Abstract
An increased awareness on neonatal pain-associated complications has led to the development of pain scales adequate to assess the level of pain experienced by newborns such as the ABC score. A commonly used analgesic procedure is to administer a 33% oral dextrose solution to newborns prior to the painful intervention. Although this procedure is very successful, not in all subjects it reaches complete efficacy. A possible explanation for the different response to the treatment could be genetic variability. We have investigated the genetic variability of the OPRM1 gene in 1077 newborns in relation to non-pharmacologic pain relief treatment. We observed that the procedure was successful in 966 individuals and there was no association between the genotypes and the analgesic efficacy when comparing individuals that had an ABC score = 0 and ABC score >0. However, considering only the individuals with ABC score>0, we found that the homozygous carriers of the G allele of the missense variant SNP rs1799971 (A118G) showed an interesting association with higher ABC score. We also observed that individuals fed with formula milk were more likely to not respond to the analgesic treatment compared to those that had been breastfed.
Similar content being viewed by others
Introduction
Until few decades ago, it was thought that newborns were unable to feel pain: for this reason, many painful procedures, such as surgical interventions, tracheal intubation or venipuncture, were performed without analgesia. However, there are overwhelming evidences supporting the fact that the ability to respond to painful stimuli starts during intrauterine life1,2,3,4,5,6. Pain related stress has been associated with poor growth and neurocognitive impairment in term and preterm infants3,5,6,7,8,9,10. Intracranial hemorrhage and periventricular leukomalacia have been described as short term complications of painful procedures while behavioral disorders, anxiety spectrum disorders, sleep disorders, reduced post-natal growth and poor neurological outcome have been identified as long-term complications of prolonged stress/pain in early life8,9. An increased awareness on neonatal pain-associated complications has led to the development of pain scales adequate to assess the level of pain experienced by newborns (ABC scale, PIPP scale). These scales are based on behavioral changes (crying, changes in facial expression) and vital signs (heart rate, respiratory rate) during painful procedures. All term infants are commonly subjected to painful procedures before discharge at home for neonatal rare disease screening purposes; preterm newborns experience more invasive procedures such as intubation, central vein catheterization and may undergo 3 or 4 blood sampling every day for the first few weeks of life. Alongside improved methods to assess pain, also clinical procedures to alleviate it have been developed. With this regard, for major painful procedures, such as tracheal intubation, opioids and benzodiazepines are recommended, while less painful procedures (e.g. venipuncture or capillary blood sampling from the heel) are generally performed under a non-pharmacological analgesia. A commonly used approach is to administer an oral dextrose solution (20 to 33% concentration) to newborns prior to the painful intervention11. Although this procedure is very successful, not in all subjects it reaches analgesic efficacy. Numerous evidences suggest the involvement of the mu opioid receptor (MOR-1) in the analgesic efficacy of the dextrose solution. However, it is not clear if this effect is achieved through a direct interaction between the sugar and the receptor or through the regulation of endogenous opioids. Taddio and colleagues in a very small study consisting in 11 preterm infants aimed at establishing a direct link between beta-endorphin increase after sucrose administration, did not report a statistically significant association12. However, several studies conducted using animal models suggest the release of endogenous opioids through the blockage of mu opioid receptor during the administration of sweet substances13. To support this hypothesis, there is also the observation that the greatest analgesic efficacy is recorded after about two minutes from the beginning of glucose administration, time lapse that coincides with that necessary for the release of endorphins14. An additional indirect association between the MOR-1 receptor and dextrose/sucrose analgesia is the observation that newborns of methadone addicted mothers did not respond to orogustatory (sucrose) stimulation15. Finally, the analgesic effect of oral dextrose may also be attributable to an increase in plasma insulin levels which in turn has been shown to have analgesic activity through the regulation of many pathways16,17. The MOR-1 receptor is encoded by the OPRM1 gene that is highly polymorphic and many studies performed in adults have suggested an association between the genetic variability in the OPRM1 gene, and the response to pain relief treatment in adults18,19,20,21,22,23,24,25. Despite all these evidences, to the best of our knowledge, there are no studies linking the effect of the dextrose/glucose treatment with the genetic variability of the gene. With these premises we have investigated for the first time the genetic variability of the OPRM1 gene in 1077 newborns collected at University Hospital of Santa Chiara, in relation to non-pharmacologic pain relief treatment.
Materials and methods
Study population
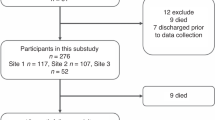
Blood samples from 1077 neonates born between 2015 and 2019 were collected at the Division of Neonatology of the Santa Chiara Hospital. For each newborn 5 ml of blood were collected from the cord at birth, in a completely not invasive way. Anthropometric measures at birth (birth weight, length, head circumference), type of feeding (exclusive breastfeeding, partial breastfeeding and exclusive formula milk) data on delivery (spontaneous vs caesarean section and mother’s pharmacological analgesia if present) and familiar history (ethnicity, mother’s age, pre-pregnancy BMI, weight increase during pregnancy, relevant diseases) were also collected. The parents of all subjects signed a written informed consent form the study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of the Meyer Children Hospital of Florence.
Pain relief determination
In order to reduce variability secondary to different type of procedures, we assessed just heel lancing for neonatal metabolic screening. According to Italian Neonatal Society guidelines26 and international guidelines for pain relief in newborns, before the procedure to all neonates a 33% dextrose solution was orally administered in order to reduce pain perception. The heel incision device used was Gentle heel™ produced by Alleset, Inc (Flowery Branch, USA). Pain level was assessed with ABC scale, which consists of three cry parameters: (A) First cry acuteness (NO = 0; YES = 2), (B) Burst rhythmicity (NO = 0; YES = 2), (C) Cry constancy (no cry or only a brief moan = 0; not constant, but more than a brief moan = 1; constant = 2). The ABC scale was validated for healthy, non-intubated term newborns27. Clinical procedures and pain recording were carried out at the Neonatology Unit of the Santa Chiara Hospital by trained personnel only.
SNPs selection
Common genetic variability in the OPRM1 gene region was investigated following a hybrid functional and tagging approach to identify candidate SNPs. For OPRM1 tagging SNPs were selected with the use of the Haploview Tagger Program (http://www.broad.mit.edu/mpg/haploview/; http://www.broad.mit.edu/mpg/tagger/)28, using pairwise tagging with a minimum r2 of 0.8. In addition, we have included in the selection the OPRM1-rs1799971 (A118G) that is a putatively functional SNP. The final selection included 11 SNPs for the OPRM1 gene.
DNA extraction and genotyping
DNA was extracted from umbilical cord blood using Quick-DNA Plus Kit (Zymo Research). Genotyping was performed using the allele-specific TaqMan PCR SNP genotyping assay (Thermo Fisher Scientific, Waltham, Massachusetts, USA) as recommended by the manufacturer. Detection of the genotyping calls was made using the QuantStudio 5 Real-Time PCR System (Applied Biosystems by Thermo Fisher Scientific, Waltham, Massachusetts, USA), 3.5% of the samples were duplicated to ensure genotyping quality.
Statistical analysis
Hardy-Weinberg equilibrium was tested by the chi square test. The association between the SNPs, the covariates considered (mentioned before) and pain relief treatment was calculated using an unconditional logistic regression computing Odds Ratio (OR) and confidence intervals (CI) considering the ABC score as a categorical variable (ABC score = 0 Vs ABC score >0). In addition, we also performed an analysis considering only the individuals that had and ABC score>0 and calculated the association between the genotypes and the ABC score (ABC score coded as 1;2;3;4;5 and 6) with a general linear model (glm). Genetic analyses were performed under a co-dominant inheritance model. For a subgroup of individuals (n = 845), we have also collected data on the person performing the sedation (so forth called operator) and we used this variable for adjustment. We performed crude analysis (without adjusting for operator) and adjusted analysis. The glm model was adjusted for gestational age and operator, since these variables were the only ones showing a borderline association with the ABC score.
Bioinformatic analysis
We used several bioinformatic tools to assess possible functional relevance for the SNPs showing significant associations. RegulomeDB (http://regulome.stanford.edu/)and HaploReg29 were used to identify the regulatory potential of the SNPs, The Genotype-Tissue Expression (GTEx)30 was used to identify potential associations between the SNP and expression levels of nearby genes (eQTL).
Results
Data filtering and quality control
All SNPs genotype distribution were in Hardy-Weinberg equilibrium with a P-value > 0.005. The average polymorphism call rate was 98.84% with a minimum of 97.62% for rs610231 and a maximum of 99.72% for rs2075572. The concordance rate for the duplicated samples was more than 99%.
Anthropometric, clinical and lifestyle variables and analgesic treatment response
Oral dextrose administration was successful in avoiding pain related to minor painful procedures in 966 individuals out of 1054; in 88 patients it was not effective: 9 had an ABC score of 1, 40 of 2, 13 of 3, 17 of 5 and 9 of 6. For 33 individuals the ABC score was not calculated. In the crude analysis we observed that the type of feeding (exclusive breastfeeding, partial breastfeeding and exclusive formula milk) had an effect on the ABC score with the tendency (P test for trend p = 0.005) of mixed types and artificial feeding to increase the chance of having an ABC score > 0 with OR 1.90 (95% CI 1.18–3.06; P-value = 0.008) for mixed type and OR 2.23 (95% CI 0.88–5.64; P-value = 0.088) for formula milk. In addition, we observed that also the age of the mother had a weak effect on the ABC score with OR 1.05 (95% CI 1.01–1.09; p = 0.036) for each year increase in the maternal age. These results are shown in Table 1. The other variables taken into consideration did not show any significant result. Adjusting for operator we observed similar results (Supplementary Table 1). Considering the subjects with ABC score > 0 none of the variables showed a statistically significant association, with gestational age and operator showing a borderline association with the ABC score (Supplementary Table 2).
SNPs effect on analgesic treatment
In the crude analysis we observed no statistically significant association as shown in Supplementary Table 3. Adjusting for operator we observed that rs510769 was close to the conventional threshold for statistical significance in the codominant model: OR 1.6 (95% CI 0.99–2.58) p = 0.055 as shown in Table 2. Considering the subgroup analysis of the individuals with an ABC score > 0, we observed that homozygous carriers of the G allele of the missense SNP rs1799971 (A118G) were associated with a higher ABC score, although the results were borderline not significant (p = 0.055) as shown in Supplementary Table 4. We also performed exploratory analysis adjusting for gestational age as shown in Table 3, operator (Supplementary Table 5) and gestational age and operator (Supplementary Table 6). OPRM1-rs1799971 (A118G) was associated with high ABC score reaching statistical significance when adjusting for gestational age (p = 0.041).
Possible functional effects
Haploreg and RegulomeDB did not show any potential functional effect for the SNPs taken into consideration and the GTEx database did not suggest any eQTLs for the rs1799971-SNP.
Discussion
In this study we have enrolled more than 1000 newborns to investigate whether genetic variability and anthropometric and lifestyle factors could influence non-pharmacologic analgesic treatment in newborns. This sample size makes it one of the largest studies on newborn genetics with the addition of meticulously collected information on anthropometric and life style factors. The efficacy of the non-pharmacologic treatment was very good affecting 966 (92%) out of 1054 individuals.
We observed an association between feeding type and analgesic efficacy of the non-pharmacologic treatment. The association was significant in the crude model and with adjustment. The trend showed a clear association between natural human milk and increased chance of effective analgesic treatment (p = 0.005). A possible explanation of this association might reside in the fact that breast feeding could have a prolonged soothing effect on newborns, decreasing their anxiety and increasing the sugar effect. The pain relief effect of breast milk could also be explained by the higher concentration of tryptophan compared to formula milk. As suggested by Heine, tryptophan is a precursor of melatonin, which can increase beta endorphin production regulating appetite, satisfaction and pain perception31. The relative relevance of the two different aspects could not be weighted because all patients that received human milk were breastfed and did not receive expressed breast milk or human milk from donors.
From a genetic point of view, we observed that in the logistic regression (ABC score > 0 vs ABC score = 0) rs510769 was close to the conventional threshold for statistical significance (p = 0.055) and that in the glm model (ABC score from 1 to 6) the carriers of the G allele of the rs1799971 showed the least benefit from the analgesic treatment. OPRM1-rs510769 is an intronic variant that has been investigated in relation to various human traits, such as onset of side effects in patients during a methadone maintenance treatment (MMT)32, smoking behavior in MMT33, susceptibility to heroin addiction34 and amphetamine-induced euphoria35. However, there are no functional evidences in the literature for this SNP; in addition, the results from the bioinformatic tools we used are inconclusive. Therefore, it is not easy to infer a mechanistic relation between the SNP and analgesic treatment.
On the other hand, rs1799971 (A118G) is the most studied variant in the OPRM1 gene and there are overwhelming evidence, spanned among a decade, supporting its role in a variety of human phenotypes including pain, analgesia and drug tolerance18,19,20,21,24,25,33,36,37,38,39. This polymorphism is a missense variant with an A to G nucleotide change that leads to an amino-acid substitution (Asn40Asp) at a putative N-glycosylation site in the extracellular receptor region. The majority of the studies support an increased pain sensitivity and worse response to pain relief therapy in individuals with the GG genotype compared to the other genotypes18,19,20,21,24,25. Changes from a basic amino acid to an acid amino acid in the OPRM1 receptor could alter its ability to bind ligands and could explain the altered effectiveness of the protein. In agreement with what suggested by the literature, we observed a tendency for GG homozygous to display less affective analgesic efficacy, even though in a subgroup analysis. This difference may be explained by the fact that pain relief treatment is a complex experience that is mediated by several variables. Indeed, a single SNP is unlikely to predict the ability to respond to the therapy, also considering the relative small size of non-responders in our population. However, our results suggest, even though with a weak statistical association, that among the individuals that do not respond to the therapy the intensity of the score could be mediated by the genotype of the rs1799971 (A118G) variant. This result should be interpreted with caution since it comes from a subgroup analysis, and therefore from a small number of individuals; in light of the multiple tests that we performed it could be statistical fluctuation. However, our findings are in line with what has been repeatedly observed for adults, i.e. the GG genotype of the rs1799971 (A118G) SNP associated with less effectiveness of pain reducing treatments.
In conclusion, this study highlights that the type of milk seems to be associated with newborn pain treatment response and also suggests a possible association between the missense variant rs1799971 (A118G) and pain reduction in newborns. These findings if further replicated could represent an important step in evaluating the possibility of a personalized analgesia in newborns.
Data availability
The data for this work will be made available to researchers who submit a reasonable and detailed request to the corresponding author, conditional to approval of the Ethics Commission of the of the Meyer Children Hospital of Florence. Data will be stripped from all information allowing identification of study participants.
References
Anand, K. J. & Hickey, P. R. Pain and its effects in the human neonate and fetus. The New England journal of medicine 317, 1321–1329, https://doi.org/10.1056/nejm198711193172105 (1987).
Bartocci, M., Bergqvist, L. L., Lagercrantz, H. & Anand, K. J. Pain activates cortical areas in the preterm newborn brain. Pain 122, 109–117, https://doi.org/10.1016/j.pain.2006.01.015 (2006).
Bouza, H. The impact of pain in the immature brain. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 22, 722–732, https://doi.org/10.3109/14767050902926962 (2009).
Fitzgerald, M. The development of nociceptive circuits. Nature reviews. Neuroscience 6, 507–520, https://doi.org/10.1038/nrn1701 (2005).
Fitzgerald, M. & Walker, S. M. Infant pain management: a developmental neurobiological approach. Nature clinical practice. Neurology 5, 35–50, https://doi.org/10.1038/ncpneuro0984 (2009).
Walker, S. M. Neonatal pain. Paediatric anaesthesia 24, 39–48, https://doi.org/10.1111/pan.12293 (2014).
Bhutta, A. T. & Anand, K. J. Vulnerability of the developing brain. Neuronal mechanisms. Clinics in perinatology 29, 357–372 (2002).
Valeri, B. O., Holsti, L. & Linhares, M. B. Neonatal pain and developmental outcomes in children born preterm: a systematic review. The Clinical journal of pain 31, 355–362, https://doi.org/10.1097/ajp.0000000000000114 (2015).
Vinall, J. et al. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain 153, 1374–1381, https://doi.org/10.1016/j.pain.2012.02.007 (2012).
Grunau, R. Early pain in preterm infants. A model of long-term effects. Clinics in perinatology 29(373–394), vii–viii, https://doi.org/10.1016/S0095-5108(02)00012-X (2002).
Bueno, M. et al. A systematic review and meta-analyses of nonsucrose sweet solutions for pain relief in neonates. Pain research & management 18, 153–161, https://doi.org/10.1155/2013/956549 (2013).
Taddio, A., Shah, V., Shah, P. & Katz, J. Beta-endorphin concentration after administration of sucrose in preterm infants. Arch Pediatr Adolesc Med 157, 1071–1074, https://doi.org/10.1001/archpedi.157.11.1071 (2003).
Segato, F. N., Castro-Souza, C., Segato, E. N., Morato, S. & Coimbra, N. C. Sucrose ingestion causes opioid analgesia. Braz J Med Biol Res 30, 981–984, https://doi.org/10.1590/s0100-879x1997000800011 (1997).
Stevens, B., Yamada, J., Ohlsson, A., Haliburton, S. & Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 7, CD001069, https://doi.org/10.1002/14651858.CD001069.pub5 (2016).
Blass, E. M. & Ciaramitaro, V. A New Look at Some Old Mechanisms in Human Newborns - Taste and Tactile Determinants of State, Affect, and Action. Monogr Soc Res Child 59, 1–81 (1994).
McLaughlin, J. P. & Chavkin, C. Tyrosine phosphorylation of the mu-opioid receptor regulates agonist intrinsic efficacy. Mol Pharmacol 59, 1360–1368, https://doi.org/10.1124/mol.59.6.1360 (2001).
Xing, Y. L. et al. Insulin decreases isoflurane minimum alveolar anesthetic concentration in rats independently of an effect on the spinal cord. Anesthesia and Analgesia 98, 1712–1717, https://doi.org/10.1213/01.Ane.0000113550.47942.47 (2004).
Campa, D., Gioia, A., Tomei, A., Poli, P. & Barale, R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clinical pharmacology and therapeutics 83, 559–566, https://doi.org/10.1038/sj.clpt.6100385 (2008).
Gong, X. D. et al. Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain. Asian Pacific journal of cancer prevention: APJCP 14, 2937–2943 (2013).
Hwang, I. C. et al. OPRM1 A118G gene variant and postoperative opioid requirement: a systematic review and meta-analysis. Anesthesiology 121, 825–834, https://doi.org/10.1097/aln.0000000000000405 (2014).
Khalil, H. et al. OPRM1 and COMT Gene-Gene Interaction Is Associated With Postoperative Pain and Opioid Consumption After Orthopedic Trauma. Biological research for nursing 19, 170–179, https://doi.org/10.1177/1099800416680474 (2017).
Lotsch, J., Geisslinger, G. & Tegeder, I. Genetic modulation of the pharmacological treatment of pain. Pharmacology & therapeutics 124, 168–184, https://doi.org/10.1016/j.pharmthera.2009.06.010 (2009).
Lotsch, J. et al. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenetics and genomics 19, 429–436 (2009).
Muriel, J. et al. OPRM1 influence on and effectiveness of an individualized treatment plan for prescription opioid use disorder patients. Annals of the New York Academy of Sciences, 82–93, https://doi.org/10.1111/nyas.13735 (2018).
Yu, Z., Wen, L., Shen, X. & Zhang, H. Effects of the OPRM1 A118G Polymorphism (rs1799971) on Opioid Analgesia in Cancer Pain: A Systematic Review and Meta-Analysis. The Clinical journal of pain 35, 77–86, https://doi.org/10.1097/ajp.0000000000000636 (2019).
Lago, P. et al. Guidelines for procedural pain in the newborn. Acta paediatrica (Oslo, Norway: 1992) 98, 932–939 (2009).
Bellieni, C. V. et al. Development and validation of the ABC pain scale for healthy full-term babies. Acta paediatrica (Oslo, Norway: 1992) 94, 1432–1436, https://doi.org/10.1111/j.1651-2227.2005.tb01816.x (2005).
Stephens, M., Smith, N. J. & Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68, 978–989, S0002-9297(07)61424-4 https://doi.org/10.1086/319501 (2001).
Ward, L. D. & Kellis, M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic acids research 40, D930–934 https://doi.org/10.1093/nar/gkr917 (2012).
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660, https://doi.org/10.1126/science.1262110 (2015).
Heine, W. E. The significance of tryptophan in infant nutrition. Advances in experimental medicine and biology 467, 705–710, https://doi.org/10.1007/978-1-4615-4709-9_91 (1999).
Wang, S. C. et al. Genetic polymorphisms in the opioid receptor mu1 gene are associated with changes in libido and insomnia in methadone maintenance patients. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 22, 695–703, https://doi.org/10.1016/j.euroneuro.2012.02.002 (2012).
Chen, Y. T. et al. OPRM1 genetic polymorphisms are associated with the plasma nicotine metabolite cotinine concentration in methadone maintenance patients: a cross sectional study. Journal of human genetics 58, 84–90, https://doi.org/10.1038/jhg.2012.139 (2013).
Levran, O. et al. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes, brain, and behavior 7, 720–729, https://doi.org/10.1111/j.1601-183X.2008.00410.x (2008).
Dlugos, A. M. et al. OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes, brain, and behavior 10, 199–209, https://doi.org/10.1111/j.1601-183X.2010.00655.x (2011).
De Gregori, M. et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. European journal of clinical pharmacology 69, 1651–1658, https://doi.org/10.1007/s00228-013-1523-7 (2013).
Liu, J., Hu, D., Jiang, Y., Xi, H. & Li, W. Association between single nucleotide polymorphisms in the OPRM1 gene and intraoperative remifentanil consumption in northern Chinese women. Pharmacology 94, 273–279, https://doi.org/10.1159/000368082 (2014).
Sia, A. T. et al. Influence of mu-opioid receptor variant on morphine use and self-rated pain following abdominal hysterectomy. The journal of pain: official journal of the American Pain. Society 14, 1045–1052, https://doi.org/10.1016/j.jpain.2013.03.008 (2013).
Yao, P. et al. Effect of gene polymorphism of COMT and OPRM1 on the preoperative pain sensitivity in patients with cancer. International journal of clinical and experimental medicine 8, 10036–10039 (2015).
Acknowledgements
The authors wish to dedicate this work to Paolo Ghirri, that passed away during the revison of the manuscript. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 04/01/2019. This work was supported by Fondazione Arpa (www.fondazionearpa.it) and by the PRA 2018–2019 grant round, with project code -PRA_2018_39 funded by the University of Pisa.
Author information
Authors and Affiliations
Contributions
I.E., R.F., M.G., C.R. and S.G. performed the experiments and statistical analysis; M.C., C.T., F.M., A.B. and M.F. contributed to enrollment and pain assessment; A.T., P.G. and D.C. designed the study; D.C. drafted the manuscript; I.E., R.F., M.G., C.R., S.G., M.C., C.T., F.M., A.B., M.F., A.T., P.G. and D.C. read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erbi, I., Ciantelli, M., Farinella, R. et al. Role of OPRM1, clinical and anthropometric variants in neonatal pain reduction. Sci Rep 10, 7091 (2020). https://doi.org/10.1038/s41598-020-63790-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-63790-2
This article is cited by
-
Polymorphic variants in Sweet and Umami taste receptor genes and birthweight
Scientific Reports (2021)