Abstract
In many ways, the complement of cell subtypes determines the information processing that a local brain circuit can perform. For example, the balance of excitatory and inhibitory (E/I) signaling within a brain region contributes to response magnitude and specificity in ways that influence the effectiveness of information processing. An extreme example of response changes to sensory information occur across Critical Periods (CPs). In primary mammalian visual cortex, GAD65 and parvalbumin inhibitory cell types in particular control experience-dependent responses during a CP. Here, we test how the density of GAD65- and parvalbumin-expressing cells may inform on a CP for complex behavioral learning. Juvenile male zebra finch songbirds (females cannot sing) learn to sing through coordinated sensory, sensorimotor, and motor learning processes distributed throughout a well-defined neural network. There is a CP for sensory learning, the process by which a young male forms a memory of his “tutor’s” song, which is then used to guide the young bird’s emerging song structure. We quantified the effect of sex and experience with a tutor on the cell densities of GAD65- and parvalbumin-expressing cells across major nodes of the song network, using ages that span the CP for tutor song memorization. As a resource, we also include whole-brain mapping data for both genes. Results indicate that inhibitory cell populations differ across sex, age, and experiential conditions, but not always in the ways we predicted.
Similar content being viewed by others
Introduction
Balanced excitatory and inhibitory (E/I) signaling is a widespread neural feature that facilitates informational integration and cognitive function1. E/I balance typically stabilizes across development to create efficient processing networks with controlled neural plasticity2,3,4. In sensory brain areas, E/I balance is particularly important for increasing the selectivity and response properties to specific stimuli5,6,7,8. An extreme example of shifts in response to sensory stimuli occur across Critical Periods (CPs), restricted developmental phases when a specific experience has profound and lasting effects on a particular brain system and patterns of resulting behavior9. E/I balance may therefore play an especially essential role in neural plasticity across CPs.
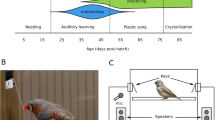
Like all songbirds, zebra finches (Taeniopygia guttata) learn their song. In this species, only males can sing, females never sing10. Despite hearing song all day every day, male zebra finches can only use song they hear during the juvenile phase that spans approximately Posthatch day (P) 30–65 to shape the structure of their own song (Fig. 1a)11,12,13,14,15 (but see16). This limited learning phase meets the criteria for a CP because males that do not hear song P30–65 can use a song they experience later to guide their song structure; learning song prevents future learning whereas lack of learning permits late learning17,18. This indicates that song is processed differentially based on sex, age, and prior tutor experience.
Zebra finch system for developmental song learning. (a) Timeline of Posthatch (P) development with male images at ages relevant to tutor song memorization, and showing the multiple types of learning required for tutor song copying and adult song crystallization. (b) Simplified schematic of the brain network for song learning and production. Major brain areas of the network quantified here are shaded. Blue = nodes necessary for sensorimotor learning during development, Area X and the lateral magnocellular nucleus of the anterior nidopallium (LMAN). Red = motor output pathway, including the robust nucleus of the arcopallium (RA). Purple = node in both sensorimotor and motor pathways (HVC, proper name), Yellow = auditory forebrain (AF) required for tutor song memorization, which has three major components (not shown): primary auditory cortex Field L, and higher order association areas caudomedial nidopallium (NCM) and caudal mesopallium (CM).
The entirety of the developmental song learning process requires integrated and distributed learning across a network of brain regions (Fig. 1b). The song network can be roughly divided into functionality for three types of learning17,19,20,21,22,23,24. The foundation of learned song structure is the memory a young male forms of an adult “tutor” male’s song, which normally occurs P30–65 and which requires the auditory forebrain (composed of primary auditory cortex Field L, and higher order association areas caudomedial nidopallium (NCM) and caudal mesopallium (CM))12,13,14,25,26. A process of sensorimotor error correction then incorporates the tutor song memory into the bird’s immature vocalizations via the Anterior Forebrain Pathway comprised of HVC (proper name), Area X, and the lateral magnocellular nucleus of the nidopallium (LMAN). Finally, through intense motor rehearsal, that song becomes highly stereotyped, or crystallized, such that each male sings one song his entire adult life, using the Posterior Motor Pathway of HVC and the robust nucleus of the arcopallium (RA)27.
Neuroanatomical, epigenetic, molecular, and physiological response properties emerge and become selective differently within the different nodes of this network as developmental song learning proceeds, and a functional contribution of local inhibitory networks has already been described in the sensorimotor song region HVC28. It is therefore possible that inhibitory cells establish and maintain E/I balance across the network.
There are multiple inhibitory cell subtypes in the brain, and diversity within each of them29,30. Here, we focused on subtypes defined by GAD65 (a GABA-synthesizing enzyme) and parvalbumin (a calcium-binding protein) expression because of their central roles in controlling the CP for plasticity in the mammalian primary visual cortex, the best-studied CP31,32,33. We asked how GAD65- and parvalbumin-expressing cell populations changed in major nodes of the network for developmental song learning. We compared males and females to gain insight into properties that may explain sex differences in song. We chose three ages that span the typical CP, and reared birds with and without tutor experience to test the interactions between maturational changes in inhibitory cell populations and experience-dependent effects. The combination of age, sex, and prior tutor experience determines whether or not a bird can memorize tutor song and sing, thus these conditions permit associations between GAD- and parvalbumin- expressing cell populations and brain function and behavior. We quantified cell density within major nodes of the song network, and profiled brain-wide expression patterns for all of the experimental groups. Our results support the broad hypothesis that inhibitory cell populations may contribute to the emergence of song processing specificity across the song network, though in sometimes unexpected ways. Future work can expand on these first findings by investigating additional inhibitory cell subtypes and measuring dynamic firing properties of these cell populations.
Methods
All procedures were conducted in accordance with the National Institute of Health guidelines for the care and use of animals for experimentation and were approved by the University of Chicago Institutional Animal Care and Use Committee (ACUP #72220).
Experimental animals
All birds used in this study were hatched in in-house breeding aviaries where males and females of all ages were housed on a 14 h:10 h light:dark cycle, with seed and water provided ad libitum. Birds reared in the ‘Normal’ condition were allowed to remain in their home aviary until collection at P25, P45, or P65. Birds that were raised for the ‘Isolate’ condition were removed from their home aviaries the day after they fledged, P21-P23, and lived with two adult females in a sound attenuation chamber until collection at P25, P45, or P65. The Isolate condition prevents juveniles from hearing song (females cannot sing), but they are exposed to conspecific vocalizations in the form of female calls and experience social interactions. We assayed males and females for all three ages and both conditions, n = 3 for each combination of age, sex and tutor experience condition.
In situ hybridization
Whole brains were rapidly dissected, embedded in OCT (Fisher), and flash frozen on dry ice. Brains were stored at −80 °C until sectioning into 12 μm sagittal slices on a cryostat in a series so that adjacent slides from each brain could be processed for GAD65 and parvalbumin across samples. The same series was used consistently across subjects for each probe, such that GAD65 and parvalbumin staining was analyzed using adjacent sections. All brain sections analyzed here were from the left hemisphere.
We hybridized with antisense-configured riboprobes generated from two zebra finch ESTs (GenBank accession numbers CK310366 and FE713884, for GAD65 and parvalbumin, respectively). Briefly, EST plasmids were grown in Luria Bertani (LB) media overnight at 37 °C and purified before further use (QIAprep Spin Miniprep Kit, Qiagen). Plasmids were linearized with PauI (New England Biolabs) restriction enzyme at 37 °C, then used as templates for in vitro transcription with RNA T3 polymerase and DIG-labeled NTPs (Roche) to generate DIG-labeled riboprobes. Probes were purified before use (RNeasy Mini Kit, Qiagen), and concentration was assessed via dot blot (Roche).
Processing followed prior protocols34,35. Sections were first dried at room temperature and then fixed for 10 min in 4% paraformaldehyde (pH 7.4). They were rinsed three times in 0.025 M KPBS (pH 7.4), equilibrated in 0.1 M Triethanolamine (TEA) for 3 min, then treated with 0.25% v/v acetic anhydride in TEA for 10 min. Sections were washed twice in 2X SSC and dehydrated in a graded ethanol series. After drying at room temperature, sections were hybridized for 16 hr at 65 °C in hybridization solution (50% formamide, 2X SSPE [pH 7.4], 2 mg/mL tRNA, 1 mg/mL bovine serum albumin, 300 ng/mL polyadenylic acid, 0.1 M DTT) containing 400 ng of either GAD65 or parvalbumin antisense-configured riboprobes. Following hybridization, sections were rinsed in 2X SSC at room temperature to remove coverslips, and then high-stringency washed in 0.1X SSC/50% formamide once and 0.1X SSC twice, all at 65 °C. Sections were blocked for 1 hr at room temperature (Roche), then rinsed three times in GBA (100 mM Tris pH 7.5, 165 mM NaCl) and incubated 2 hr at room temperature with a 1:5000 dilution of alkaline phosphatase-conjugated anti-DIG primary antibody in block solution (Roche, cat #11-082-736-103, cat #11-585-762-001). Sections were then rinsed four times in GBA and once in GBB (100 mM Tris pH 9.5, 100 mM NaCl, 100 mM MgCl2) before incubating with BCIP/NBT alkaline phosphatase detection substrate (Sigma, cat #B5655), and rinsing in deionized water before cover-slipping in aqueous mounting media.
Imaging and quantification
All images were captured using microscopes at the University of Chicago Integrated Light Microscopy Core Facility. Images for each region that was quantitated (Field L, NCM, CM, HVC, Area X, LMAN, RA) were obtained using a 4X objective on an Olympus IX81 microscope (Olympus Corporation of the Americas, Center Valley, PA) with a Hamamatsu Orca Flash 4.0 sCMOS camera (Hamamatsu Photonics, Skokie, IL) running Slidebook 5.0 software (Intelligent Imaging Innovations). Images were captured to include the song region of interest and surrounding brain region, used as background control. All images were captured and analyzed at the same magnification and illumination, though image contrast was modified for clarity when presented in figures.
For each and all images, a threshold was applied in FIJI (National Institutes of Health) to exclude background staining. We then used the particle analysis function to obtain data only for positively-stained cells in each region. Quantification was done within song areas and adjacent surrounding brain regions to control for inter-section variation in staining intensity as follows: auditory forebrain (Field L, NCM, and CM) and Hippocampus (HP); HVC and nidopallium; RA and arcopallium; LMAN and nidopallium; Area X and medial striatum. LMAN could not be consistently differentiated between core and shell components across sections, so the entire region was quantified as one unit. We did not quantify Area X for females as they lack an easily identifiable Area X-like structure within the medial striatum with this staining (but see34). Because we sectioned the brains in series, adjacent sections were hybridized with GAD65 and parvalbumin riboprobes; that along with immunostaining of another series of sections (not analyzed for this project but useful for identifying boundaries of song areas) allowed researchers blind to the condition of the individual reliably and consistently capture the regions of interest. Within the auditory forebrain of each bird, we separately analyzed the one primary thalamorecipient region (Field L2a, hereafter referred to as “Field L”) and two secondary auditory regions (NCM and CM), from midline to 1 mm lateral of midline. Neuroanatomical landmarks and specific boundaries used for consistent quantification of these regions were aided by the Zebra Finch Expression Brain Atlas (ZEBrA, Oregon Health and Science University; zebrafinchatlas.org).
Data were first calculated as cell counts divided by the total area of the selected region to give a cell density measure. This density calculation was initially performed on the surrounding brain areas that we used as control for background staining, and data were used to test for effects of age, sex, and prior experience. With one exception, no significant differences were found (results below; Table 1). Second, the cell density measure of a particular song region was then divided by a cell density measure of its adjacent non-song region to give us a normalized cell density measure for each brain section35. These normalized section measures were averaged by song area within each subject to give one normalized average cell density measure per subject per song network node36,37. The normalized average cell density is the measure used in the following statistical analysis and in Fig. 2. Statistical analysis was also performed on the non-normalized cell densities for each song area, and for the average area measured for each song area (Supplementary Materials).
Quantification of inhibitory cell densities across sex, age, tutor experience conditions, and brain area. Quantification of in situ hybridization labeling for GAD65 (top) and parvalbumin (bottom) revealed that cell type densities are determined by age, sex, and tutor experience differently across major nodes of the song network. Plotted are means ± s.e.m. Bullets are individual data values.
Statistics
Statistical tests within each region for main effects of age, sex, condition, and the age * condition interaction were run with the anova function with Type III analysis in R (R version 3.5.1, stats package v 3.6.0) with α = 0.05. Parametric post hoc analyses were performed using the TukeyHSD function in the stats package.
Gene expression mapping
To create a comprehensive profile of hybridization across the brain, relative abundance of staining was subjectively determined and divided into four categories: high (+++), medium (++), low (+), and absent (−−). First, brain areas of consistently high and low staining were identified to serve as standards for each gene. Then, a brain region that showed an intermediate hybridization level was designated medium. These standards held for one gene across age, sex, and rearing environment but were not necessarily the same between GAD65 and parvalbumin datasets. Observations across multiple sections were averaged within an individual. Hybridization staining was mapped across the entire brain for subjective and relative intensity of staining using published and online canary and zebra finch atlases as guides, and the revised avian brain nomenclature (the ZEBrA database, Oregon Health & Science University, Portland, OR 97239; http://www.zebrafinchatlas.org38,39,40.
Results
All main effect and interaction statistical results are listed in Table 1.
In males, significant effects of age and age * condition interaction are detected only in Field L for GAD65
To test if tutor experience has an effect on the typical organization of GAD65 cell populations, we asked if cell densities changed across age in Normal males, and whether or not at each age tutor experience affected those densities. The only brain region with statistically significant main effect across the three ages in Normal males is Field L (F(2,6) = 9.38, p = 0.01); pairwise posthoc comparisons revealed P65 is significantly different from P25 (p = 0.02) and P45 (p = 0.04) density measures. Similarly, the only brain region with a main effect of tutor experience in males is Field L (F(1,12) = 5.48, p = 0.04; Figs. 2 and 3). There were no significant interactions between age and condition in males, although we observed a trend towards increasing density across age through P65 in Normal males in auditory regions Field L, NCM, CM but decreasing in sensorimotor and motor regions HVC, RA, LMAN, and Area X (Fig. 2). Interestingly, an age difference was detected in the arcopallium area used as the control region for RA, with P25 GAD cell densities greater than those at P45 (Table 1). This is unlikely to have altered the conclusion that there is no age change in RA, however, because the non-normalized GAD65 cell densities in Normal males across age are non-significant (p = 0.61).
Representative images of auditory forebrain showing GAD65 and parvalbumin staining. Images show major significant statistical effects between Normal and Isolate males for both genes, and between Normal males and females for GAD65. Schematic overlay in top left panel shows the three major auditory forebrain components Field L, NCM, CM, and the adjacent hippocampus (HP). Dorsal = up, Rostral = left. Scale bars = 500 µm.
In males, significant effects of age and tutor experience are distributed across the song network for parvalbumin
As for GAD65, we asked if and how parvalbumin cell populations shifted across the CP for tutor song memorization in Normal males, and if tutor experience affected cell densities. Several brain areas show significant changes in Normal males across the three ages sampled. In the auditory forebrain, Field L and NCM showed significant age fluctuations (Field L: (F(2,6) = 4.91, p = 0.05, posthoc test showed P25 and P45 are different, p = 0.05; NCM: F(2,6) = 5.79, p = 0.04; posthoc tests revealed marginal differences between P25 and P45 (p = 0.06) and P65 (p = 0.06)), though CM did not reach significance threshold (p = 0.08). In the Anterior Forebrain Pathway, only Area X showed significant differences by age (F(2,6) = 25.86, p = 0.001), with P65 densities distinct from those at P25 (p = 0.001) and P45 (p = 0.01). Parvalbumin cell densities in RA also showed an effect of age (F(2,6) = 22.42, p = 0.002), with densities higher at P25 than the older ages (P25-P45: p = 0.002; P25-P65: p = 0.005; Fig. 2). There were no significant main effects of tutor experience on any brain area (Table 1).
Several brain areas, however, revealed significant interactions between age and condition in males. All three major components of the auditory forebrain had significant interactions (Field L: F(2,12) = 7.85, p = 0.007; NCM: F(2,12) = 7.72, p = 0.007; CM: F(2,12) = 3.94, p = 0.05. In all three components, cell densities in Normal males were higher than those in Isolate males at P25, but lower than densities in Isolate males at P45 and P65 (Figs. 2, 3). Area X also had a significant interaction (F(2,12) = 4.50, p = 0.03). Cell densities in Normal males were greater than those in Isolate males at P25 and P65, but lower than densities in Isolate males at P45 (Fig. 2).
Sex significantly affects cell densities in motor pathway nodes and Field L
We therefore asked how GAD65 and parvalbumin cell densities compared between males and females, because males, but not females, sing and it is an open question about how many and which differences in the song network contribute to the behavioral sex difference17,25,41.
When we compared Normal males and females, we found significant main effects of sex in Field L (F(1,12) = 15.50, p = 0.002), HVC (F(1,12) = 21.48, p < 0.001), and RA (F(1,12) = 4.73, p = 0.05032) for GAD65 cell densities, and in HVC (F(1,12) = 38.05, p = 4.812e-05) and RA (F(1,12) = 65.78, p = 3.267e-06) for parvalbumin cell densities. In all cases, females had greater cell densities than males (Figs. 2, 3 and 4).
Representative images of HVC and RA showing GAD65 and parvalbumin staining. Images show major significant statistical effects between Normal males and females and across age. Schematic overlay in top left panel shows the telencephalic regions of nidopallium and arcopallium that include HVC and RA, respectively. Dorsal = up, Rostral = left. Scale bars = 500 µm.
We also tested for an interaction of age and sex between Normal males and females, and found a significant effect in GAD65 densities only in Field L (F(4,12) = 11.11, p < 0.001), and significant effects on parvalbumin densities in Field L (F(4,12) = 3.23, p = 0.05), CM (F(4,12) = 3.56, p = 0.04), RA (F(4,12) = 4.51, p = 0.02), and LMAN (F(4,12) = 6.19, p = 0.006), with a trend in NCM (F(4,12) = 2.91, p = 0.07) but not HVC (F(4,12) = 1.69, p = 0.22; Fig. 2).
Cell densities in female song network areas are minimally affected by age and tutor experience
Finally, we asked if age and tutor experience affected the density of GAD65 and parvalbumin-expressing cells in females, which would reveal new maturational and experience-dependent effects on the organization of the female song network. When we compared Normal females by age, we found two significant effects, one for GAD65 (Field L: F(1,12) = 12.46, p = 0.007, posthoc tests showed P65 densities were significantly different from those at P25 (p = 0.04) and P45 (p = 0.006) measures) and one for parvalbumin (LMAN: F(4,12) = 14.94, p = 0.005, posthoc tests showed P25 densities significantly different from those at P45 (p = 0.004) and at P65 (p = 0.03)). The Normal and Isolate females had significantly different parvalbumin-expressing cell densities in LMAN only (F(1,12) = 6.24, p = 0.03; Figs. 2, 5). The female age * condition interaction was also significant for parvalbumin-expressing cells (F(2,12) = 7.33, p = 0.008; Fig. 2).
Representative images of parvalbumin-labeled cells in male Area X, and male and female LMAN staining. Images show comparison between Normal and Isolate birds across age. Schematic overlay in top left panel shows LMAN, positioned within the rostral nidopallium, and Area X within the medial striatum (MSt). Dorsal = up, Rostral = left. Scale bars = 500 µm.
Neuroanatomical distribution and subjective quantification across whole brains
Subjective mappings of in situ hybridization labeling revealed widespread distribution of GAD65- and parvalbumin-expressing cells throughout the brain. As a resource, comprehensive tables for both genes, in Normal and Isolate males and females, across all three ages, are included as Supplementary Materials.
Discussion
Effective behavioral acquisition requires that brain areas are organized appropriately to process information effectively. E/I balance underlies much of this progression, especially during development, because it facilitates specificity in response magnitudes and selectivity. Inhibitory cell types, GAD65 and parvalbumin in particular, are sufficient to gate an extreme example of developmental switches in experience-dependent plasticity: CP. Here, we asked if the density of these two cell types in major areas of a neural network required for developmental song learning in the zebra finch songbird are consistent with a CP for tutor song memorization and the distributed learning that occurs throughout the network.
Our primary prediction was that higher order auditory association areas required for tutor song memorization, NCM and CM, would show age and experience-dependent changes in males. Our data do not fully support this prediction. We found no significant differences based on age or tutor experience condition for GAD65-expressing cells. We did detect a significant effect of age in NCM and a trend in CM for parvalbumin-expressing cell densities, but no effect of condition. In Field L, however, we found significant effects of age and tutor experience on GAD65-expressing cells and of age for parvalbumin-expressing cells. We also found a significant interaction between age and tutor experience condition in male Field L; densities at P25 are higher in Normal compared to Isolate males, but this relationship is flipped at P45 and P65.
The differences found in Field L raise the possibility that primary sensory cortices may be particularly sensitive to the effects of age and experience; the bulk of the work describing functionality of GAD65 and parvalbumin cell subtypes in CPs comes from the primary visual cortex in mammals33,42,43 where GAD65 signaling and parvalbumin cell populations have direct influence on neural plasticity31,32. The current results are intriguing because on the one hand, Field L does not demonstrate distinct response magnitude or selectivity properties for tutor song compared to conspecific song in normally-reared males, yet on the other hand, early song experience does alter the balance of responsiveness and selectivity to song compared to non-song sounds in Field L44,45,46. Notably, other studies that measured parvalbumin protein-labeled cells did not detect alterations in Normally reared males across age47,48. It can, however, be difficult to link different levels of analysis with simple linear relationships. For example, hearing song evokes the same electrophysiological responsiveness in auditory forebrain neurons at P20 and P30, but results in a significant difference in molecular cascade responses between P23 and P3025,49. Additional work to link cell populations with activity metrics is needed to reconcile these apparent discrepancies. However, we also note that Field L is highly interconnected with NCM and CM, and may thus still exert an influence on higher order sensory integrating processing23. Future comparative work across species or investigation of the auditory forebrain in the context of other sensory processing functions may also further clarify relationships between cell types and behavior.
Interestingly, in Normal males the qualitative trend for GAD65-expressing cells in the three major regions of the auditory forebrain was for density to increase from P25 to P65, but parvalbumin-expressing cell densities were highest at P25 compared to P45 and P65. Perhaps this reflects shifting proportions of inhibitory cell subtypes. In addition to parvalbumin, evidence for somatostatin and VIP inhibitory cell subtype involvement in CP plasticity is accumulating50,51,52. Because we performed in situ hybridization for both GAD65 and parvalbumin on the same brains, on adjacent sections, these patterns accurately reflect relative population changes within individuals. Assays for other inhibitory cell subtypes would test for the possibility that, for example, somatostatin or VIP cell populations were increasing as parvalbumin subtypes decline.
We were interested in inhibitory cell populations in the female auditory forebrain because it is unclear whether or not females, like males, can memorize tutor song but simply can’t “tell” us because they can’t sing a representation of this memory. Alternatively, tutor song memorization might be male-specific, a reflection of a set of cell types or experience-dependent activation profiles in the auditory forebrain that are sexually dimorphic. Juvenile females can discriminate songs and maintain a trace of their dad’s songs into adulthood53,54, but interestingly, while early song isolation creates deficits in behavioral discrimination of songs, distinct electrophysiological responses in Field L to hearing song only occurred in song-isolated females and not in control females46,55,56. The bulk of molecular evidence is that there is are no sex differences in molecular responses to hearing song (but see57), but recent data do reveal a significant sex difference at P30 but not before, coincident with the onset of the CP for tutor song memorization25.
Here, we did not detect a significant effect of condition in any auditory forebrain component in females. In Normal females, the only significant effect of age was for GAD65-expressing cells in Field L. Females, like males, had higher cell densities at P65 compared to P25 and P45; there was also a significant sex difference in GAD65-expressing cells in Field L. This pattern may thus represent an inhibitory cell population with different overall levels in males and females, but which is regulated similarly by experience-independent maturational mechanisms.
Although the study design was focused on the CP for tutor song memorization, the auditory forebrain projects to nodes in the Anterior Forebrain and Posterior Motor Pathways58,59,60. It was therefore possible that song isolation, by preventing the sensorimotor error correction process, for example, would affect inhibitory cell populations throughout the network.
While we did find significant effects of age in Normal males, with higher parvalbumin-expressing cell densities at P25 than at P45 and P65 in RA, and a denser population at P65 compared to P25 and P45 in Area X, we found no main effect of tutor experience on any component of the Anterior Forebrain or Posterior Motor Pathways in males. This finding is consistent with prior work that showed almost no genomic effect of song isolation in these areas61. It is, however, possible that our ages were too early to see the effects of song isolation on inhibitory cell populations in the sensorimotor and motor network. For example, in HVC, densities of parvalbumin cells are higher in Isolate males than Normal males by P120, but not at P6062.
Both HVC and RA had significant sex differences in GAD65- and parvalbumin-expressing cells. In all cases, females had higher densities than males. Lower levels of inhibitory signaling are more permissive for neural plasticity in HVC28. If indeed sparser inhibitory cell population is permissive for flexible syllables, then perhaps the greater density in females is part of the cellular substrate that does not allow learned song production in females.
Within females, we did find main effects of age and condition, and in their interaction, on parvalbumin-expressing cell densities within the Anterior Forebrain Pathway node LMAN. We found no such effects in males. The effects derive primarily from differences at P25. It is possible that one of the underlying mechanisms that alters the volume of LMAN from P25 to P45 is a decrease in the inhibitory cell population63,64,65,66.
In fact, we observed that several measures showed apparent magnitude differences between Isolate and Normal birds at P25. This is interesting because we removed birds from their home aviaries at P21 to create the Isolate group, whereas the Normal birds remained undisturbed in their home aviaries. While formally possible that a dramatic change in cell subtype populations could occur based on differential song exposure within four days, and that this could alter song learning16, it is also possible that the distinctions at P25 is a result of this social stressor. Future experiments could employ more balanced control conditions successfully used to demonstrate epigenetic influences of tutor experience: Tutored condition with one adult male and one adult female in comparison to the two adult female Isolate condition, as used here. This comparison thus controls for aviary removal and standardizes social complexity while still varying tutor experience. There is precedence for these conditions causing distinctions in epigenetic- and systems-levels of analysis in juveniles males36,67.
Our initial predictions were based on the evidence that the NCM and CM portions of the auditory forebrain are required for tutor song memorization and that this is the type of learning that is gated by a CP17,25,26,68,69,70. But largely, it was Field L, not NCM and CM, that showed effects of age and tutor experience. Perhaps our predictions relied too strictly on CP mechanisms described for a CP in visual field organization, which is distinct from tutor song memorization in several ways.
First, the experiential conditions that gate CP plasticity is sensory deprivation in the primary visual cortex but the CP for tutor song memorization is closed only by exposure to a tutor; female calls, which have very similar acoustic features to simple song elements, and the bird’s own song, are not sufficient auditory stimuli to close the CP12,13,15,18,71. Second, the CP for tutor song memorization is for learning, not perception. In fact, tutor song memorization is not rote, it is optimal when song is presented in social interactions and multimodal sensory experiences72,73. Third, a feature of the CP in primary visual cortex is that extended experience-dependent plasticity is a delay of maturation, but epigenetic evidence from juvenile male auditory forebrain is not consistent with this idea33,36. Fourth, the cellular organization of avian brains is not the same as in mammals. Much of the study of local inhibitory circuits in mammals is related to their effect on pyramidal cells. While gene analysis has uncovered strong parallels between bird forebrain and mammalian neocortex, cell types are organized distinctly, and more distributed in bird brains74,75,76. Thus local circuits may also require a different configuration to have similar effects. However, both Field L and primary visual cortex in mammals receive major thalamic inputs, which are essential for patterning the receptive fields of sensory cells. It is possible that this property explains the effects we detected here23.
The current study represents an initial foray into assessing the potential role of local inhibitory circuits in the emergent response and selectivity properties across the brain network required for developmental song learning in zebra finches. The results indicate specific alterations to the density of inhibitory cell subtypes depending on the age, sex, and tutor experience of the individual, and differed based on the brain region quantified. Important next steps include investigation of additional cell subtypes and the measurement of dynamic synaptic function of these networks. The zebra finch song network has already provided key insights into neural properties that promote and limit the ability to learn and produce complex behaviors; additional investigation into the role of local inhibitory circuits is thus likely to be fruitful.
Data availability
Corresponding author will share materials, data and associated protocols available to readers without undue qualifications upon request.
References
Zhou, S. & Yu, Y. Synaptic E-I Balance Underlies Efficient Neural Coding. Frontiers in Neuroscience 12, https://doi.org/10.3389/fnins.2018.00046 (2018).
Isaacson Jeffry, S. & Scanziani, M. How Inhibition Shapes Cortical Activity. Neuron 72, 231–243, https://doi.org/10.1016/j.neuron.2011.09.027 (2011).
Rubenstein, J. L. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior 2, 255–267 (2003).
Wehr, M. & Zador, A. M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446, https://doi.org/10.1038/nature02116 (2003).
Moore, A. K. & Wehr, M. Parvalbumin-Expressing Inhibitory Interneurons in Auditory Cortex Are Well-Tuned for Frequency. The Journal of Neuroscience 33, 13713–13723, https://doi.org/10.1523/jneurosci.0663-13.2013 (2013).
Natan, R. G., Rao, W. & Geffen, M. N. Cortical Interneurons Differentially Shape Frequency Tuning following Adaptation. Cell Rep 21, 878–890, https://doi.org/10.1016/j.celrep.2017.10.012 (2017).
Vickers, E. D. et al. Parvalbumin-Interneuron Output Synapses Show Spike-Timing-Dependent Plasticity that Contributes to Auditory Map Remodeling. Neuron 99, 720–735.e726, https://doi.org/10.1016/j.neuron.2018.07.018 (2018).
Wood, K. C., Blackwell, J. M. & Geffen, M. N. Cortical inhibitory interneurons control sensory processing. Current opinion in neurobiology 46, 200–207, https://doi.org/10.1016/j.conb.2017.08.018 (2017).
Knudsen, E. I. Sensitive periods in the development of the brain and behavior. Journal of cognitive neuroscience 16, 1412–1425, https://doi.org/10.1162/0898929042304796 (2004).
Zann, R. A. The Zebra Finch: A Synthesis of Field and Laboratory Studies. (1996).
Böhner, J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav 39, 369–374, https://doi.org/10.1016/S0003-3472(05)80883-8 (1990).
Eales, L. A. Song Learning in Zebra Finches - Some Effects of Song Model Availability on What Is Learnt and When. Anim Behav 33, 1293–1300, https://doi.org/10.1016/S0003-3472(85)80189-5 (1985).
Eales, L. A. Song Learning in Female-Raised Zebra Finches - Another Look at the Sensitive Phase. Anim Behav 35, 1356–1365, https://doi.org/10.1016/S0003-3472(87)80008-8 (1987).
Roper, A. & Zann, R. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112, 458–470, https://doi.org/10.1111/J.1439-0310.2005.01169.X (2006).
Slater, P. J. B., Eales, L. A. & Clayton, N. S. In Advances in the Study of Behavior 18 1–34 (1988).
Adret, P., Meliza, C. D. & Margoliash, D. Song tutoring in presinging zebra finch juveniles biases a small population of higher-order song-selective neurons toward the tutor song. Journal of neurophysiology 108, 1977–1987, https://doi.org/10.1152/jn.00905.2011 (2012).
London, S. E. Developmental song learning as a model to understand neural mechanisms that limit and promote the ability to learn. Behavioural Processes, https://doi.org/10.1016/j.beproc.2017.11.008 (2017).
Morrison, R. G. & Nottebohm, F. Role of a Telencephalic Nucleus in the Delayed Song Learning of Socially Isolated Zebra Finches. J Neurobiol 24, 1045–1064, https://doi.org/10.1002/Neu.480240805 (1993).
Brainard, M. S. Contributions of the Anterior Forebrain Pathway to Vocal Plasticity. Annals of the New York Academy of Sciences 1016, 377–394, https://doi.org/10.1196/annals.1298.042 (2004).
Deregnaucourt, S. et al. Song Development: In Search of the Error-Signal. Annals of the New York Academy of Sciences 1016, 364–376, https://doi.org/10.1196/annals.1298.036 (2004).
Fee, M. S., Kozhevnikov, A. A. & Hahnloser, R. H. R. Neural Mechanisms of Vocal Sequence Generation in the Songbird. Annals of the New York Academy of Sciences 1016, 153–170, https://doi.org/10.1196/annals.1298.022 (2004).
Hahnloser, R. H. R. & Kotowicz, A. Auditory representations and memory in birdsong learning. Current opinion in neurobiology 20, 332–339, https://doi.org/10.1016/j.conb.2010.02.011 (2010).
Theunissen, F. E. et al. Song Selectivity in the Song System and in the Auditory Forebrain. Annals of the New York Academy of Sciences 1016, 222–245, https://doi.org/10.1196/annals.1298.023 (2004).
Williams, H. Birdsong and Singing Behavior. Annals of the New York Academy of Sciences 1016, 1–30, https://doi.org/10.1196/annals.1298.029 (2004).
Ahmadiantehrani, S. & London, S. E. Bidirectional manipulation of mTOR signaling disrupts socially mediated vocal learning in juvenile songbirds. Proceedings of the National Academy of Sciences 114, 9463–9468, https://doi.org/10.1073/pnas.1701829114 (2017).
London, S. E. & Clayton, D. F. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci 11, 579–586, https://doi.org/10.1038/nn.2103 (2008).
Simpson, H. & Vicario, D. Brain pathways for learned and unlearned vocalizations differ in zebra finches. The Journal of Neuroscience 10, 1541–1556, https://doi.org/10.1523/jneurosci.10-05-01541.1990 (1990).
Vallentin, D., Kosche, G., Lipkind, D. & Long, M. A. Inhibition protects acquired song segments during vocal learning in zebra finches. Science (New York, N.Y.) 351, 267–271, https://doi.org/10.1126/science.aad3023 (2016).
Blankvoort, S., Witter, M. P., Noonan, J., Cotney, J. & Kentros, C. Marked Diversity of Unique Cortical Enhancers Enables Neuron-Specific Tools by Enhancer-Driven Gene Expression. Current Biology 28, 2103–2114.e2105, https://doi.org/10.1016/j.cub.2018.05.015 (2018).
Murphey, D. K., Herman, A. M. & Arenkiel, B. R. Dissecting inhibitory brain circuits with genetically-targeted technologies. Frontiers in Neural Circuits 8, https://doi.org/10.3389/fncir.2014.00124 (2014).
Davis, M. F. et al. Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision. Neuron 86, 1055–1066, https://doi.org/10.1016/j.neuron.2015.03.062 (2015).
Hensch, T. K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Takesian, A. E. & Hensch, T. K. In Progress in Brain Research 207 3–34 (2013).
London, S. E., Boulter, J. & Schlinger, B. A. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. The Journal of comparative neurology 467, 496–508, https://doi.org/10.1002/cne.10936 (2003).
London, S. E., Dong, S., Replogle, K. & Clayton, D. F. Developmental shifts in gene expression in the auditory forebrain during the sensitive period for song learning. Dev Neurobiol 69, 437–450 (2009).
Kelly, T. K., Ahmadiantehrani, S., Blattler, A. & London, S. E. Epigenetic regulation of transcriptional plasticity associated with developmental song learning. Proceedings of the Royal Society B: Biological Sciences 285, https://doi.org/10.1098/rspb.2018.0160 (2018).
Mello, C. V., Vicario, D. S. & Clayton, D. F. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA 89, 6818–6822 (1992).
Nixdorf-Bergweiler, B. & Bischof, H. (Bethesda (MD): National Center for Biotechnology Information (US), (2007).
Reiner, A., Perkel, D. J., Mello, C. V. & Jarvis, E. D. Songbirds and the revised avian brain nomenclature. Ann N Y Acad Sci 1016, 77–108, https://doi.org/10.1196/annals.1298.013 (2004).
Stokes, T. M., Leonard, C. M. & Nottebohm, F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. The Journal of comparative neurology 156, 337–374, https://doi.org/10.1002/cne.901560305 (1974).
Shaughnessy, D. W., Hyson, R. L., Bertram, R., Wu, W. & Johnson, F. Female zebra finches do not sing yet share neural pathways necessary for singing in males. Journal of Comparative Neurology 527, 843–855, https://doi.org/10.1002/cne.24569 (2019).
Blakemore, C. & Cooper, G. F. Development of the Brain depends on the Visual Environment. Nature 228, 477–478, https://doi.org/10.1038/228477a0 (1970).
Wiesel, T. N. & Hubel, D. H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of neurophysiology 26, 1003–1017, https://doi.org/10.1152/jn.1963.26.6.1003 (1963).
Gehr, D. D., Hofer, S. B., Marquardt, D. & Leppelsack, H. Functional changes in field L complex during song development of juvenile male zebra finches. Brain research. Developmental brain research 125, 153–165 (2000).
Maul, K. K. et al. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Dev Neurobiol 70, 28–40, https://doi.org/10.1002/dneu.20751 (2010).
Woolley, S. M. N., Hauber, M. E. & Theunissen, F. E. Developmental experience alters information coding in auditory midbrain and forebrain neurons. Developmental Neurobiology 70, 235–252, https://doi.org/10.1002/dneu.20783 (2010).
Braun, K., Scheich, H., Heizmann, C. W. & Hunziker, W. Parvalbumin and calbindin-D28K immunoreactivity as developmental markers of auditory and vocal motor nuclei of the zebra finch. Neuroscience 40, 853–869 (1991).
Cornez, G. et al. Perineuronal nets and vocal plasticity in songbirds: A proposed mechanism to explain the difference between closed-ended and open-ended learning. Dev Neurobiol 77, 975–994, https://doi.org/10.1002/dneu.22485 (2017).
Stripling, R., Kruse, A. A. & Clayton, D. F. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol 48, 163–180 (2001).
Hensch, T. K. & Quinlan, E. M. Critical periods in amblyopia. Vis Neurosci 35, E014–E014, https://doi.org/10.1017/S0952523817000219 (2018).
Trachtenberg, J. T. Competition, inhibition, and critical periods of cortical plasticity. Current opinion in neurobiology 35, 44–48, https://doi.org/10.1016/j.conb.2015.06.006 (2015).
van Versendaal, D. & Levelt, C. N. Inhibitory interneurons in visual cortical plasticity. Cell Mol Life Sci 73, 3677–3691, https://doi.org/10.1007/s00018-016-2264-4 (2016).
Braaten, R. F., Petzoldt, M. & Colbath, A. Song perception during the sensitive period of song learning in zebra finches (Taeniopygia guttata). J Comp Psychol 120, 79–88, https://doi.org/10.1037/0735-7036.120.2.79 (2006).
Riebel, K. In Advances in the Study of Behavior Vol. 40 197–238 (2009).
Diez, A., Cui, A. & MacDougall-Shackleton, S. A. The neural response of female zebra finches (Taeniopygia guttata) to conspecific, heterospecific, and isolate song depends on early-life song exposure. Behavioural Processes, https://doi.org/10.1016/j.beproc.2017.12.022 (2017).
Lauay, C., Gerlach, N. M., Adkins-Regan, E. & Devoogd, T. J. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim Behav 68, 1249–1255, https://doi.org/10.1016/j.anbehav.2003.12.025 (2004).
Bailey, D. J. & Wade, J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Brain Res Mol Brain Res 116, 147–154 (2003).
Bauer, E. E. et al. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci 28, 1509–1522, https://doi.org/10.1523/jneurosci.3838-07.2008 (2008).
Mandelblat-Cerf, Y., Las, L., Denisenko, N. & Fee, M. S. A role for descending auditory cortical projections in songbird vocal learning. eLife 3, e02152, https://doi.org/10.7554/eLife.02152 (2014).
Vates, G. E., Broome, B. M., Mello, C. V. & Nottebohm, F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. The Journal of comparative neurology 366, 613-642, 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7 (1996).
Mori, C. & Wada, K. Audition-independent vocal crystallization associated with intrinsic developmental gene expression dynamics. J Neurosci 35, 878–889, https://doi.org/10.1523/JNEUROSCI.1804-14.2015 (2015).
Cummings, J. A. A Developmental Timeline of Parvalbumin-Expressing Neuron Addition to the Zebra Finch HVC During the Critical Period. (2016).
Bottjer, S. W., Glaessner, S. L. & Arnold, A. P. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J Neurosci 5, 1556–1562 (1985).
Bottjer, S. W. & Sengelaub, D. R. Cell death during development of a forebrain nucleus involved with vocal learning in zebra finches. J Neurobiol 20, 609–618, https://doi.org/10.1002/neu.480200702 (1989).
Johnson, F. & Bottjer, S. W. Afferent influences on cell death and birth during development of a cortical nucleus necessary for learned vocal behavior in zebra finches. Development 120, 13–24 (1994).
Korsia, S. & Bottjer, S. W. Developmental changes in the cellular composition of a brain nucleus involved with song learning in zebra finches. Neuron 3, 451–460, https://doi.org/10.1016/0896-6273(89)90204-3 (1989).
Layden, E. A., Schertz, K. E., Berman, M. G. & London, S. E. Functional connectivity strength within the auditory forebrain is altered by song learning and predicts song stereotypy in developing male zebra finches. bioRxiv, 657825, https://doi.org/10.1101/657825 (2019).
Gobes, S. M., Zandbergen, M. A. & Bolhuis, J. J. Memory in the making: localized brain activation related to song learning in young songbirds. Proceedings. Biological sciences/The Royal Society 277, 3343–3351, https://doi.org/10.1098/rspb.2010.0870 (2010).
Phan, M. L., Pytte, C. L. & Vicario, D. S. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. P Natl Acad Sci USA 103, 1088–1093, https://doi.org/10.1073/Pna5.0510136103 (2006).
Yanagihara, S. & Yazaki-Sugiyama, Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nature Communications 7, 11946, https://doi.org/10.1038/ncomms11946 http://www.nature.com/articles/ncomms11946#supplementary-information (2016).
Hensch, T. K. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience 6, 877–888, https://doi.org/10.1038/nrn1787 (2005).
Adret, P. Vocal imitation in blindfolded zebra finches (Taeniopygia guttata) is facilitated in the presence of a non-singing conspecific female. Journal of Ethology 22, 29–35, https://doi.org/10.1007/s10164-003-0094-y (2004).
Deregnaucourt, S., Poirier, C., Kant, A. V., Linden, A. V. & Gahr, M. Comparisons of different methods to train a young zebra finch (Taeniopygia guttata) to learn a song. J Physiol Paris 107, 210–218, https://doi.org/10.1016/j.jphysparis.2012.08.003 (2013).
Calabrese, A. & Woolley, S. M. N. Coding principles of the canonical cortical microcircuit in the avian brain. Proceedings of the National Academy of Sciences 112, 3517–3522, https://doi.org/10.1073/pnas.1408545112 (2015).
Dugas-Ford, J., Rowell, J. J. & Ragsdale, C. W. Cell-type homologies and the origins of the neocortex. Proceedings of the National Academy of Sciences 109, 16974–16979, https://doi.org/10.1073/pnas.1204773109 (2012).
Karten Harvey, J. Neocortical Evolution: Neuronal Circuits Arise Independently of Lamination. Current Biology 23, R12–R15, https://doi.org/10.1016/j.cub.2012.11.013 (2013).
Acknowledgements
We thank Sarah Greta for assistance with image quantification. Supported by the University of Chicago and the Institute for Mind and Biology.
Author information
Authors and Affiliations
Contributions
Investigation, Analysis, Visualization, Writing: J.V.G., E.O.G., S.E.L.; Conceptualization, Resources, Supervision: S.E.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gogola, J.V., Gores, E.O. & London, S.E. Inhibitory cell populations depend on age, sex, and prior experience across a neural network for Critical Period learning. Sci Rep 9, 19867 (2019). https://doi.org/10.1038/s41598-019-56293-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-56293-2