collection units in the chronologies table under the most generous interpretation possible.
Data Records
The primary repository for the data generated in this project, consisting of the table of geochronologic dates compiled in stage 1 and the chronologies generated in stage 2, is the Neotoma Paleoecology Database, where they have been integrated into the relational structure of the database. They can be accessed from the Neotoma API as an aggregated dataset52. The new dates and new chronologies, exactly as uploaded at publication, are also archived in the Zenodo release of the Github repository53,54.
We have provided an example of how the new chronologies for a single site are represented in the Neotoma database schema (open.neotomadb.org/dbschema) in Table 2. Raw geochronologic dates (in this case uncalibrated radiocarbon ages and errors, lab identification numbers, and associated δ13C values) are stored in the table geochronology, and the related sample metadata (material, preparation methods, associated specimens) are linked to it via the table samples. The geochronologic data sets can be viewed using the Neotoma Explorer web interface or downloaded with the get_table() function in the neotoma2 R package50. Within Neotoma, the geochronologic dates added to the database from this project are publicly available, but have not been marked in any way that allows them to be distinguished from the other data in the table.
The chronologies table stores the chronology name, model, and the estimated maximum and minimum ages for the collection unit. It links to the constituent analysis unit ages (maximum, minimum, and central) in the sampleages table, and to the chronology control points (calibrated radiocarbon ages and all other age estimates or dates used in chronology construction) in the chroncontrols table. Each radiocarbon-based chronology control point is referred back to its corresponding raw geochronologic dates in geochronology via the linking table geochroncontrols. For further details of chronology definition in Neotoma, see Williams et al.24 and the Neotoma manual (open.neotomadb.org/manual). For the relational structure of all tables linked to chronologies, see open.neotomadb.org/dbschema/ndb/tables/chronologies.html, and for tables linked to geochronological dates, see open.neotomadb.org/dbschema/ndb/tables/geochronology.html.
The chronologies from this project have the chronology name “Syverson-Blois: bounds” or “Syverson-Blois: event” and the age model names “Bayesian unit bounds” or “Bayesian event distribution” in the chronologies table. Each individual chronological control (i.e. entry in the chroncontrols table) for each chronology is linked via the sampleages table to a particular date in the geochronology table. The set of chronologies for any site in Neotoma, including the top-level collection unit age boundaries and all constituent analysis unit ages and control points, can be viewed using the function “chronologies” in the neotoma2 R package50. The “Syverson-Blois: bounds” chronologies have been set as the default chronologies for all sites included in this update, which means that they are also returned by default in the Neotoma Explorer “samples” tab and dataset download and automatically used for age-based queries from the API.
The Zenodo-archived Github repository contains the updated dates53 and chronologies54 unlinked from the rest of the Neotoma relational database. It also contains seven RMarkdown notebooks containing the entire workflow, as well as various.csv files containing data and R scripts containing functions that are called from the notebooks. Scripts 1–5 guide the user through the process of downloading geochronological dates from Neotoma and generating chronologies for all Late Pleistocene through Holocene small mammal collections, as was done in this project. Script 6 is a concise version of the previous five scripts that is designed to retrieve the current chronological and taxonomic information for all collections in a single site or a small set of sites specified by their Neotoma site ID, and is likely to be the most useful to any readers interested in recomputing the chronology for particular collections, for instance if they have added more radiocarbon dates to the database for their study site. Script 7 uses the outputs of the other scripts to generate the figures in this paper. All code was written by VJPS on Debian Linux 12 and tested by JLB on Mac OS 15, using R v4.2.0 or later with libraries neotoma2 v1.0.550 and oxcAAR v1.151.
Technical Validation
New and updated radiocarbon dates
Following the cleaning procedures, we made the following improvements52,53 to the available set of radiocarbon dates for small mammal sites in Neotoma (Fig. 2):
-
Added 2074 new dates to 264 collections at 254 existing sites
-
Corrected or updated 452 dates already in Neotoma
-
Added 42 new sites with dates
-
Updated 22 existing collection units with new small mammal fossil occurrences
To examine the effects of our update on temporal coverage, we binned the dates into four intervals, designated LH (Late Holocene, 6000 - 0 cal BP), EH (Early Holocene, 6,000–11,700 cal BP), DG (“deglacial”, 11,700–16,000 cal BP), and LGM (Last Glacial Maximum, 16,000–30,000 cal BP). The majority of dates added were from the Late Holocene, as is true of the overall data set we used for computing the new chronologies. However, we have significantly added to the number of available Pleistocene (2.588 Ma - 11,700 cal BP) dates with this update, increasing the number of LGM dates by nearly 70% and more than doubling the number of DG dates. The distribution of dates in the dataset is significantly influenced by geographic bias in collection and data upload, most obviously evident in the underrepresentation of fossil sites from Mexico. Taphonomic factors also influence the availability of dates; for example, northern areas covered by continental ice sheets at the Last Glacial Maximum lack Pleistocene dates. The representation of Level 1 EPA ecoregions55 is also temporally uneven; for example, dates from Great Plains and Northern forest sites are overrepresented in the Holocene, while most dates from taiga environments are of Pleistocene age. This largely reflects known taphonomic and sampling biases (Bellvé et al., in review.). A cross-tabulation of ecoregions and time periods is given in Fig. 2c.
Our sampling effort compiles legacy data from original literature dating back to the earliest published radiocarbon lab date lists, which confines its scope to those dates that were at some point published in accessible literature with sufficient metadata for identification. Updates to some sites or dates based on later work may have occurred in the museum context but never been published in any public-facing literature; these would not be captured by our process. Additionally, some dates may have been missed by our process due to inconsistencies in the metadata used to identify sites and dates (e.g., sites with multiple names, differences between paleontological and archeological stratigraphic practices, failure to report lab numbers) and in the date values themselves (e.g., failure to report reservoir correction or calibration status). These inconsistencies should be taken as motivation for better standardization of date reporting and application of permanent identifiers to radiocarbon dates in order to improve interoperability between museum repositories, authors and scientific publishers, and online aggregators like Neotoma56 (SJG, VJPS, JLB et al., in prep.).
Updated chronologies
We computed and uploaded new calibrated radiocarbon chronologies52,54 for a total of 3648 analysis units from 1,553 collection units containing small vertebrate fossils. 2,731 analysis units were directly dated from samples, while age estimates for a further 928 analysis units were inferred based on their stratigraphic relationships. 67 of the collection units with new chronologies from this project had no existing chronology in Neotoma; of the remaining 1,486 collection units, 1,158 of them previously had default chronologies based on uncalibrated radiocarbon dates, and 333 were based on calibrated radiocarbon age or other calendar age methods. While more work remains, this update significantly improves standardization and completeness of the vertebrate faunal sites in Neotoma. The geographic locations and age ranges of the new analysis unit age ranges are summarized in Fig. 5.
All analysis unit age estimates from the radiocarbon chronologies calculated in this project (event chronology only). Each circle corresponds to one analysis unit; multiple concentric circles indicate several analysis units from the same site. Color indicates median age of the analysis unit, as shown in legend; roughly, yellow dots are Late Holocene, green are Early Holocene to Deglacial, and blue/purple are Pleistocene. Circle size indicates age precision, with larger circles indicating analysis units with wider estimated age boundaries; the largest circles shown correspond to ~1 kyr age ranges.
For those collections and analysis units that previously relied on uncalibrated radiocarbon chronologies, we can directly assess the effects of date calibration and Bayesian modeling of age distributions (Table 3; Fig. 6). Calibrating the radiocarbon ages, as expected from the fact that the best-fit slope of the intcal20 curve is less than −1, increases the central age estimate for each dated analysis unit by a median of 7 years for those analysis units with existing median age estimates. Compared to the previous uncalibrated chronologies, our re-estimation of the analysis unit age ranges decreased the ranges of the analysis units by a median of 198 years using the “event” method and increased them by a median of 490 years using the “bounds” method (Fig. 6a). Results are similar for the overall chronology (collection unit) age bounds: the new “event” chronologies start on average 6 years later and end 96 years earlier than the old ones, while the new “bounds” chronologies extend a median of 404 years before and 176 years after the old uncalibrated chronology bounds (Fig. 6b). While the “bounds” chronology does downgrade the apparent precision of sample age estimates, this more conservative estimate more closely reflects realistic limits to the accuracy with which sample ages can be inferred from a small number of time-averaged dates.
Graphical comparisons of age ranges between chronologies. (a) Plot of distribution of analysis units age ranges (bounds chronology only). For each analysis unit, its maximum calculated age is indicated with a blue dot, minimum age with a red dot, and the two are connected with a horizontal black line. Vertical black line indicates the Pleistocene-Holocene boundary (11,700 cal BP). Eight units with maximum sample ages >150,000 cal BP have been omitted from this plot for visual clarity. Note that while age ranges are on average much larger for older analysis units, many Pleistocene and early Holocene units are dated to relatively high precision. (b) Graphical depiction of data in Table 2, comparing the mean differences in older and younger age boundary estimates between the previous (uncalibrated) default chronologies and the two new chronologies described in this document, for both dated analysis units (left) and collection units (right). Compared to the uncalibrated chronologies, the bounds chronologies are generally less precise and the event chronologies are more precise; also, both new chronologies skew older than the previous chronologies due to the calibration.
Usage Notes
Our chronologies have been set as the default in Neotoma because the calibrated age basis allows them to be directly compared to other datasets in the database, such as pollen cores or varve sequences, that are dated on the basis of calibrated radiocarbon or calendar age. These chronologies are not suitable for all research projects. Many studies require detailed species- or site-level chronologies based on individually dated specimens and high-quality dates with detailed stratigraphies, such as inferring extinction timing1. Our chronologies are intended to inform regional to continental level studies, which benefit from a uniform set of chronological inferences with reasonable error estimates.
As discussed in the subsection “Dated analysis units” above, this project produced two different chronologies for each collection unit evaluated. The “Syverson-Blois: bounds” chronology gives broader (more conservative) age ranges and does not provide a central age estimate. This chronology is recommended for most uses, and provides the best representation available of the uncertainty associated with the ages of samples within each analysis unit. The “Syverson-Blois: event” chronology provides narrower (less conservative) age ranges, and additionally gives a central age estimate for each radiocarbon-dated sample in the collection. This chronology may be suitable for applications where a single age for each analysis unit is needed, with the caveat that this usage masks the temporal uncertainty of the samples, which can be substantial in some cases. We have therefore set the “bounds” chronology as the default chronology in Neotoma, but uploaded both chronologies to Neotoma so that individual researchers can choose the chronology best suited for their research.
Data availability
The aggregated dataset in Neotoma, including both dates and chronologies along with all other data for the affected sites, is available for download from the Neotoma API at the endpoint api.neotomadb.org/v2.0/data/aggregatedatasets/1352. The newly added dates and chronologies are separately available as dates pub copy.xlsx53 and chronologies pub copy.xlsx54 in the Zenodo release of the Github repository: https://doi.org/10.5281/zenodo.17064489.
Code availability
The R and OxCal code used in this project is available in the Zenodo release of the Github repository: https://doi.org/10.5281/zenodo.17064489.
References
O’Keefe, F. R. et al. Pre–Younger Dryas megafaunal extirpation at Rancho La Brea linked to fire-driven state shift. Science 381, eabo3594 (2023).
Short, R. A., McGuire, J. L., Polly, P. D. & Lawing, A. M. Trophically integrated ecometric models as tools for demonstrating spatial and temporal functional changes in mammal communities. Proc. Natl. Acad. Sci. 120, e2201947120 (2023).
Finsinger, W., Bigler, C., Schwörer, C. & Tinner, W. Rates of palaeoecological change can inform ecosystem restoration. Biogeosciences 21, 1629–1638 (2024).
Shuman, B. N. Patterns of centennial to millennial Holocene climate variation in the North American mid-latitudes. Clim. Past 20, 1703–1720 (2024).
Blois, J. L., Williams, J. W. J., Grimm, E. C., Jackson, S. T. & Graham, R. W. A methodological framework for assessing and reducing temporal uncertainty in paleovegetation mapping from late-Quaternary pollen records. Quat. Sci. Rev. 30, 1926–1939 (2011).
Blaauw, M., Christen, J. A., Bennett, K. D. & Reimer, P. J. Double the dates and go for Bayes — Impacts of model choice, dating density and quality on chronologies. Quat. Sci. Rev. 188, 58–66 (2018).
Cao, X. et al. A taxonomically harmonized and temporally standardized fossil pollen dataset from Siberia covering the last 40 kyr. Earth Syst. Sci. Data 12, 119–135 (2020).
Zimmerman, S. R. H. & Wahl, D. B. Holocene paleoclimate change in the western US: The importance of chronology in discerning patterns and drivers. Quat. Sci. Rev. 246, 106487 (2020).
Flantua, S. G. A. et al. A guide to the processing and standardization of global palaeoecological data for large-scale syntheses using fossil pollen. Glob. Ecol. Biogeogr. 32, 1377–1394 (2023).
Lovelace, D. M. et al. An age-depth model and revised stratigraphy of vertebrate-bearing units in Natural Trap Cave, Wyoming. Quat. Int. 647–648, 4–21 (2023).
Parnell, A. C., Buck, C. E. & Doan, T. K. A review of statistical chronology models for high-resolution, proxy-based Holocene palaeoenvironmental reconstruction. Quat. Sci. Rev. 30, 2948–2960 (2011).
Bronk Ramsey, C. Methods for summarizing radiocarbon datasets. Radiocarbon 59, 1809–1833 (2017).
Bronk Ramsey, C. Deposition models for chronological records. Quat. Sci. Rev. 27, 42–60 (2008).
Blaauw, M. 14C age modeling. in Encyclopedia of Quaternary Science (Third edition) (ed. Elias, S.) 618–627, https://doi.org/10.1016/B978-0-323-99931-1.00076-3 (Elsevier, Oxford, 2025).
Bronk Ramsey, C. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009).
Hajdas, I. et al. Radiocarbon dating. Nat. Rev. Methods Primer 1, 1–26 (2021).
Reimer, P. J. Composition and consequences of the IntCal20 radiocarbon calibration curve. Quat. Res. 96, 22–27 (2020).
Reimer, P. J. et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020).
Stuiver, M. et al. INTCAL98 radiocarbon age calibration, 24,000–0 cal BP. Radiocarbon 40, 1041–1083 (1998).
Reimer, P. J. et al. IntCal04 terrestrial radiocarbon age calibration, 0-26 cal kyr BP. Radiocarbon 46, 1029–1058 (2004).
Reimer, P. J. et al. IntCal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon 51, 1111–1150 (2009).
Reimer, P. J. et al. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55, 1869–1887 (2013).
re3data.org: Neotoma Paleoecology Database. re3data.org - Registry of Research Data Repositories, https://doi.org/10.17616/R3PD38 (2025).
Williams, J. W. et al. The Neotoma Paleoecology Database, a multiproxy, international, community-curated data resource. Quat. Res. 89, 156–177 (2018).
Wang, Y., Goring, S. J. & McGuire, J. L. Bayesian ages for pollen records since the last glaciation in North America. Sci. Data 6, 176 (2019).
FAUNMAP Working Group, Graham, R. W. & Lundelius, E. L. Faunmap: A Database Documenting Late Quaternary Distributions of Mammal Species in the United States. (Illinois State Museum, Springfield, 1994).
Graham, R. W. et al. Spatial response of mammals to late Quaternary environmental fluctuations. Science 272, 1601–1606 (1996).
Stuart, A. J. & Lister, A. M. Extinction chronology of the woolly rhinoceros Coelodonta antiquitatis in the context of late Quaternary megafaunal extinctions in northern Eurasia. Quat. Sci. Rev. 51, 1–17 (2012).
McDonald, H. G., Dundas, R. G. & Chatters, J. C. Taxonomy, paleoecology and taphonomy of ground sloths (Xenarthra) from the Fairmead Landfill locality (Pleistocene: Irvingtonian) of Madera County, California. Quat. Res. 79, 215–227 (2013).
White, L. C., Saltre, F., Bradshaw, C. J. A. & Austin, J. J. High-quality fossil dates support a synchronous, Late Holocene extinction of devils and thylacines in mainland Australia. Biol. Lett. 14, 20170642 (2018).
Wendt, J. A. F., McWethy, D. B., Widga, C. & Shuman, B. N. Large-scale climatic drivers of bison distribution and abundance in North America since the Last Glacial Maximum. Quat. Sci. Rev. 284, 107472 (2022).
Blois, J. L., McGuire, J. L. & Hadly, E. A. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature 465, 771–774 (2010).
Smith, F. A. et al. Unraveling the consequences of the terminal Pleistocene megafauna extinction on mammal community assembly. Ecography 39, 223–239 (2016).
Martindale, A. et al. Canadian Archaeological Radiocarbon Database (CARD 2.1) (2016).
Gajewski, K. et al. The Canadian Archaeological Radiocarbon Database (CARD): archaeological 14C dates in North America and their paleoenvironmental context. Radiocarbon 53, 371–394 (2011).
Kelly, R. L. et al. A new radiocarbon database for the lower 48 states. Am. Antiq. 87, 581–590 (2022).
David G. Anderson, J. W. Digital Index of North American Archaeology (DINAA). Open Context, https://doi.org/10.6078/M7N877Q0 (2015).
Borden, C. A uniform site designation scheme for Canada. Anthropol. Br. Columbia 3, 44–48 (1952).
Bird, D. et al. p3k14c, a synthetic global database of archaeological radiocarbon dates. Sci. Data 9, 27 (2022).
Bird, D., Bocinsky, R. K. & Miranda, L. p3k14c (2022).
Peters, S. et al. A new tool for deep-down data mining. Eos, https://doi.org/10.1029/2017EO082377 (2017).
Bronk Ramsey, C. Radiocarbon dating: revolutions in understanding. Archaeometry 50, 249–275 (2008).
Rodríguez-Rey, M. et al. Criteria for assessing the quality of Middle Pleistocene to Holocene vertebrate fossil ages. Quat. Geochronol. 30, 69–79 (2015).
Brown, T., Nelson, D., Vogel, J. & Southon, J. Improved collagen extraction by modified Longin method. Radiocarbon 30, 171–177 (1988).
Fuller, B. T. et al. Ultrafiltration for asphalt removal from bone collagen for radiocarbon dating and isotopic analysis of Pleistocene fauna at the tar pits of Rancho La Brea, Los Angeles, California. Quat. Geochronol. 22, 85–98 (2014).
Stafford, T. Jr, Jull, A., Brendel, K., Duhamel, R. & Donahue, D. Study of bone radiocarbon dating accuracy at the University of Arizona NSF Accelerator Facility for Radioisotope Analysis. Radiocarbon 29, 24–44 (1987).
Gove, H. E. Some comments on accelerator mass spectrometry. Radiocarbon 42, 127–135 (2000).
Schiffer, M. B. Radiocarbon dating and the “old wood” problem: The case of the Hohokam chronology. J. Archaeol. Sci. 13, 13–30 (1986).
Holden, A. R. et al. A 50,000 year insect record from Rancho La Brea, Southern California: Insights into past climate and fossil deposition. Quat. Sci. Rev. 168, 123–136 (2017).
Vidaña, S. D. & Goring, S. J. neotoma2: An R package to access data from the Neotoma Paleoecology Database. J. Open Source Softw. 8, 5561 (2023).
Bronk Ramsey, C. Radiocarbon calibration and analysis of stratigraphy: the OxCal program. Radiocarbon 37, 425–430 (1995).
Syverson, V. J. P. & Blois, J. L. Aggregated dataset for sites with updated dates and chronologies. Neotoma https://api.neotomadb.org/v2.0/data/aggregatedatasets/13.
Syverson, V. J. P. & Blois, J. L. New radiocarbon dates. Zenodo, https://doi.org/10.5281/zenodo.17064489.
Syverson, V. J. P. & Blois, J. L. New chronologies. Zenodo, https://doi.org/10.5281/zenodo.17064489.
Omernik, J. M. & Griffith, G. E. Ecoregions of the conterminous United States: evolution of a hierarchical spatial framework. Environ. Manage. 54, 1249–1266 (2014).
Herrando-Pérez, S. & Stafford, T. W. Making vertebrate fossil radiocarbon dates more useful for global scientific research. J. Quaternary Sci. https://doi.org/10.1002/jqs.70012 (2025).
Author information
Authors and Affiliations
Contributions
J.L.B. and M.A.J. conceptualized the project; V.J.P.S. carried it out and wrote the manuscript, with substantial contributions by J.L.B. and edits by J.L.B., M.A.J. and A.M.B. AM facilitated access to data from CARD and provided information on data status. S.J.G. provided technical support and facilitated bulk upload of new dates and chronologies to Neotoma; and N.C. handled corrections, new sites, and all other changes to Neotoma data. All authors have reviewed, edited, and approved the manuscript. We thank the original data contributors and the Neotoma Paleoecology Database for providing access to the data. This work was supported by National Science Foundation (NSF) Division of Earth Sciences (EAR) 2149416, NSF EAR 1948579, and NSF EAR 2410965 to J.L.B.; NSF EAR 2149419 to M.A.J.; and N.S.F. EAR 2410961 to S.J.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syverson, V.J.P., Goring, S.J., Cullen, N. et al. Updated chronologies for North American small mammal fossil localities in the Neotoma Paleoecology Database. Sci Data 13, 173 (2026). https://doi.org/10.1038/s41597-025-06491-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-06491-7