Abstract
Pyrrolizidine alkaloids (PAs) are persistent mutagenic and carcinogenic compounds produced by many common plant species. Health authorities recommend minimising human exposure via food and medicinal products to ensure consumer health and safety. However, there is little awareness that PAs can contaminate water resources. Therefore, no regulations exist to limit PAs in drinking water. This study measured a PA base concentration of ~ 70 ng/L in stream water adjacent to an invasive PA-producing plant Petasites hybridus (Asteraceae). After intense rain the PA concentration increased tenfold. In addition, PAs measured up to 230 ng/L in seepage water from groundwater wells. The dominant PAs in both water types corresponded to the most abundant PAs in the plants (senkirkine, senecionine, senecionine N-oxide). The study presents the first discovery of persistent plant toxins in well water and their associated risks. In addition, it for the first time reports monocrotaline and monocrotaline N-oxide in Petasites sp.
Similar content being viewed by others
A popular fairytale The Ugly Duckling written in Danish by Hans Christian Andersen in 1843 narrates that the ducklings were born under the leaves of a butterbur: the plant such tall that small children could stand upright under the largest ones1. The customized name of this regional plant was not translated into foreign languages. However, the English name butterbur also has a local customary background. It is supposed to have originated from the large leaves that were used to wrap butter during hot weather2. Invasive plants, such as the butterbur, and their adverse roles are little recognized by society, especially when they are inseparable parts of the culture or folklore.
Common butterbur (Petasites hybridus (G. Gaertn., B. Mey. & Scherb.)) is a flowering plant found in damp nutrient-rich soil and around the shores of fresh water bodies in northern Europe and parts of North America3,4. With the aid of powerful rhizomes it can form large populations; its leaves reach up to 70 cm in width on 1 m stems that shade other vegetation and thereby cause soil erosion5. In these regions the species is regarded as invasive since it is originally native to southern Europe and western Asia and was introduced to northern Europe around 1350 as a medicinal plant by monks5,6,7. The butterbur expanded from cultivation sites in monasteries, castles and estates to natural habitats across northern and western Europe. Nowadays, it is also marketed and deliberately planted for ornamental purposes, e.g. around local community fire safety ponds or in garden lakes as portrayed in H.C. Andersen’s The Ugly Duckling.
The bioactive sesquiterpene constituents in butterbur (petasin, isopetasin) express antispasmodic properties and offer a variety of medicinal applications where the relaxation of muscle and vascular spasms is desired4,8,9,10. Historically, the plant’s leaves and rhizomes were also used to treat or prevent plague, rashes, arthritis, kidney and bladder stones. Its most documented evidence-based modern therapeutic application is the treatment of migraine headaches and allergic rhinitis4,5,9,10,11,12. However, commercialization of unrefined butterbur extracts is prohibited due to numerous cases of intensive liver damage caused by high concentrations of unsaturated pyrrolizidine alkaloids4,5,11,13,14.
Alkaloids are a large class of naturally occurring diverse organic compounds structurally determined by a heterocyclic ring structure containing at least one nitrogen atom. They are produced as secondary metabolites primarily by fungi and plants, also by bacteria and animals15,16,17,18,19,20. Many alkaloids are highly toxic and some contain properties desired for therapeutic or recreational purposes21. Atropine and coniine rich extracts have been used for homicide since ancient Rome. Strychnine and anabasine were used as insecticides before the development of a wide range of synthetic pesticides that are comparatively less toxic to humans22. For centuries alkaloids have been used as pharmaceuticals (morphine, codeine) and stimulants (cocaine, nicotine)16,23. Health authorities regulate specific alkaloid groups to ensure safety of food, tea, medicine, honey, supplements and other products17,24,25,26.
Heterocyclic rings and ample hydrogen bonds determine alkaloid stability and contribute to their high water solubility. The polarity and persistence of alkaloids make them compatible with persistent and mobile organic compounds (PMOCs). PMOCs are emerging contaminants that are rarely detected in the environment due to the lack of available analytical techniques27. PMOCs include surfactants, polar industrial chemicals, pharmaceuticals and personal care products28,29. Alkaloids applied as pharmaceuticals or stimulants are also commonly found in waste water and sewage sludge, whereas groundwater surveys reveal alkaloid caffeine among the most frequently encountered compounds23,30,31,32,33,34.
Pyrrolizidine alkaloids (PA) comprise several hundred compounds based on the heterocyclic two-ring structure necine16,35,36 (Fig. 1). PAs are among the most common natural toxins produced by plants as defence chemicals against insects and herbivores37,38,39,40. The PA composition in PA-producing plants is not necessarily constant and can vary due to climatic and environmental conditions, the age and the part of the plant, as well as discrete genotypes and chemotypes25,41,42. The mode of PA toxicity depends on the molecular structure. When ingested, PAs with an unsaturated bond in 1,2 position of the necine unit (Fig. 1) (hereafter called unsaturated PAs) metabolically oxidize and react with proteins and nucleic acids43,44,45,46 resulting in hepatotoxic, mutagenic and carcinogenic effects16,37,41,46,47,48,49,50.
Oxidized PAs (PA N-oxides, Fig. 1) are present in plants in nearly equal quantities as free base PAs51. The toxicity of PA N-oxides has been demonstrated but most are suggested to be less toxic than the PAs from which they originate42,51,52. However, metabolic reduction of N-oxides to their corresponding free base PAs has been reported to take place in the gut of animals42,53. Studies on human liver microsomal system confirm that the results from animal studies are also relevant to humans18,51,54. N-oxides are more polar than free base PAs, implying potentially greater aquatic emissions from plants and a higher mobility in soils and sediments.
Plants with high concentrations of unsaturated PAs are responsible for the death of cattle18,55. Toxic effects in humans such as hepatic sinusoidal obstruction syndrome and incidents of acute and subacute food poisoning with high morbidity and mortality have been reported from many countries24,25,37,38,44,45,50,55,56. The World Health Organization (WHO) recommends minimizing PA exposure to humans as much as possible57. Due to the high toxicity, national health authorities in e.g. Germany and United Kingdom recommended a daily total PA intake of not more than 7 ng/kg bodyweight applying a margin of exposure (MOE) of 10,00037,58,59,60,61. The European Food Safety Authority (EFSA) recommends minimizing exposure and in 2017 established a benchmark dose lower confidence limit 10% (BMDL10) of 237,000 ng/kg body weight per day. Applying the same MOE as above, this results in a recommended threshold of 23.7 ng/kg body weight per day24,62,63,64. The daily limit of 23.7 ng/kg body weight will be used in this study as a reference point.
It is estimated that 3% of all flowering plants contain at least one unsaturated PA65. In regions where these plants are prevalent there is an emerging concern of PAs highly exceeding safety limits in honey20,24,66,67,68,69. Despite their abundance in flora, the degree of natural PA emissions and their fate in the environment remains largely unknown. A recent study measured up to 3800 μg/kg of PAs in topsoil and up to 530 μg/L in pond water. The study associated the compounds with adjacent densely growing common ragwort (Jacobaea vulgaris (Gaertn.))70.
We hypothesize that PAs can naturally leach from butterbur into water and make it unsafe to drink. The stability of PAs and frequently reported cases of groundwater contamination by structurally similar caffeine imply that PAs can leach into groundwater. Since there are no regulations that require PA monitoring, these and other toxic alkaloids may be overlooked as emerging environmental contaminants. In order to test the hypothesis we measured the content of 21 PAs in a stream, seepage and ground water over a period of four months in a butterbur affected catchment system.
Results
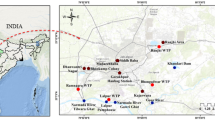
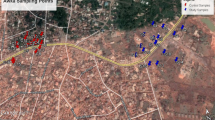
A site with a channelized surface water stream and a system of groundwater abstraction wells was selected in eastern Denmark (Fig. 2). The shores of the stream and the land surface surrounding two of the wells (G2 and G3) were infested with butterbur. Every well consisted of a vertical pipe providing access to deep groundwater (estimated water level of approximately 60 m depth) and a surrounding casing supported by concrete rings that naturally filled with seepage water from the soil (observed water level 2.2–3.0 m depth) (Fig. 3). The waters from different depths are not mixed and the deep groundwater is pumped for municipal water supply.
A qualitative test revealed 9 cyclic diester unsaturated PAs in butterbur leaves sprouting near well G2 in April 2019: jacobine, jacobine N-oxide, monocrotaline, monocrotaline N-oxide, senecionine, senecionine N-oxide, senecivernine, senecivernine N-oxide and senkirkine (hereafter referred to as type 1 PAs). Subsequent quantitative analyses of matured butterbur leaves and rhizomes sampled near wells G2 and G3 on the dates listed in Table 2 of the supplementary material (SM) revealed the same PA compositions with concentrations provided in Fig. 4. To the best of our knowledge, monocrotaline and monocrotaline N-oxide have not been reported in Petasites sp. before.
Average concentrations of PAs detected in butterbur plants, stream and seepage waters ± SDs (in alphabetical order). The graphs of PAs in the plants have different scales on the y-axes. The empty values represent no detection. “Other sites” in the stream water column refer to additional surface water sampling sites mapped in Fig. 2. The dates of the sampling events are listed in Table 2 of the SM.
A total of 21 PAs were detected in the stream water and in the seepage water of wells G2 and G3. They comprised all the type 1 PAs, together with lower concentrations of 12 unsaturated PAs that were not detected in the butterbur (hereafter referred to as type 2 PAs). Type 2 PAs comprised cyclic diesters (erucifoline, retrorsine, retrorsine N-oxide, seneciphylline), open chain diesters (echimidine, echimidine-N-oxide, lasiocarpine) and monoesters (europine, europine N-oxide, heliotrine, intermedine, intermedine N-oxide). The concentrations of individual type 1 PAs measured in the stream water and in the seepage water in wells G2 and G3 are presented in Fig. 4. No PAs were detected in the deep groundwater, in seepage waters of the control wells G1 and G4, or in blank water sampled as the field controls.
PAs were detected in the stream water and in the seepage water in wells G2 and G3 during all sampling events. In all sampled matrices the concentrations of N-oxides were similar to the concentrations of the equivalent free base compounds. The most frequently found and abundant PAs in the various sampled water corresponded to the most abundant PAs in the plants (senkirkine, senecionine, and senecionine N-oxide). No other PA metabolites were detected, whereas N-oxides are known to be the subject to metabolic back-transformation to corresponding free base PAs42,53. In order to be compatible with the recommended thresholds set by health authorities for PAs in orally consumed products24,57,62, further risk assessment will be based on the total unsaturated PA concentrations.
The base stream flow (sampling events No. 1, 6 and 7 (Table 2 of the SM)) had average total PA concentrations in the surface water monitoring site from 49 to 91 ng/L (Fig. 5). Intense rapid rain between sampling events No. 1 and 2 (13 mm recorded by the Danish Meteorological Institute) resulted in a tenfold increase in the total PA concentration, implying simple and quick toxin washoff and release from the plants or the soil. The segmentation of the stream into stagnant water patches due to summer drought conditions (sampling event No. 4) resulted in PA concentrations approximately 3 times higher than at the base flow.
Concentrations of total PAs in the stream at the surface water monitoring site (A) and in seepage waters in the wells (B) ± SDs of the total PA concentrations in triplicate samples. The type 1 PAs represent the sum of nine PAs that were detected in plants and water (Fig. 4), whereas the type 2 PAs represent the sum of twelve PAs that were detected in water only (Table 1 of the SM). The dates of the sampling events are listed in Table 2 of the SM, the seepage water sampling locations mapped in Fig. 2.
Total PA concentrations in the well seepage waters were highest during sampling event No. 3 (Fig. 5) which corresponded with the largest biomass of adjacent butterbur. The greater plant coverage of the well (well G3, Fig. 2) was associated with higher PA concentrations in the well water. There were fewer plants around the wells at subsequent sampling events due to regular biomass cutting and removal by a maintenance company to ensure access to the wells. This biomass removal possibly caused reduced PA concentrations.
The concentrations of individual type 2 PAs that are presented in Fig. 5 did not exceed 10 ng/L, except in the stream water under non-base flow conditions (108 ng/L of retrorsine and 27 ng/L of seneciphylline in sampling event No. 2, and 56 ng/L of erucifoline and 46 ng/L of retrorsine N-oxide in sampling event No. 4). Type 1 PAs comprised cyclic diesters only, whereas type 2 PAs also included open-chain diesters and monoesters (Table 1 of the SM). Open-chain diester and monoester PAs have not been reported in butterbur in this or in previous studies4.
Discussion
This research reports PAs from butterbur in surface water for the first time, and provides evidence for our starting hypothesis that PAs can naturally leach from butterbur into water and make it unsafe to drink. Several previous studies identified PAs as soil or surface water contaminants70,71,72. Experimentally determined octanol–water partition coefficients infer little potential PA sorption to soil73. The surface waters adjacent to PA-producing plants may show relatively high PA concentrations (up to 530 μg/L70) and thus contribute to the overall exposure of farm and wildlife animals to PAs. Furthermore, this study illustrates PA leaching from PA-producing plants to groundwater wells via seepage water. Open bottomed shallow groundwater wells are common in remote one-household settlements with no centralized water supply in both developing and developed countries. These wells are frequently subject to pollution of organic matter, nitrate and pesticides that seep from farming areas and noncentralized sanitation. To the best of our knowledge PAs in well water have not been examined before.
Other known PA producing plants were not observed in the studied area and the control wells contained neither PA types. That implies that all PAs in water likely originated from butterbur. The highest concentrations of type 2 PAs in the seepage water were observed in sampling event No. 7 (October) at which time there were the highest proportion of naturally wilted and decaying butterbur. The type 2 PAs may have originated from other unknown sources, transformed in decaying biomass or in the water from the type 1 PAs.
According to the recommended maximum daily intake of 23.7 ng/kg body weight of unsaturated PAs, daily intake of 2 L of well water with a PA concentration of 233 ng/L, or 2 L of surface water with 523 ng/L corresponds to 30% and 60% of the maximum allowable intake for a 70 kg healthy human. The stream and shallow well waters reported in this study caused no harm to human health only because these waters were not utilized for drinking. The analysed exploited deep groundwater contained no PAs because this water was pumped from beneath impermeable geological layers with no contact with leachates from the butterbur. However, the groundwater pipes immersed in contaminated seepage water (Fig. 3C) pose a potential risk to groundwater. Poorly drilled or maintained deep groundwater wells are subject to the downward transmission of water carrying microbiological and hazardous anthropogenic contaminants74,75,76.
Microbiological and anthropogenic chemical pollution is methodically monitored and regulated in drinking water by law. The Directive of the European Parliament and of the Council on the protection of groundwater against pollution and deterioration (2006/118/EC) imposes EU member states to terminate exploitation of water from groundwater wells if active substance of any pesticide, including its relevant metabolites, degradation and reaction products exceeds the 100 ng/L threshold77. Threshold violations have been reported in Italy, the Netherlands, Belgium, Sweden, Finland and Denmark, leading to the closure of many drinking water wells74.
Plant-produced toxic compounds can locally outcompete anthropogenic chemicals in quantity because of their continuous production and comparatively high concentrations in plants78. Invasive and foreign ornamental species tend to contain highly toxic natural compounds. Inedible parts of agricultural crops contain natural toxins that could possibly also be released to larger land areas such as carcinogenic quinolizidine alkaloids in leaves and blossoms of lupin, and the acute toxic steroidal alkaloid solanine in leaves and stems of potato79,80,81. To some extent, the natural toxins are regulated in food. Plant toxins not entering the human food chain are generally regarded as not harmful to humans. However, their aquatic toxicity and waterborne ingestion by humans remain largely unknown. In addition to direct harm, ingested plant toxins can interact with other contaminants in humans creating synergies and cumulative adverse health effects82.
Anthropogenic activities directly or indirectly stimulate the spread of many invasive species. Studies show that among toxic invasive plants, plants producing PAs and other alkaloids are the most common83. For example, the genus Cytisus comprises about 50 species of invasive PA-producing flowering plants. Many other plant-produced toxic alkaloids can be stable in water, like alkaloids entailing toxic persistent heterocyclic triazole rings.
The PA releasing butterbur tends to invade moist and disturbed lands like shaded sides of roads, bridge embarkments, the shores of artificial surface water bodies, edges of ditches, channelized streams and water wells. According to the Global Biodiversity Information Facility, the species is omnipresent in most of western and northern Europe, common in the rest of the Europe and occasionally found across North America84. No mitigation operations of these species exist. In northern Europe climate change is leading to an increase in precipitation that together with various land management activities will further stimulate the spread of butterbur, leading to an increased risk of PA contaminated water. The absence of regulations on natural toxins in water has resulted in a lack of suitable methodologies for their monitoring and identification. This study illustrates the need for the advancement of procedures to monitor persistent plant toxins and indicates the need for water cleaning technologies to facilitate their removal.
Methods
Sample collection
Stream water was sampled over a 4-month period from maturation to decay of adjacently growing butterbur. To identify any PA washoff effects, stream sampling events occurred during base flow conditions, shortly after intense precipitation and during drought conditions. In addition, groundwater and seepage water inside groundwater wells were sampled from two wells with adjacently growing butterbur and two wells without. The three water types, the leaves and the rhizomes of the plant were analysed. The concentrations of individual and total PAs were examined and compared.
Butterbur plants were sampled by cutting 3 whole average-height leaves from the ground surface and digging 10–15 cm segments of 3 rhizomes. Each sample was collected approximately 5 m from one another. The sampled biomass was frozen within 8 h at – 20 °C prior to analyses. The stream water was sampled at the surface water monitoring site during the dates listed in Table 2 of the SM. Intense precipitation resulted in a sudden flow increase, whereas dry periods from July to September resulted in no water movement or dry stream bed. Supplementary stream water samples were irregularly taken at 3 additional surface water sampling sites (Fig. 2).
The seepage water was sampled by manually scooping it from the top 50 cm in 1 L bottles with an extension stick. Deep groundwater was sampled using a pump. The samples of all water types were taken in triplicates of 1 L in polypropylene bottles and frozen within 8 h at – 20 °C prior to analyses. To avoid any possible cross-contamination, no plant samples were taken during groundwater sampling events (Table 2 of the SM). A PA-free blank water was sampled, processed and analysed among all surface and groundwater samples as a field control.
Analytical
All plant and water samples were prepared by the method described in Hama and Strobel70. In short, three plants from each sampling point were finely chopped into 2 mm pieces, vigorously mixed, 0.1 g was measured into 10 ml amber glass tubes, 10 ml of MeOH was added and the mixture was sonicated for 15 min. Subsequently, the supernatants were transferred to 50 ml centrifuge tubes. Extraction cycles with additional MeOH and sonication from the remaining plant materials were repeated twice, resulting in a total of 30 ml of supernatant per sample. The supernatants were centrifuged for 10 min at 2100 g and dried under gentle nitrogen flow in a heating block at 40 °C. The dried extracts were dissolved in 1 ml 40% (V/V) MeCN and filtered through 0.2 μm polytetrafluoroethylene (PTFE) membrane filters prior to analysis.
The water samples were passed through a 2.5 μm filter paper (Whatman quantitative-Grade 42) under a vacuum and acidified to a pH of 3.0 with 0.1 mol/L formic acid. Solid phase extraction cartridges Oasis MCX (6 cc, 150 mg 30 µm particle) were conditioned with 5 ml MeOH followed by 5 ml H2O, and then 1.0 L of the acidified water samples were loaded at a flow rate of 10 ml/min. The loaded cartridges were washed with 5 ml 0.065 mmol/L formic acid and successively eluted with 5 ml MeOH and with 10 ml 3:1 (V/V) mixture of A: MeOH and B: 10% ammonia solution. A gentle nitrogen flow dried the eluates in a heating block at 40 °C. The dried extracts were dissolved in 1.0 ml 40% (V/V) MeCN and filtered through a 0.2 μm PTFE membrane prior to analysis.
The PAs were identified and quantified from individual fragmentation patterns with UPLC-MS/MS as described in Hama and Strobel70. All PAs were detected in a single chromatographic run with the mass spectrometer set for multiple reaction monitoring (MRM). The ion traces were obtained for apex retention time (tR) ± 0.15 min. Each MRM mode recorded only one PA by predefined parent and product ions85. In total 30 PAs were monitored based on reported PAs in the literature and from Toxic Plants–Phytotoxins Database83 whereas the application of tR would have also recorded other PAs. However, only 21 PAs were detected in the samples. When an analyte was detectable but not quantifiable its concentration was set equal to its limit of detection (LOD)85.
The instrumental limits of detection and quantification of different PAs were in the range of 2–7 and 5–9 µg/L, respectively. The solid phase extraction provided a 1000 times concentration of the water samples with a 90% recovery rate70,85. Field blanks consisted of laboratory grade deionised water that was brought to the field during each sampling event, sampled, handled, prepared and analysed along with the water samples in order to monitor any possible contamination by the PAs during the sampling and preparation steps. All concentrations of PAs in water blanks were below the limits of detection. The average recovery rate of surrogate (caffeine) was 94% ± 11 (n = 3). The concentrations of all the free base PAs and N-oxides were quantified against certified external standards purchased from Phytolab (Germany).
The concentrations of individual PAs were reported as the average concentrations quantified in triplicate samples. In cases when the individual PAs were quantified in only 2 of the triplicate samples (close to the approximate 5–9 ng/L limit of quantification of the full method), the concentrations of individual PAs were reported as the average of 2 positive samples. If individual PAs were quantified in only 1 of the triplicate samples, the concentrations were not quantified and reported as trace. Total PA concentrations (Fig. 5) were reported as the averages of the sums of all PAs quantified in triplicate samples.
References
Andersen, H. C. Den grimme ælling. In Nye Eventyr. Første Bind. Første Samling (C.A. Reitzels Forlag, 1843).
Brøndegaard, V. J. Folk og flora: dansk etnobotanik (Rosenkilde og Bagger, 1987).
Giles, M. et al. Butterbur. J. Herb. Pharmacother. 5, 119–143 (2009).
Aydin, A. A., Zerbes, V., Parlar, H. & Letzel, T. The medical plant butterbur (Petasites): analytical and physiological (re)view. J. Pharm. Biomed. Anal. 75, 220–229 (2013).
Giversen, I., Brimer, L., & Kristiansen, B. Danmarks Vilde Lægeplanter (Gyldendal A/S, 2014).
Asen, A. Plants of possible monastic origin, growing in the past or present, at medieval monastery grounds in Norway. In Plants and Culture: Seeds of the Cultural Heritage of Europe (ed. Morel, J.P.) 227–238 (Edipuglia, 2009).
Solberg, S. O. More than just weeds. NordGen’s work with Cultural Relict Plants and Bernt Løjtnant’s inventories from Denmark (Nordic Genetic Resource Center, 2014).
Thomet, O. A., Wiesmann, U. N., Blaser, K. & Simon, H. U. Differential inhibition of inflammatory effector functions by petasin, isopetasin and neopetasin in human eosinophils. Clin. Exp. Allergy 31, 1310–1320 (2001).
Sutherland, A. & Sweet, B. V. Butterbur: an alternative therapy for migraine prevention. Am. J. Health Syst. Pharm. 67, 705–711 (2010).
Benemei, S., De Logu, F., Li, Puma, S., Marone, I.M., Coppi, E., Ugolini, F., Liedtke, W., Pollastro, F., Appendino, G., Geppetti, P., Materazzi, S., Nassini, R. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol. 174, 2897–2911 (2017).
Anderson, N. & Borlak, J. Hepatobiliary events in migraine therapy with herbs: the case of petadolex, a petasites hybridus extract. J. Clin. Med. 8, 652 (2019).
Kozlov, V., Lavrenova, G., Savlevich, E. & Bazarkina, K. Evidence-based phytotherapy in allergicrhinitis rhinitis. Clin. Phytosci. 4, 1–8 (2018).
Avula, B., Wang, Y. H., Wang, M., Smillie, T. J. & Khan, I. A. Simultaneous determination of sesquiterpenes and pyrrolizidine alkaloids from the rhizomes of Petasites hybridus (L.) GM et Sch and dietary supplements using UPLC-UV and HPLC-TOF-MS methods. J. Pharm. Biomed. Anal. 70, 53–63 (2012).
Kalin, P. The common butterbur (Petasites hybridus)—portrait of a medicinal herb. Forsch Komplementarmed Klass Naturheilkd 10, 41–44 (2003).
Roberts, M. F. & Wink, M. Alkaloids: Biochemistry, Ecology, and Medicinal Applications (Springer, Berlin, 1998).
Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications 2nd edn. (Elsevier, Hoboken, 2015).
International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Some Traditional Herbal Medicines, Somemycotoxins, Naphthalene and Styrene (IARC Press, Lyon, 2002).
Xia, Q., He, X., Ma, L., Chen, S. & Fu, P. P. Pyrrolizidine alkaloid secondary pyrrolic metabolites construct multiple activation pathways leading to DNA adduct formation and potential liver tumor initiation. Chem. Res. Toxicol. 31, 619–628 (2018).
Yang, L. & Stockigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 27, 1469–1479 (2010).
Wang, T. et al. Pyrrolizidine alkaloids in honey: quantification with and without standards. Food Control 98, 227–237 (2019).
Scholtz, S., MacMorris, L., Krogmann, F. & Auffarth, G. U. Poisons, drugs and medicine: on the use of atropine and scopolamine in medicine and ophthalmology: an historical review of their applications. J. Eye Stud. Treat. 1, 51–58 (2019).
Matolcsy, G., Nadasy, M. & Andriska, V. Pesticide Chemistry 32nd edn. (Elsevier, Hoboken, 1989).
Dembitsky, V. M., Gloriozova, T. A. & Poroikov, V. V. Naturally occurring plant isoquinoline N-oxide alkaloids: their pharmacological and SAR activities. Phytomedicine 22, 183–202 (2015).
Europen Food Safety Authority. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA Journal. https://www.efsa.europa.eu/en/efsajournal/pub/4908 (2017).
European Medicines Agency, Committee on Herbal Medicinal Products. Public Statement on Contamination of Herbal Medicinal Products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids—Transitional Recommendations for Risk Management and Quality Control. https://www.ema.europa.eu/en/documents/public-statement/public-statement-contamination-herbal-medicinal-products/traditional-herbal-medicinal-products-pyrrolizidine-alkaloids_en.pdf (2016).
López-Pacheco, I. Y. et al. Anthropogenic contaminants of high concern: existence in water resources and their adverse effects. Sci. Total Environ. 690, 1068–1088 (2019).
Angeles, L. F. & Aga, D. S. Catching the elusive persistent and mobile organic compounds: novel sample preparation and advanced analytical techniques. Trends Environ. Anal. Chem. 25, e00078 (2020).
Reemtsma, T. et al. Mind the gap: persistent and mobile organic compounds—water contaminants that slip through. Environ. Sci. Technol. 50, 10308–10315 (2016).
Furlong, E. T. et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: pharmaceuticals. Sci. Total Environ. 579, 1629–1642 (2017).
Boxall, A. B. A. The environmental side effects of medication. EMBO Rep. 5, 1110–1116 (2004).
Gao, J. et al. Stability of alcohol and tobacco consumption biomarkers in a real rising main sewer. Water Res. 138, 19–26 (2018).
Lian, L., Yan, S., Yao, B., Chan, S. & Song, W. Photochemical transformation of nicotine in wastewater effluent. Environ. Sci. Technol. 51, 11718–11730 (2017).
Stuart, M., Lapworth, D., Crane, E. & Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 416, 1–21 (2012).
Turner, R. D. R., Warne, M. S., Dawes, L. A., Thompson, K. & Will, G. D. Greywater irrigation as a source of organic micro-pollutants to shallow groundwater and nearby surface water. Sci. Total Environ. 669, 570–578 (2019).
Robertson, J. & Stevens, K. Pyrrolizidine alkaloids. Nat. Prod. Rep. 31, 1721–1788 (2014).
Rosemann, G. M., Botha, C. J. & Eloff, J. N. Distinguishing between toxic and non-toxic pyrrolizidine alkaloids and quantification by liquid chromatography–mass spectrometry. Phytochem. Lett. 8, 126–131 (2014).
European Medicines Agency, Committee on Herbal Medicinal Products. Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs). https://www.ema.europa.eu/en/use-herbal-medicinal-products-containing-toxic-unsaturated-pyrrolizidine-alkaloids-pas (2014).
Prakash, A. S., Pereira, T. N., Reilly, P. E. & Seawright, A. A. Pyrrolizidine alkaloids in human diet. Mutat. Res. 433, 53–67 (1999).
Bolechova, M., Caslavsky, J., Pospichalova, M. & Kosubova, P. UPLC-MS/MS method for determination of selected pyrrolizidine alkaloids in feed. Food Chem. 170, 265–270 (2015).
van Egmond, H. P. Natural toxins: risks, regulations and the analytical situation in Europe. Anal. Bioanal. Chem. 378, 1152–1160 (2004).
Hoogenboom, L. A. P. et al. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. A 28, 359–372 (2011).
Mattocks, A.R. Chemistry and toxicology of pyrrolizidine alkaloids. Academic Pr. (1986).
Molyneux, R. J., Gardner, D. L., Colegate, S. M. & Edgar, J. A. Pyrrolizidine alkaloid toxicity in livestock: a paradigm for human poisoning?. Food Addit. Contam. A 28, 293–307 (2011).
Ebmeyer, J. et al. Human CYP3A4-mediated toxification of the pyrrolizidine alkaloid lasiocarpine. Food Chem. Toxicol. 130, 79–88 (2019).
He, X. et al. Primary and secondary pyrrolic metabolites of pyrrolizidine alkaloids form DNA adducts in human A549 cells. Toxicol. In Vitro 54, 286–294 (2019).
Chen, T., Mei, N. & Fu, P. P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 30, 183–196 (2010).
Huxtable, R. J. Activation and pulmonary toxicity of pyrrolizidine alkaloids. Pharmacol. Therapeut. 47, 371–389 (1990).
McLean, E. K. The toxic actions of pyrrolizidine senecio-D alkaloids. Pharmacol. Ther. 47, 371–389 (1990).
Mori, H. et al. Some toxic properties of a carcinogenic pyrrolizidine alkaloid, petasitenine. J. Toxicol. Sci. 9, 143–149 (1984).
European Food Safety Authority. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 9, 2406 (2011).
Wang, Y. P., Yan, J., Fu, P. P. & Chou, M. W. Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol. Lett. 155, 411–420 (2005).
Yang, M. et al. Intestinal and hepatic biotransformation of pyrrolizidine alkaloid N-oxides to toxic pyrrolizidine alkaloids. Arch. Toxicol. 93, 2197–2209 (2019).
Chou, M. W. et al. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 145, 239–247 (2003).
Xia, Q., Chou, M. W., Kadlubar, F. F., Chan, P. C. & Fu, P. P. Human liver microsomal metabolism and DNA adduct formation of the tumorigenic pyrrolizidine alkaloid, riddelliine. Chem. Res. Toxicol. 16, 66–73 (2003).
Moreira, R., Pereira, D. M., Valentao, P. & Andrade, P. Pyrrolizidine alkaloids: chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 19, 1668 (2018).
Edgar, J. A., Colegate, S. M., Boppre, M. & Molyneux, R. J. Pyrrolizidine alkaloids in food: a spectrum of potential health consequences. Food Addit. Contam. A 28, 308–324 (2011).
International Programme on Chemical Safety (WHO). Pyrrolizidine Alkaloids. Environmental Health Criteria 80 (1988).
European Medicines Agency. Committee on Herbal Medicinal Products (HMPC), EMA/HMPC/893108/2011 Rev. 1. Public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (PAs) including recommendations regarding contamination of herbal medicinal products with pyrrolizidine alkaloids (2020).
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (United Kingdom). COT Statement on Pyrrolizidine Alkaloids in Food (2008).
Chen, L. H. et al. Simultaneous determination and risk assessment of pyrrolizidine alkaloids in Artemisia capillaris Thunb by UPLC-MS/MS together with chemometrics. Molecules 24, 1077 (2019).
The German Federal Institute for Risk Assessment (BfR) (in German: Bundesinstitut für Risikobewertung). Analytik und Toxizität von Pyrrolizidinalkaloiden sowie eine Einschätzung des gesundheitlichen Risikos durch deren Vorkommen in Honig (2013).
The German Federal Institute for Risk Assessment (in German: Bundesinstitut für Risikobewertung). Updated risk assessment on levels of 1,2-unsaturated pyrrolizidine alkaloids (2020).
The German Federal Institute for Risk Assessment (in German: Bundesinstitut für Risikobewertung). Frequently asked questions on pyrrolizidine alkaloids in foods (2020).
Kopp, T., Abdel-Tawab, M. & Mizaikoff, B. Extracting and analyzing pyrrolizidine alkaloids in medicinal plants: a review. Toxins. 12, 320 (2020).
Smith, L. W. & Culvenor, C. C. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 44, 129–152 (1981).
Edgar, J. A., Roeder, E. & Molyneux, R. J. Honey from plants containing pyrrolizidine alkaloids: a potential threat to health. J. Agric. Food Chem. 50, 2719–2730 (2002).
Kempf, M., Reinhard, A. & Beuerle, T. Pyrrolizidine alkaloids (PAs) in honey and pollen-legal regulation of PA levels in food and animal feed required. Mol. Nutr. Food Res. 54, 158–168 (2010).
Dubecke, A., Beckh, G. & Lullmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. A 28, 348–358 (2011).
Gottschalk, C. et al. Spread of Jacobaea vulgaris and occurrence of pyrrolizidine alkaloids in regionally produced honeys from Northern Germany: inter- and intra-site variations and risk assessment for special consumer groups. Toxins 12, 1–19 (2020).
Hama, J. R. & Strobel, B. W. Pyrrolizidine alkaloids quantified in soil and water using UPLC-MS/MS. RSC Adv. 9, 30350–30357 (2019).
Selmar, D. et al. Horizontal natural product transfer: intriguing insights into a newly discovered phenomenon. J. Agric. Food Chem. 67, 8740–8745 (2019).
Gunthardt, B. F. et al. “Is there anybody else out there?”—First insights from a suspect screening for phytotoxins in surface water. Chimia 74, 129–135 (2020).
Schonsee, C. D. & Bucheli, T. D. Experimental Determination of octanol−water partition coefficients of selected natural toxins. J. Chem. Eng. 65, 1946–1953 (2020).
Ellegaard-Jensen, L., Horemans, B., Raes, B., Aamand, J. & Hansen, L. H. Groundwater contamination with 2,6-dichlorobenzamide (BAM) and perspectives for its microbial removal. Appl. Microbiol. Biotechnol. 101, 5235–5245 (2017).
Stuart, M., Lapwortha, D., Cranea, E. & Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 416, 1–21 (2012).
Lapworth, D. J., Baran, N., Stuart, M. E. & Ward, R. S. Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ. Pollut. 163, 287–303 (2012).
Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration.
Bucheli, T. D. Phytotoxins: environmental micropollutants of concern?. Environ. Sci. Technol. 48, 13027–13033 (2014).
Wink, M. & Hartmann, T. Sites of enzymatic synthesis of quinolizidine alkaloids and their accumulation in Lupinus polyphyllus. Zeitschrift für Pflanzenphysiologie 102, 337–344 (1981).
Hama, J. R. & Strobel, B. W. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ. Sci. Eur. 32, 126 (2020).
Anonymous. Solanine poisoning. Br. Med. J. 2, 1458–1459. https://doi.org/10.1136/bmj.2.6203.1458-a (1979).
Aichinger, G., Pantazi, F. & Marko, D. Combinatory estrogenic effects of bisphenol A in mixtures with alternariol and zearalenone in human endometrial cells. Toxicol. Lett. 319, 242–249 (2020).
Gunthardt, B. F., Hollender, J., Hungerbuhler, K., Scheringer, M. & Bucheli, T. D. Comprehensive toxic plants–phytotoxins database and its application in assessing aquatic micropollution potential. J. Agric. Food Chem. 66, 7577–7588 (2018).
Global Biodiversity Information Facility. https://www.gbif.org/species/9490132. Accessed 6th February 2020.
Hama, J.R. & Strobel, B.W. Occurrence of pyrrolizidine alkaloids in ragwort plants, soils and surface waters at the field scale in Grassland. Sci. Total Environ (Article reference:STOTEN_142822) (Accepted). Available online 16 October 2020, 142822 (2020).
Acknowledgements
The authors would like to thank Ann-Katrin Pedersen and Steen Dam Larsen from Greater Copenhagen Utility HOFOR for providing access to groundwater wells and for technical support.
Funding
This project was funded by the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska-Curie Grant Agreement No. 722493.
Author information
Authors and Affiliations
Contributions
V.K. and L.H.R. conceived and directed the study, V.K. and N.S. performed sampling, V.K. and J.R.H. performed analysis, H.C.B.H and B.W.S supervised, V.K. wrote the paper with feedback from L.H.R., J.R.H., H.C.B.H and B.W.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kisielius, V., Hama, J.R., Skrbic, N. et al. The invasive butterbur contaminates stream and seepage water in groundwater wells with toxic pyrrolizidine alkaloids. Sci Rep 10, 19784 (2020). https://doi.org/10.1038/s41598-020-76586-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-76586-1
This article is cited by
-
Allelochemical run-off from the invasive terrestrial plant Impatiens glandulifera decreases defensibility in Daphnia
Scientific Reports (2023)
-
Natural toxins: environmental contaminants calling for attention
Environmental Sciences Europe (2021)