Abstract
The development of environmentally friendly plant protection products (PPPs), including pesticides, is a challenge nowadays. A commercial herbicide combining terbuthylazine and nicosulfuron as active substances (a.s.) was selected as a model PPP. The suitability of manipulating the ratio between a.s. towards alternative formulations with reduced impacts in a non-target indicator (Lemna minor) was assessed. The efficacy of such eco-friendlier a.s. ratios was then assessed using a target weed, Portulaca oleracea. Single and mixture toxicity testing with L. minor revealed an antagonistic joint action of the a.s., suggesting an environmentally protective effect of the combination compared to single dosing of a.s. The efficacy testing against the target weed of single and combined treatments of the a.s. showed that (i) the a.s. behave antagonistically throughout the whole P. oleracea response surface; (ii) there were no environmentally safe a.s. combinations ensuring target-efficacy; (iii) terbuthylazine alone was effective in controlling P. oleracea with no environmental hazardous potential, dosed at concentrations 10-fold lower than those involved in commercially recommended application doses. Overall, this case-study suggests that modelling tools widely used in the field of environmental risk assessment of PPPs may also have application in PPP design stages for a more efficient meeting of efficacy and environmental friendliness requirements.
Similar content being viewed by others
Introduction
Agriculture relies on plant protection products (PPPs) to ensure improvements in quality and yield of crops1,2. However, the use of PPPs may involve risks to human and environment health, reflecting into significant costs3,4. Regulatory agencies worldwide have already recognized this problem and have been developing tight screening protocols before the marketing of PPPs can be authorized, as well as supporting tools. Amongst these latter, modelling tools have been developed addressing PPP transport and inputs in aquatic systems. In the European Union, for example, the FOCUS platform is used to assess Predicted Environmental Concentrations (PEC values) in surface and groundwater, depending on PPP application doses and their physicochemical properties. PEC values can be compared with ecotoxicological benchmarks retrieved following ecotoxicity tests, allowing conclusions on PPP environmental hazard potential5,6. Still, PPPs have been reaching surface water through different transport pathways, e.g. runoff and leaching, often causing hazardous contamination scenarios7,8,9,10, with exposure to pesticide residues being often clearly linked to significant ecosystem risks7,8,11,12,13.
In order to counteract these environmentally hazardous scenarios, the agrochemicals industry has been impelled to innovate in the formulation of its products14. Common strategies to develop eco-friendlier PPP formulations include (i) the use of natural products or greener equivalents in PPP formulations15,16; (ii) the improvement of PPP application techniques and target delivery17,18,19,20; (iii) and the combination of already licensed active substances (a.s.)21, based on a putative synergic behavior that intends the use of lower a.s. quantities with the same levels of efficacy against the target. This latter solution is often used also to improve the control of a broader range of weeds22 but the success of such an approach is controversial. Whether some argued that synergic behavior can be reached, experimental evidence exists that pesticide mixtures rarely result in synergic effects23. Additionally, the interactive effects between formulants should be considered while assessing the toxic potential of each formulated PPP, rather than focusing only each single formulant,to predict joint action24,25,26,27. This is feasibly achievable using well-developed and established mixture toxicity assessment tools currently available (e.g.28,29). This rationale motivated the present study, where we focused on the manipulation of the ratio between two a.s. of a commercial herbicide to preliminarily evaluate the suitability of this strategy as an additional approach to formulate eco-friendlier products.
This commercial herbicide is the 2-way formulation Winner Top® (Selectis®, Portugal), with nicosulfuron and terbuthylazine as a.s. plus undisclosed formulants20,30, applied in crops to control weeds such as Portulaca oleracea and Amaranthus spp. Its action is systemic and residual following a single application per year, the effects in target weeds being perceptible 7–10 days after the application through a visible weeds control31. Nicosulfuron and terbuthylazine belong to sulfonylureas and 1,3,5-triazines chemical groups, respectively. Nicosulfuron prevents the growth of susceptible plants by blocking the synthesis of branched-chain amino acid through the inhibition of acetohydroxyacid synthase, and terbuthylazine inhibits photosynthesis by acting as a photosystem II blocker32.
Portulaca oleracea, commonly known as purslane, was selected as a representative target weed within the study since this species is a major target of the focused commercial herbicide as defined in the product documentation31. This is a major worldwide weed affecting several crops, including maize, rice, wheat, cotton and sugarcane33, which contributes to its representativeness as a test species. Adding to its small size, rapid growth and tolerance to a relatively wide range of culturing conditions33, purslane is easy to find in seeds given that there is some tendency to cultivate the species for its medicinal properties and traditional use as salad and soup vegetable34, all contributing for the species suitability for handling and testing in the laboratory. Taking into account the susceptibility of freshwater ecosystems to PPP residues contamination (see above), the macrophyte Lemna minor was selected as the non-target test species to address the possibility of establishing eco-friendlier alternatives to the commercial a.s. combination. This option was ruled by (i) the established status of Lemna sp. as standard ecotoxicological test species35; (ii) the herbicidal and systemic nature of the PPP, which a priori suggests that macrophytes should be more sensitive (thus more environmentally protective) than non-plant indicators and equivalent indicators lacking a vascular system such as microalgae; (iii) the available database on the ecotoxicity of each a.s. confirming Lemna sp. as a very sensitive species32,36,37.
Given that only the a.s. terbuthylazine and nicosulfuron are disclosed as active components of the model commercial formulation, these were the components considered for testing within the present study, which was structured following a tiered approach through the accomplishment of sequential specific aims. In a first tier, the response of the non-target L. minor was assessed following single exposure to each a.s. to feed reference mixtures toxicity models of Concentration Addition (CA38) and Independent Action (IA39), and further prediction of mixture toxicity response surfaces. CA assumes that mixture components act as dilutions of each other since they have a similar toxicological mode of action, while IA assumes that the components of a mixture act independently through a dissimilar mode of action, thus the effect of one of the components in the mixture should remain unchanged in the presence of another component (see details on the mixture toxicity theory in e.g.28,29,40). Responses of L. minor to mixtures as predicted by these reference models allowed the definition of the mixture treatments for further testing, triggering the second tier of the study. Here we aimed specifically at defining the actual response surface of L. minor following exposure to the mixture of terbuthylazine and nicosulfuron towards spotting deviations (synergism/antagonism, dose-level and dose-ratio29) from the reference models of mixture toxicity. The least hazardous mixtures between the a.s. (e.g. antagonistic combinations) would represent eco-friendlier alternatives to the ratio between a.s. used in the commercial formulation. The third tier of the study then aimed at testing the efficacy, towards the target weed P. oleracea, of these eco-friendlier mixtures of nicosulfuron and terbuthylazine, thus validating their suitability to control a model weed.
Results and Discussion
The response of L. minor to single exposures allowed to define the mixture scheme for the second tier of the study, and provided an overview on the relative toxicity of the mixture components. Single-chemical concentration-response curves were well covered by experimental data (Fig. 1) and significant fitting of the nonlinear decay model to the experimental results was achieved (F4, 127 = 237.4; p < 0.001). Maximal inhibitory effects of 76% and 56% were reached at 69 and 257 µg/L nicosulfuron and terbuthylazine, respectively, and the higher toxicity of nicosulfuron to L. minor was confirmed by an estimated 7 d-EC50 value for frond yield inhibition (3.31 µg/L) lower by one order of magnitude compared to the corresponding benchmark of 74.5 µg/L found for terbuthylazine (Fig. 1).
Effects of nicosulfuron (A) and terbuthylazine (B) dosed singly in mean Lemna minor frond number yield, with error bars representing the standard error (n = 3). The allosteric decay model was fit to the raw data to feed mixture toxicity models and to calculate EC x values; EC50 values and corresponding 95% confidence interval within brackets is shown for indicative purposes, while EC1, EC5 and EC20 can be found in Table S1 as they were used as references for establishing mixture treatments tested with Portulaca oleracea.
This difference in toxicity - as read from an integrative endpoint such as biomass yield - between substances designed to have an efficient herbicidal action should result from the interplay between intake suitability, the metabolic pathway affected and the meaning of the impairment for the overall physiological performance of the organism. Terbuthylazine has a higher Kow than nicosulfuron (3.4 vs. 0.61 at 20 °C and pH = 732), meaning that the former is much more lipid-soluble thus of easier internalization through cell membranes41. However, L. minor is a rooted macrophyte, and the translocation of substances absorbed through the roots should compensate for a lower direct intake of nicosulfuron via inner frond surface contact compared to terbuthylazine (see Cedergreen and Madsen42 for a revision on chemical’s absorption in Lemna). Thus, it is conceivable that the impairment of aminoacid synthesis by nicosulfuron can more prominently constrain L. minor growth than photosystem II inhibition by terbuthylazine at equivalent doses, hence the lower sensitivity of L. minor biomass to the latter.
Taking into account different statistical parameters, the mixture toxicity model that best fitted the mixture exposure of L. minor to nicosulfuron and terbuthylazine was the IA model incorporating dose-level dependent effects (DL-IA; Table 1). The predictive ability of DL- IA in the present case is additionally illustrated in Fig. 2 by the strong association between observed data and their counterparts as predicted by the model. The value found for the ‘a’ parameter considering the dose-level deviation of IA was slightly above zero (0.002; see Table 1), which indicates a tendency for an antagonistic behaviour of the mixture at low dose level and a synergic behaviour at high dose level29. This latter was unperceived in the corresponding isobologram (Fig. 2), where antagonism throughout the whole response surface at the mixture strength range focused is rather evident by the convex shape of the isoboles. The value found for the ‘b’ parameter was heavily negative (−1231; Table 1), confirming that the shift from antagonism into synergism as expected by the DL-IA model is not likely to occur and that the magnitude of antagonism is effect level dependent29. The isobologram denotes that stronger antagonism (higher degree of convexity) occurred consistently at lower nicosulfuron doses (Fig. 2), which is consistent at all but the lowest effect level (bluish areas of the isobologram).
Lemna minor response (frond number) following a 7-d exposure to binary mixtures between nicosulfuron and terbuthylazine, according to the dose-level dependent IA model. The left-hand panel shows the agreement between experimental responses and those predicted by the model; the regression equation and corresponding coefficient are given embedded within the graph. The right-hand panel shows the isobologram illustrating the mixtures response surface as predicted by the model under a TU mixture strength scaling; the colour gradient is indicative of the level of effect, the graphical legend referring to frond number.
Nicosulfuron and terbuthylazine are herbicides with dissimilar modes of action32. Because both metabolic pathways are common between target weeds and L. minor, and provided that this non-target species was proven sensitive to both compounds (see the above regarding single-chemical toxicity), a good description of the mixture toxicity by an IA model was somewhat expected taking into account the basics on mixtures toxicity theory (e.g.40). Also, although no previous studies were found regarding the toxicity of mixtures between sulfonylureas (such as nicosulfuron) and triazines (such as terbuthylazine) for a more direct discussion, antagonistic effects of pesticide mixtures are not rare. For example, they were already found following exposure of fish to glyphosate and cypermethrin43, of midges to pyrethroids and neonicotinoids44, and of bacteria to quaternary mixtures between ionic liquids structurally much resembling new-generation pesticide molecules with long carbon-based alkyl chains45. Despite these examples and the fact that pesticide mixtures are rarely synergic against biological targets23, the finding of an antagonistic behavior in the present study was somewhat surprising since we worked with the components of a PPP formulation (Winner Top®) and such formulations are, in principle, designed towards the best efficacy against the target. Under this ruling principle, it seems logical that antagonistic interaction between a.s. should be avoided. Given that L. minor shares with the target weed the metabolic pathways involved in their toxicity, antagonistic effects of the a.s. combination within the commercial formulation in P. oleracea should reasonably be expected, unless there is a susceptibility modulation by a non-identified mechanism. Although nothing could be found in the literature regarding L. minor or P. oleracea, some maize hybrid crops are tolerant to nicosulfuron46, which is related to different rates of absorption/translocation and/or better detoxification metabolism47,48.
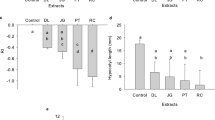
The mixture design applied to P. oleracea involved additional single-chemical treatments, thus allowing direct interpretation of the weed sensitivity to each mixture component (see Fig. 3 for an overview). Nicosulfuron and terbuthylazine applied singly were able to significantly depress P. oleracea growth (p < 0.001) both on the basis of dry weight (One-way ANOVA: F10, 17 = 15.01 and F6, 15 = 28.74 for each chemical, respectively; Fig. 3) and leaf number (One-way ANOVA: F10, 17 = 11.48 and F6, 15 = 8.18 for each chemical, respectively). Given that there were some difficulties while recording the number of leafs at the end of the tests, and provided that the allosteric decay model used to estimate concentration-response curves fitted better to the experimental dry weight data from single-chemical testing (r2 = 0.869; F4, 41 = 67.71 with p < 0.001), further interpretation will be based on dry weight records only. As applied singly, nicosulfuron induced significant decrease in P. oleracea dry weight only at 1.42 and 2.84 Toxic Units (TU) (Fig. 3), corresponding to application doses of 60 and 120 g/ha, respectively (Table S1), which translate into surface water concentrations higher than the L. minor EC20 (safety) benchmark and is inconsistent with regulatory environmental safety demands5,6. Terbuthylazine significantly impaired P. oleracea dry weight at application doses corresponding to values lower than EC20 to Lemna minor (0.85 and 1.2 TU, i.e. 50 and 72.74 g/ha; Table S1 and Fig. 3). A slightly higher sensitivity to the latter was found based on a lower 16 d-EC50 value estimated with non-overlapping 95% confidence intervals of 42.22 (7.87) compared to 58.22 (5.77) g/ha. This was in line to the relative sensitivity order found for the non-target indicator L. minor, further supporting the possibility of the occurrence of similar tendencies between species in the response to the mixture challenge.
Dry weight average of Portulaca oleracea 16 days after treatment with nicosulfuron, terbuthylazine their binary mixtures (a.s. mixture). The effects of Winner Top® are also represented in a treatment equivalent to one of the a.s. mixtures tested. Error bars represent the standard error. The dashed lines were added for clarity purposes only. The asterisks assign significant differences in dry weight relative to the control treatment (Dunnet test; p < 0.05).
The mixture between nicosulfuron and terbuthylazine was also able to significantly suppress the species growth (One-way ANOVA: F10, 8 = 13.65 with P = 0.001; Fig. 3). The LOEC found for terbuthylazine was the lowest compared to the counterparts retrieved for nicosulfuron and the mixture. And, at treatment strengths between 1.0 and 1.5 TU, the efficacy of terbuthylazine dosed singly (88% dry weight inhibition at 1.25 TU compared to the control) was higher than that of nicosulfuron (86% inhibition at 1.42 TU) and the equitoxic mixture combining the doses equivalent to L. minor EC20 values (70% inhibition at 1.37 TU), which was the only mixture tested that was able to significantly impair P. oleracea biomass yield. This suggests that there is no advantage of dosing a mixture rather than one of the a.s. singly when the intent is the control of the weed. The apparent decrease of the mixture efficacy compared to terbuthylazine dosed singly was confirmed throughout the whole response surface. The mixtures toxicity model best fitting the experimental data based on several indicators was the dose-ratio dependent IA (Table 1), and the antagonistic behaviour of the mixture throughout the modelled response surface is evidenced by the strongly convex shape of the isoboles in Fig. 4. The positive value of the parameter ‘a’ in dose-ratio dependent IA confirms that the mixture behaves antagonistically throughout the whole predicted response surface and the values found for the ‘b’ parameters denote that the antagonism is mostly due to terbuthylazine, which scored higher (Table 129). Although the significant fitting of the mixture toxicity models (Table 1) was not constrained, a note is due on the establishment of the mixture treatments in this case, which lead to an uneven distribution of experimental data across the response surface preventing a proper support to the predictions in certain areas (see in the left-hand panel of Fig. 4). Still, our results confirm that the a.s. are not synergists as argued in the label of the commercial formulation Winner Top®31.
Portulaca oleracea response (aerial biomass, i.e. dry weight of leaves and shoots) following a 16-d growth period following treatment with binary mixtures between nicosulfuron and terbuthylazine, according to the dose-ratio dependent IA model. The left-hand panel shows the agreement between experimental responses and those predicted by the model; the regression equation and corresponding coefficient are given embedded within the graph. The right-hand panel shows the isobologram illustrating the mixtures response surface as predicted by the model under a TU mixture strength scaling; the colour gradient is indicative of the level of effect, the graphical legend referring to aerial biomass at the end of the assay.
At this point it is reasonable to assume that a terbuthylazine alone would exert better and environmentally safer herbicidal toxicity than a two-way formulation with nicosulfuron as used in the model commercial compound Winner Top®. Terbuthylazine alone, applied at much lower concentration (at 72 g/L), would be more effective in reducing P. oleracea dry weight (practically to 0 g) than the concentration involved in the recommended application dose of Winner Top® (625 g/ha terbuthylazine, considering the lower application rate 2.5 L/ha), which is inherently environmentally unsafe considering that 72 g/ha delivers the L. minor EC20 in surface waters. Such overdosing recommendation in the commercial product is likely linked to the recognition that significant off-target losses may occur during application even with the great efforts to improve formulations in this sense, but this paradoxically implies a raised hazardous potential towards non-target species and edge-of-field ecosystems receiving the residues that do not reach the target4,49,50,51. On the other hand, nicosulfuron alone exerted significant toxicity towards P. oleracea only at 60 g/ha (Fig. 3), corresponding to a contaminant concentration in surface water (19.66 µg/L; Table S1) which is more than one order of magnitude above the L. minor EC20 safety benchmark (1.59 µg/L translating into an application dose of 4.86 g/ha; Table S1). Based on our results, a formulation composed only by nicosulfuron could be effective against the target species but disregarding environmental safety, thus it would not constitute a suitable eco-friendly alternative to the combination between the a.s. used in Winner Top®. Curiously, a formulation containing only nicosulfuron is currently marketed by Selectis® (Winner®52) with recommended application rates corresponding to 40–60 g/ha. Based on our results (limited by the laboratory scale used and the dosage of a.s. rather than whole formulations), no relevant effects in P. oleracea should be expected at these doses and they can represent significant environmental hazard.
A relevant argument counteracting the above interpretation could be given on the putative role of the formulants other than the a.s. present in the commercial formulation in reversing the antagonistic effects between the a.s. However, we tested on this and remarkably observed a much lower inhibition caused by Winner Top® compared to the equivalent mixture combination of its a.s, both combining 72.74 g/ha of terbuthylazine and 4.86 g/ha of nicosulfuron (Fig. 3, the open triangle representing the dosing of the commercial formulation while the black triangles represent the a.s. combination cleared of other formulants). Thus, the formulants other than the a.s. appear to greatly decrease the efficacy of the pesticides towards the target weed, reinforcing that the option for a mixture formulation as used in Winner Top® was not appropriate, thus possibly not accurately assessed during PPP design stages. One can only speculate on the reasoning behind the use of this formulation in the marketed product because there is no disclosed information in this arena that we could reach. PPP formulations are designed in such a way that rapid and uniform dispersion through large treatment areas with minimum amounts of active ingredient is ensured53. Besides these application and effectiveness features, improved handling, storage and safety (of the operator and the environment) is also taken into account54,55. Under this context, emulsifying concentrates were for long a primary option because they constitute chemical solutions and the formulation tends a priori to be stable. However, most emulsifying concentrates contain volatile aromatic solvents which are intrinsically hazardous53,56,57, and their replacement with new formulation types including suspension concentrates has been favoured56. Suspensions generally perform worst per se because the larger suspension particles are less likely to penetrate cuticles, and adjuvant dispersants and/or surfactants are commonly added to the formulations since they can facilitate this part of the job56,58,59,60 although the stabilization of such a system is often a challenge56,61.
Oil dispersions such as Winner Top® are essentially suspensions with built-in oil adjuvants. Since they join surfactants and oils as adjuvants within the formulation, better performance in the field is expected often with the advantage of dispensing tank mixing55,62. However, considering our results, the use of an oil dispersion formulation in Winner Top® was apparently not advantageous since the addition of adjuvants seems to lower the a.s. efficacy, impelling for the raise of their application concentrations into environmentally unsafe levels. And, as much terbuthylazine was added to the formulation (recommended application doses represent 625 g/ha terbuthylazine, which translate into 10.7 TU that score almost one order of magnitude higher than the 1.25 TU in Winner Top® treatment used in this study; Table S1 and Fig. 3), the higher the magnitude of the antagonism expected given the role of terbuthylazine as a driver of this mixture behaviour (see above). It may be that the surfactant(s) selected is not effective in assisting cuticle penetration of the a.s. in P. oleracea, but this is highly unlikely given that the species is a major target weed of Winner Top® as immediately indicated in the label31, and considering that surfactants are seen as enhancers of biological activity within the formulation thus their selection tends to be careful and well equated55,58,59,60.
Conclusions
The initial expectations for this study involved the assumption that the a.s. used in Winner Top® would behave as synergists in affecting the selected non-target indicator L. minor given the species physiological similarity with target weeds. On the contrary, antagonistic behaviour was found towards the macrophyte. This can be taken as an environmentally protective feature of the combination compared to the single dosing of each a.s. at a first glance, but the recommended application doses clearly deliver edge-of-field surface water PECs above corresponding L. minor safety benchmarks. Moreover, antagonistic behaviour was found between the a.s. regarding effects in the major target weed P. oleracea, with nicosulfuron apparently being useless for the overall herbicide activity, which was surprising given that a.s. are assumed synergists in the label of the commercial formulation31. Still, we were able to signal alternative a.s. formulation options. There were no combination ratios effective against P. oleracea that can concomitantly ensure environmental safety. Our results rather show that terbuthylazine alone, dosed at concentrations 10-fold lower than those involved in recommended application doses, is effective in controlling P. oleracea and translates into safe PEC levels, i.e. representing lessened environmental hazardous potential.
The huge costs of research and development to place new a.s. in the market - which have been increasing due to a major contribution of environmental chemistry and toxicology regulatory demands63,64- have been making the reformulation of existent ones an option since it can be a time- and cost-effective alternative21. Still, research and development stages (see Husby et al.65 for an overview on typical product development stages), namely those involving laboratory and greenhouse tests, eventually field trials, for confirming the biological activity of the renewed product should not be disregarded. This applies also to the formulation solutions applied following the establishment of the a.s. combination. In fact, formulation technology is seen as an enabling arena within agrochemicals development, adding value to the products as to their stability, convenience, human and environmental safety, but primarily by enhancing the a.s. efficacy56,61. On the contrary, our results suggest that the formulants used in Winner Top® rather spoil the a.s. efficacy. They indicate further that assessment methods merely based in predictive approaches (e.g. in silico tools and QSAR modelling), as those that have been for long used to assist formulation design66,67,68,69, may be short in capturing the actual efficacy of the designed product and the most favourable formulation for further development, both from an economic (important savings would be made by producing a single-way terbuthylazine formulation) and an environmental (PEC references can apparently be met with formulation changes) point of view. It is worth remarking in this context that our workflow considered simple and affordable (time- and cost-effective) laboratory testing, as well as modelling tools (FOCUS and mixture toxicity assessment tools) of free web access with familiar interfaces.
Methods
Chemicals
The chemicals used in the toxicity assays were the commercial formulation Winner Top® (Selectis®, Portugal), terbuthylazine (Pestanal®, Sigma-Aldrich®, Steinheim) and nicosulfuron (Pestanal®, Sigma-Aldrich®, Steinheim). Winner-Top is a two-way herbicide formulating terbuthylazine (250 g/L) and nicosulfuron (16.75 g/L). Stock solutions were prepared immediately before each assay by dissolving the a.s. or diluting the commercial formulation in culture medium or in tap water for testing with L. minor and P. oleracea, respectively. No solvent carriers were used.
Test organisms and general test protocols
Cultures of the non-target aquatic macrophyte L. minor were maintained in 500-mL Erlenmeyer vessels filled with ca. 200 mL of Steinberg medium35, at 20 °C with a photoperiod of 16 hL:8 hD (light intensity: ≈2000 Lux). Cultures were renewed once a week. Growth inhibition tests with L. minor followed the OECD guideline 22135 adapted to 6-well microplate use70,71, at 23 °C, under continuous illumination (intensity: ≈1700 Lux), for 7 days. In brief, tests started by inoculating each well with 3 healthy colonies with 3 fronds each, which were then allowed to grow under defined treatment ranges (Table S1). Each chemical treatment was run in triplicate and 6 replicates were used for the control. After 7 days of exposure, fronds were counted and frond yield was calculated through the difference with inoculating frond number.
Seeds of the weed P. oleracea (Flora Lusitana Lda., Portugal) were purchased from a local supplier. Vegetative vigour tests with the target weed P. oleracea were run by adapting from the OECD guideline 22772, using three replicates per treatment (see Table S1 for treatment ranges). Each replicate was set-up by adding 200 g dry-weight LUFA soil (Speyer, Germany) into a plastic pot (95 cm2 circular area) holed at the bottom for the placement of a cotton rope allowing bottom watering throughout the test. Although LUFA is a natural soil widely accepted as a standard matrix for soil toxicity tests, three samples of the test soil were briefly characterized (n = 3): pH in H2O = 5.32 ± 0.02 SD, conductivity = 309 µS/cm ± 17.8 SD, water content = 6.18% ± 0.08 SD, loss-on-ignition organic matter content = 2.32% ± 0.22 SD, determined according to standard protocols73. LUFA was hence found adequate for testing with P. oleracea, after pH adjustment using CaCO3 (500 mg/Kg dry-weight soil) to reach the plant optimal range33. Ten seeds were evenly distributed within each replicated pot with moistened soil and the pots were then placed at 20 ± 3 °C under a 16 hL:8 hD photoperiod (light intensity and humidity of 19600 Lux and 50 ± 2%, respectively). Nutrients (Substral®, Scotts Celaflor GmbH) were added to the bottom watering supply vessel once after seedling (14 mL/L).
Only the first 5 seeds emerging were kept in each replicated pot, and the plants were left to grow for 10 days, i.e. until reaching the 4-true leaf stage (Winner Top® application should be performed while the weed plants have 3–5 leaves31). The plants were then treated by spraying over the leaves (Turn’n’ spray, Bürkle, with 1.2 ± 0.1 mL spray volume) with the test solutions (see Table S1 for test concentrations). The concentration of test solutions was adjusted so that all replicates within all treatments could receive the same treatment volume, including in the control, where tap water was used instead. Sixteen days following treatment, the assay was finished, the leaves were counted and shoots were harvested for dry weight records after drying until constant weight at 60 °C. These experimental results were directly analysed via one-way ANOVA, followed by the post-hoc Dunnet test as applicable. This allowed addressing the effects of single and combined treatments of nicosulfuron and terbuthylazine in P. oleracea vegetative vigour. A significance level of 0.05 was always used.
Mixture modelling and mixture toxicity analysis
Frond yield records following single exposure of L. minor to terbuthylazine and nicosulfuron were fitted to the nonlinear decay model28. Significant fitting was always achieved, with model accuracy being assessed through adjusted coefficient of determination (r2) and residual distribution74; the significance of regressions was validated through by the F-test of overall significance75. Prediction of the joint action of chemicals was carried out by integrating these experimental data into the reference mixture models of CA and IA assuming no interaction between the components of the mixture. CA is mathematically represented in equation 1, where Ci represents the individual concentrations of each component present in the mixture with a total effect of x% and ECxi are those concentrations of the components that would alone cause the same effect xi as observed for the mixture. IA was calculated by multiplying the probability of non-response of each ith component of the mixture, following equation 2, where Ci represents the individual concentrations of each component in the mixture and E(Ci) is the effect of Ci when the ith component is dosed singly. The effective mixture concentrations are commonly presented using the dimensionless TU scaling allowing a measure of the toxic strength. The sum of the quotients Ci/EC50i was applied for the purpose following the CA principles for ith mixture components29.
Based on the expected response curves resulting from the combination of responses to single chemical exposures, a 5 × 5 incomplete factorial (Ray) design (nicosulfuron x terbuthylazine) was used to test mixtures with L. minor (Table S1). The concentrations were established ensuring coverage of a wide range of responses, from minimum to high (see details in e.g.40). The experimental responses (L. minor frond yield) were compared to the reference CA and IA models to assess the most suitable modelling approach. Deviation functions to the reference models adapted from Jonker et al.29 were fitted to the experimental data to identify if synergistic/antagonistic (S/A) effects, dose-level (DL) or dose-ratio (DR) dependent effects were more suitable to describe the experimental data. These effects can be denoted by the values of two additional model parameters, ‘a’ and ‘b’, as detailed in Jonker et al.29. Comparisons between different models fitting significantly to the dataset were carried out using the F-test under the assumption that a simpler model (i.e. with less parameters) represents a better fitting solution than a more complex one; the rank from the simpler to the most complex is as follows: Baseline > S/A > DL > DR. The Akaike’s Information Criterion (AIC) was also calculated as a complementary approach to express model fit in a quickly comparable way, with a lower AIC value denoting higher likelihood associated with the model76. Mixture toxicity modelling and analysis was run in a customized MS®Excel® spreadsheet (ToxCalcMix, version 1.0, last rev. 20/01/2016; AJA Nogueira, unpublished).
In order to assess the efficacy of eco-friendly formulations between nicosulfuron and terbuthylazine, the target weed P. oleracea was tested against mixtures eliciting up to 20% L. minor frond inhibition (mixture EC20) estimated based on the best fit of mixture models to the experimental data (see above); note that the EC20 is a standard protective benchmark5. Namely, application doses of nicosulfuron and terbuthylazine were combined, corresponding to concentrations composing L. minor mixture EC1, EC5 and EC20; additional single chemical treatments were added to the treatments range to better complete the exposure design by covering more widely single chemical concentration-response curves (see Table S1). Step 1 (Winner Top® is typically applied only once a year and the present study was not based on any specific transport scenario) of the EU-FOCUS platform (http://eusoils.jrc.ec.europa.eu/projects/stepsonetwo) was used to calculate application doses for testing with P. oleracea corresponding to L. minor EC x , assuming that these later correspond to surface water PEC values. FOCUS predicts pesticide residue concentrations in edge-of-field waterbodies following given application doses and taking into account pesticide properties constraining their fate through the soils. Since FOCUS runs backwards compared to our needs (the user inserts the application doses and the platform provides PEC values), a previous calibration step was necessary by simulating several application doses for each a.s. and retrieving corresponding PEC values at day 1 (simulation parameters were retrieved from dedicated EU reports and are exposed in Table S236,37). Obtained regressions were as follows for nicosulfuron (equation 3; n = 9 with r2 = 1.00) and terbuthylazine (equation 4; n = 7 with r2 = 1.00).
The experimental responses (P. oleracea dry weight) were compared with CA and IA and then added the deviation functions as described above for L. minor mixture toxicity results.
Winner Top® was also tested against P. oleracea as an additional non-customized mixture treatment for comparative purposes. The recommended application dose for susceptible species (41.88 g/ha nicosulfuron × 625 g/ha terbuthylazine, corresponding to 2.5 L/ha31) was down-ranged allowing direct comparison with one of the combinations between a.s. (marked in Table S1). This comparison was set to gain a complementary insight on the actual role of formulants other than the a.s. in the efficacy of the PPP against one its major target weeds.
Data availability
All data generated or analysed during this study are generally included in this published article (and its Supplementary Information files). Specific data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Carlile, W. R. Pesticide Selectivity, Health and the Environment. 1st ed, (Cambridge University Press, Cambridge, 2006).
Grube, A., Donaldson, D., Kiely, T. A. & Wu, L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. Washington DC (2011).
Hossard, L. et al. Effects of halving pesticide use on wheat production. Sci. Rep. 4, 4405 (2014).
Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Environ. Dev. Sustain. 7, 229–252 (2005).
ECB. Technical guidance document on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No. 1488/94 on risk assessment for existing substances. Part II. EUR 20418 EN/2, European Chemicals Bureau (2003).
ECHA. Guidance on information requirements and chmical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment, European Chemicals Agency (2008).
Abrantes, N., Pereira, R. & Gonçalves, F. Occurrence of pesticides in water, sediments, and fish tissues in a lake surrounded by agricultural lands: Concerning risks to humans and ecological receptors. Water Air Soil Pollut. 212, 77–88 (2010).
Carriger, J. F. & Rand, G. M. Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: I. Hazard assessment and problem formulation. Ecotoxicology 17, 660–679 (2008).
Papadakis, E. N. et al. Pesticides in the surface waters of Lake Vistonis Basin, Greece: Occurrence and environmental risk assessment. Sci Total Environ. 536, (793–802 (2015).
Silva, E., Daam, M. A. & Cerejeira, M. J. Aquatic risk assessment of priority and other river basin specific pesticides in surface waters of Mediterranean river basins. Chemosphere 135, 394–402 (2015a).
Antunes, S. C. et al. Structural effects of the bioavailable fraction of pesticides in soil: Suitability of elutriate testing. J. Hazard. Mater. 184, 215–225 (2010).
Santos, M. J. G., Ferreira, M. F. L., Cachada, A., Duarte, A. C. & Sousa, J. P. Pesticide application to agricultural fields: effects on the reproduction and avoidance behaviour of Folsomia candida and Eisenia andrei. Ecotoxicology 21, 2113–22 (2012).
Silva, E., Daam, M. A. & Cerejeira, M. J. Predicting the aquatic risk of realistic pesticide mixtures to species assemblages in Portuguese river basins. J Environ Sci 31, 12–20 (2015b).
EC. EC regulation No.1107/2009, Official Journal of the European Union (2009).
Cantrell, C. L., Dayan, F. E. & Duke, S. O. Natural products as sources for new pesticides. J. Nat. Prod. 75, 1231–1242 (2012).
Gerwick, B. C. & Sparks, T. C. Natural products for pest control: An analysis of their role, value and future. Pest Manag Sci. 70, 1169–1185 (2014).
Castro, M. J. L., Ojeda, C. & Cirelli, F. A. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 12, 85–95 (2014).
Dayan, F. E., Cantrell, C. L. & Duke, S. O. Natural products in crop protection. Bioorganic Med. Chem. 17, 4022–4034 (2009).
Felsot, A. S. et al. Agrochemical spray drift; assessment and mitigation - A review. J. Environ. Sci. Heal. B 46, 1–23 (2011).
Singh, B., Sharma, D. K. & Dhiman, A. Environment friendly agar and alginate-based thiram delivery system. Toxicol. Environ. Chem. 95, 567–578 (2013).
Müller, U. Chemical crop protection research. Methods and challenges. Pure. Appl. Chem. 74, 2241–2246 (2002).
Kudsk, P. Optimising herbicide dose: a straightforward approach to reduce the risk of side effects of herbicides. Environmentalist 28, 49–55 (2008).
Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS One 9, e96580 (2014).
Cedergreen, N. & Streibig, J. C. The toxicity of herbicides to non-target aquatic plants and algae: assessment of predictive factors and hazard. Pest Manag. Sci. 61, 1152–1160 (2005).
EC. Discussion paper on Co-formulants (rev. 3). Implementation rules for the inclusion of unacceptable coformulants in Annex III of the Regulation (EC) No 1107/2009 [WWW Document]. DG SANTE. URL http://ec.europa.eu/dgs/health_food-safety/dgs_consultations/docs/ag/advisory_plenary_20160429_09-discuss-ppp.pdf (accessed 2.2.17) (2016).
Pereira, J. L. et al. Toxicity Evaluation of Three Pesticides on Non-Target Aquatic and Soil Organisms: Commercial Formulation versus Active Ingredient. Ecotoxicology 18, 455–463 (2009).
Vidal, T., Pereira, J. L., Abrantes, N., Soares, A. M. V. M. & Gonçalves, F. Reproductive and developmental toxicity of the herbicide Betanal® Expert and corresponding active ingredients to Daphnia spp. Environ Sci Pollut Res 23, 13276–13287 (2016).
Barata, C., Baird, D. J., Nogueira, A. J. A., Soares, A. M. V. M. & Riva, M. C. Toxicity of binary mixtures of metals and pyrethroid insecticides to Daphnia magna Straus. Implications for multi-substance risks assessment. Aquat. Toxicol. 78, 1–14 (2006).
Jonker, M. J., Svendsen, C., Bedaux, J. J. M., Bongers, M. & Kammenga, J. E. Significance Testing of Synergistic/Antagonistic, Dose Level-Dependent, or Dose Ratio-Dependent Effects in Mixture Dose-Response Analysis. Environ. Toxicol. Chem. 24, 2701–2713 (2005).
Selectis. Winner Top Safety data sheet (2012) [PT].
Selectis. WINNER TOP [WWW document]. Win. Top label [PT]. URL http://www.selectis.pt/fichas_man_dig_herbi/Winner_Top.pdf (accessed 1.5.17) (2016a).
Lewis, K. A., Tzilivakis, J., Warner, D. J. & Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. 22, 1050–1064 (2016).
Chauhan, B. S. & Johnson, D. E. Seed germination ecology of Portulaca oleracea L.: An important weed of rice and upland crops. Ann. Appl. Biol. 155, 61–69 (2009).
Zhou, Y. et al. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Biomed Res. Int. 2015, 11 (2015).
OECD. OECD Guidelines for the testing of chemicals. Lemna sp. Growth Inhibition Test (2006a).
EFSA. Conclusion regarding the peer review of the pesticide risk assessment of the active substance nicosulfuron. Eur. Food Saf. Auth. J. 6, 91 (2008).
EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance terbuthylazine. Eur. Food Saf. Auth. 9, 1–133 (2011).
Berenbaum, M. C. The expected effect of a combination of agents: the general solution. J. Theor. Biol. 114, 413–431 (1985).
Bliss, C. I. The Toxicity of Poisons Applied Jointly. Ann. Appl. Biol. 26, 585–615 (1939).
Altenburger, R., Nendza, M. & Schuurmann, G. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ. Toxicol. Chem. 22, 1900–1915 (2003).
Reddy, K. N. & Locke, M. A. Molecular properties as descriptors of octanol-water partition coefficients of herbicides. Water. Air. Soil Pollut. 86, 389–406 (1996).
Cedergreen, N. & Madsen, T. V. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol. 155, 285–292 (2002).
Brodeur, J. C. et al. Toxicities of glyphosate- and cypermethrin-based pesticides are antagonic in the tenspotted livebearer fish (Cnesterodon decemmaculatus). Chemosphere 155, 429–435 (2016).
Kunce, W., Josefsson, S., Örberg, J. & Johansson, F. Combination effects of pyrethroids and neonicotinoids on development and survival of Chironomus riparius. Ecotoxicol. Environ. Saf. 122, 426–431 (2015).
Zhang, J., Liu, S. S., Xiao, Q. F., Huang, X. H. & Chen, Q. Identifying the component responsible for antagonism within ionic liquid mixtures using the up-to-down procedure integrated with a uniform design ray method. Ecotoxicol. Environ. Saf. 107, 16–21 (2014).
Cavalieri, S. D. et al. Tolerance of Corn Hybrids to Nicosulfuron. Planta Daninha 26, 203–214 (2008).
Carey, J. B., Penner, D. & Kells, J. J. Physiological Basis for Nicosulfuron and Primisulfuron Selectivity in Five Plant Species. Weed Sci. 45, 22–30 (1997).
Fonné-Pfister, R., Gaudin, J., Kreuz, K., Ramsteiner, K. & Ebert, E. Hydroxylation of primisulfuron by an inducible cytochrome P450-dependent monooxygenase system from maize. Pestic. Biochem. Physiol. 37, 165–173 (1990).
Fox, R. D., Derksen, R. C., Zhu, H., Brazee, R. D. & Svensson, S. A. A history of air-blast sprayer development and future prospects. Trans. ASABE 51, 405–410 (2008).
NRC. The Future Role of Pesticides in US Agriculture. Washington DC (2000).
Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 8, 17–29 (1995).
Selectis. WINNER. URL http://www.selectis.pt/herbicidasx93.asp?id_produtos=93 (accessed 1.1.17) (2016b).
Blanco, M., Zamora, D., Planells, J. & Mulero, R. Analytical control of adjuvants in herbicide formulations by NIR spectroscopy. Anal. Bioanal. Chem. 395, 839–844 (2009).
Martin, A., Whitford, F., Jordan, T. Pesticides and Formulation Technology, Purdue Pesticide Program. Purdue University Cooperative Extension Service (2011).
Seaman, D. Trends in the formulation of pesticides - an overview. Pestic. Sci. 29, 437–449 (1990).
Knowles, A. Global trends in pesticide formulation technology: The development of safer formulations in china. Outlooks Pest Manag. 20, 165–170 (2009).
Rathburn, C. B. Insecticide Formulations-Types and Uses: a Review. J. Am. Mosque. Control Assoc. 1, 80–84 (1985).
Baur, P., Grayson, B. T. & Schönherr, J. Polydisperse Ethoxylated Fatty Alcohol Surfactants as Accelerators of Cuticular Penetration. 1. Effects of Ethox y Chain Length and the Size of the Penetrants. Pestic. Sci. 51, 131–152 (1997).
Baur, P., Schonherr, J. & Grayson, B. T. Polydisperse ethoxylated fatty alcohol surfactants as accelerators of cuticular penetration. 2: Separation of effects on driving force and mobility and reversibility of surfactant action. Pestic. Sci. 55, 831–842 (1999).
Haas, S., Hasslin, H.-W. & Schlatter, C. Influence of polymeric surfactants on pesticidal suspension concentrates: dispersing ability, milling efficiency and stabilization power. Colloids Surfaces A Physicochem. Eng. Asp. 183–185, 785–793 (2001).
Mulqueen, P. Recent advances in agrochemical formulation. Adv. Colloid Interface Sci. 106, 83–107 (2003).
Castelani, P., Antunes, M. C. F., Leal, F. L. S. Oil Dispersion Formulations: Stability Assessment and Field Trials, in: Goss, G. (Ed.), 35th Symposium on Pesticide Formulation and Delivery Systems: Pesticide Formulations, Adjuvants and Spray Characterization. ASTM, New Orleans LA, pp. 1–14 (2016).
McDougall P. R & D trends for chemical crop protection products and the position of the European market. A consultancy study undertaken for ECPA. Saughland (2013).
McDougall P. The cost of new agrochemical product discovery, development and resgistration in 1995, 2000, 2005-8 and 2010 to 2014. R&D expenditure in 2014 and expectations for 2019. A Consultancy Study for CropLife International, CropLife America and the European Crop. Saughland (2016).
Husby, J., Bjerre, N., Schiodt, J. Development of a Plant Protection Product - From Invention to Use (2013).
Botts, M. F. Using Computers in the Development of Pesticide Formulations. In: H. B. Scher (Ed.). Advances in Pesticide Formulation Technology. (pp. 89–104. American Chemical Society, Washington DC, 1984).
Mookerjee, P. K. Computer-assisted correlation analysis in the development of pesticide formulations. In: H. B. Scher (Ed.). Advances in Pesticide Formulation Technology. (pp. 105–119. American Chemical Society, Washington DC, 1984).
Scher, H. B. Advances in Pesticide Formulation Technology. An overview. In: H. B. Scher (Ed.). Advances in Pesticide Formulation Technology (pp. 1–7. American Chemical Society, Washington DC, 1984).
Smith, K., Evans, D. A. & El-Hiti, G. A. Role of modern chemistry in sustainable arable crop protection. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 623–37 (2008).
Kaza, M., Mankiewicz-Boczek, J., Izydorczyk, K. & Sawicki, J. Toxicity Assessment of Water Samples from Rivers in Central Poland Using a Battery of Microbiotests – a Pilot Study. Polish J. Environ. Stud. 16, 81–89 (2007).
Kolasińska, J., Bielińska, M. & Nałęcz-Jawecki, G. Assessment of Fluoroquinolones Toxicity with Application to Lemna minor Microbiotest. Fresenius Environ. Bull. 19, 1453–1457 (2010).
OECD OECD Guideline for theTesting of Chemicals Terrestrial Plant Test: Vegetative Vigour Test. Terrestrial Plant Test: vegetative VigourTest (2006b).
SPAC. Soil Analysis Handbook of Reference Methods. Soil and Plant Analysis Council, Inc. Soil science. (CRC Press, Boca Raton, Florida, 1999).
Quinn, G. & Keough, M. Experimental design and data analysis for biologists. (Cambridge University Press, Cambridge, United Kingdom, 2002).
Draper, N. R. & Smith, H. Applied Regression Analysis, 3rd editio. ed. John Wiley & Sons, Inc (1998).
Motulsky, H. & Christopoulos, A. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. (Oxford University Press, Oxford, 2004).
Acknowledgements
The Portuguese Foundation for Science and Technology (FCT, Portugal) financed Tânia Vidal (SFRH/BDP/94562/2013) and Joana Pereira (SFRH/BPD/101971/2014) by means of individual grants. Thanks also due to CESAM (UID/AMB/50017) for financial support and FCT through national funds and co-funding FEDER, within the PT2020 Partnership Agreement.
Author information
Authors and Affiliations
Contributions
L.Q. and J.L.P. wrote the main manuscript text; J.L.P., A.J.A.N. and F.J.M.G. conceived the experiments and L.Q. conducted the experiments with the assistance and supervision of J.L.P. and T.V. J.L.P. and L.Q. analyzed the general results with the assistance of A.J.A.N. in the discussion of the mixture toxicity results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Queirós, L., Vidal, T., Nogueira, A.J.A. et al. Mixture toxicity assisting the design of eco-friendlier plant protection products: a case-study using a commercial herbicide combining nicosulfuron and terbuthylazine. Sci Rep 8, 5547 (2018). https://doi.org/10.1038/s41598-018-23883-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-018-23883-5