Abstract
Noninvasive ventilation (NIV) is beneficial in acute respiratory failure (ARF) caused by chest trauma; however, NIV-related complications affect the efficacy. We evaluated whether NIV with helmet decreases the incidence of complications and improves its effects in a single center. Patients with ARF after chest trauma were randomized to receive NIV with helmet or face mask. The primary outcome was the rate of NIV-related complications. Secondary outcomes were PaO2/FiO2, patient’s tolerance, intubation rate, length of intensive care unit (ICU) stay, and ICU mortality. The trial was terminated early after an interim analysis with 59 patients. The incidence of complications was lower in the helmet group [10% (3/29) vs 43% (13/30), P = 0.004], and PaO2/FiO2s were higher at 1 h and at the end of NIV (253.14 ± 64.74 mmHg vs 216.06 ± 43.86 mmHg, 277.07 ± 84.89 mmHg vs 225.81 ± 63.64 mmHg, P = 0.013 and 0.012) compared with them in face mask group. More patients reported excellent tolerance of the helmet vs face mask after 4 h of NIV [83% (24/29) vs 47% (14/30), P = 0.004] and at the end of NIV [69% (20/29) vs 30% (9/30), P = 0.03]. Differences in intubation rate, ICU stay, and mortality were non-significant (P = 0.612, 0.100, 1.000, respectively). NIV with helmet decreased NIV-related complications, increased PaO2/FiO2, and improved tolerance compared with NIV with face mask in patients with chest trauma.
Trial registration: Registered in the Chinese Clinical Trial Registry (ChiCTR1900025915), a WHO International Clinical Trials Registry Platform (http://www.chictr.org.cn/searchprojen.aspx).
Similar content being viewed by others
Introduction
Chest injury is common in patients with trauma1 and accounts for 25–40% of all trauma-related fatalities2,3. Acute respiratory failure (ARF) caused by pulmonary contusion, rib fractures, pneumothorax, and hemothorax occur frequently, despite the use of oxygen therapy and regional analgesia4,5,6. Post-traumatic ARF within 72 h is associated with a high mortality rate7,8, and as a result, patients require rapid and efficient ventilatory management. Endotracheal intubation and invasive ventilation is performed in 23–75% of patients with chest trauma9; however, these procedures are associated with increased complications, and prolonged ventilation and hospitalization times9,10,11,12.
Recent studies demonstrated that patients with ARF after chest trauma responded favorably to noninvasive ventilation (NIV)13,14,15,16, which could reduce the need for intubation, the incidence of pneumonia, and mortality rates10,17,18. Nonetheless, complications and discomfort associated with NIV delivered via a face mask are common and result in NIV failure rates of up to 14.8% in patients with chest trauma19.
Chest trauma itself causes pain and patient irritability, and may be associated with facial injuries, which interfere with the patient’s ability to cooperate with NIV with a mask. A recently-developed helmet has been used effectively to deliver NIV in select patients20,21,22,23. The helmet does not touch the patient’s face, and allows patients to eat and talk, which improves tolerance and prevents skin lesions22. We hypothesized that NIV with the helmet might reduce NIV-related complications, be easier to tolerate (especially for patients with mild facial injuries), and improve the effects of NIV in patients with chest trauma. However, to date, there are no data describing this important issue; therefore, we performed a randomized controlled trial (RCT) to investigate our hypothesis.
Methods
This single-center trial was registered in the Chinese Clinical Trial Registry (ChiCTR1900025915, 14/09/2019) and was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No: SS-2019-002). This teaching hospital has 6500 beds and a provincial trauma center with 20 intensive care beds for patients with severe trauma. All patient management was performed in accordance with relevant guidelines and regulations15,24,25. Consecutive patients admitted to the emergency intensive care unit (ICU) of this hospital from 1 September 2018 to 1 July 2019 were screened according to the eligibility criteria. Informed consent was obtained from all included patients or their relatives.
Participants
The inclusion criteria were as follows: (1) Older than 18 years; (2) within the first 72 h after chest trauma; (3) trauma confirmed by imaging; (4) moderate to severe hypoxemic respiratory failure, which was defined as the ratio of the partial pressure of oxygen in arterial blood to the fraction of inspired oxygen (PaO2/FiO2) < 200 mmHg while receiving standard oxygen therapy with an oxygen flow rate ≥ 10 L/min via a face mask at least for 15 min; and (5) informed consent signed by the patient or a close relative.
Patients meeting any of the following criteria were excluded: (1) impending cardiopulmonary arrest or the need for emergency intubation; (2) unable to accept NIV treatment because of decreased consciousness (Glasgow coma scale score ≤ 11)26,27,28; (3) contraindications for NIV (active gastrointestinal bleeding, upper airway obstruction, or severe hemodynamic instability); (4) severe facial trauma with pneumocephalus or involving a sinus, skull base fracture, or orbital fracture; (5) cervical injury; (6) increased intracranial pressure; and (7) declined to provide signed informed consent.
Randomization and masking
We randomized patients in a 1:1 ratio using a computer random number generator. The random numbers were generated and secured in sequentially numbered, opaque, sealed envelopes kept by the head nurse. After determining that a potential research participant was eligible for inclusion according to the inclusion and exclusion criteria, the researchers obtained a sealed envelope from the head nurse, and the patient randomly received a helmet (odd number) or a face mask (even number) according to the disclosed random number. Data were recorded in the medical record system and the pre-piloted forms by the nurses and therapist who were blinded to the randomization. The study investigators collected the data, and the nurses and related medical staff managed patients without discrimination.
Basic treatment protocol
Basic treatment constituted regional analgesia (mainly epidural analgesia with fentanyl plus bupivacaine) unless contraindicated. The efficacy of analgesia was measured using a visual analog scale. Contraindications for regional analgesia were skin injury or infection at the puncture site, or coagulopathy. When epidural administration was impossible, patient-controlled intravenous analgesia was used. Both groups were treated with similar fluid management protocols, and patients’ respiratory and clinical status were monitored during NIV.
Ventilation protocol
Patients in both groups received noninvasive ventilation (Servo-I; Maquet, Rastatt, Germany) in the pressure support (PS) mode. Patients in the intervention group received NIV with a suitably-sized helmet (dual-connector for breathing circuits) (CaStar; StarMed, Mirandola, Italy), which was made of transparent latex-free polyvinyl chloride and which was secured via two armpit braces to four hooks on a plastic ring. A helmet without armpit braces, with an annular openable ring placed underneath an inflatable cushion, could be used for patients with rib fractures, if necessary. Patients in the control group received NIV with a suitably-sized air-filled oronasal face mask (Tuoren Medical Instrument Co., Xinxiang, China) to ensure a tight and comfortable seal. PS was initially set at 8 cm H2O, positive end-expiratory pressure at 5 cm H2O, and FiO2 at 40%. According to the patient’s clinical symptoms and their percutaneous blood oxygen saturation (SpO2), NIV supports were sequentially increased in 1–2-cm H2O increments. If respiratory distress and SpO2 did not improve, FiO2 was progressively increased in 5% increments to achieve an SpO2 > 92%. SpO2 monitoring was performed strictly according to the equipment manufacturer’s instructions to minimize the impact of factors, such as movement of the patient’s finger to which the monitor was applied or the presence of nail polish, on the accuracy of SpO2. In cases of discrepancy between the SpO2 reading and a patient’s clinical presentation, arterial blood gas analysis was performed for further confirmation. Short interval disconnections to increase the patient’s tolerance or to clear respiratory secretions were allowed.
Weaning protocol
NIV support and FiO2 were reduced gradually in 1–2 cm H2O increments and 5% increments, respectively, if necessary. Weaning from NIV was considered possible when the patient felt dyspnea relief, SpO2 was > 92%, PS was decreased to < 8 cm H2O, and FiO2 was set at 40% with stable hemodynamics.
Criteria for endotracheal intubation
Patients meeting any of the following criteria were intubated immediately: (1) breathing too weak to trigger the ventilator, or cardiac arrest; (2) severely altered level of consciousness making patients unable to cooperate with NIV; (3) severe hemodynamic instability (defined as mean arterial pressure < 60 mmHg and no response to vasoactive agents); (4) excessive sputum beyond the patient’s expectoration capacity; (5) refractory hypoxemia (SpO2 < 85% despite a high oxygen fraction); and (6) unable to tolerate the helmet or face mask. The final decision to intubate was made by consensus among all physicians, excluding the investigators.
Outcomes
The primary outcome was the rate of complications related to NIV during the ICU stay, which was defined as the ratio of patients who developed any complication to the total number of patients. NIV-related complications included claustrophobia, skin lesions, severe air leakage, eye irritation, gastric distension, and poor tolerance, which were evaluated by nurses and the therapist as part of the daily routine care, and who were blinded to the randomization. Complications were evaluated at 1 h, 4 h, and at the end of NIV, or recorded directly from patients at any time after starting NIV. The secondary outcomes were PaO2/FiO2, patient tolerance, respiratory rate (evaluated at 1 h, 4 h, and at the end of NIV), intubation rate, duration of NIV, length of ICU stay, and ICU mortality (evaluated at ICU discharge). Skin lesions were defined as a score of at least 1 using the following scale: 0: no lesions, 1: area of redness, 2: moderate skin breakdown, 3: skin ulcer, and 4: skin necrosis, according to a previous study29. As air leakage affected the triggering, breathing cycle, and synchrony between the patient and the ventilator, in cases of air leakage, we adjusted the interface and fixation, replaced the helmet or face mask with the appropriate size of interface, and changed the trigger sensitivity. If air leaks still caused an unsuitable PS flow cycle, we changed to noninvasive pressure-controlled ventilation, which we considered to indicate severe air leakage30,31,32. Eye irritation was defined as excessive eye secretions, conjunctival congestion and edema, and any damage to the cornea or iris caused by the interface. Patients’ tolerance was recorded at planned observation time points after beginning the study, and tolerance was evaluated using the following patient tolerance scale: poor, patients try to remove the mask or helmet; moderate, ventilation with a face mask or helmet is successful with suggestions for the patient; good, face mask or helmet use is slightly uncomfortable for the patients, but they want to use it; excellent, complete tolerance21. Nurses evaluated complications related only to skin care; clinical indicators such as ICU stay and patients’ tolerance were assessed by doctors and the investigators.
Statistical analysis
We calculated the sample size based on the primary outcome of the pre-experiment in 16 patients with chest trauma-related ARF. With an expected incidence of complications of 37.5% in the face mask group and 12.5% in the helmet group with 80% power and a two-sided α of 0.05, the required sample size was 88 patients (44 in each group) using PASS 11.0 software (NCSS, Kaysville, UT). An interim analysis was performed at the halfway point in the planned 20-month duration of the study (from 1 September 2018 to 1 May 2020). The predefined NIV discontinuation determinant was a significant (P < 0.005) difference in the incidence of complications between the two groups in the interim analysis according to the O'Brien–Fleming method33.
We reported our results in accordance with the CONSORT guidelines34. All pre-specified analyses were performed by an intention-to-treat analysis. For continuous outcomes, data with a normal distribution were presented as mean ± standard deviation (SD), and these data were analyzed with an independent samples t test; otherwise, the data were reported as median [interquartile range (IQR)], and data were analyzed with the Mann–Whitney U test. For categorical variables, the outcomes were compared using the Chi square or Fisher’s exact test. The time courses for arterial blood gas variables (PaO2/FiO2, partial pressure of arterial carbon dioxide, and pH) and respiratory rates were compared using two-way analysis of variance for repeated measures within both groups. The level of significance was set at 0.05, and all analyses were performed using SPSS statistical software version 22.0 (IBM Corp., Armonk, NY).
Results
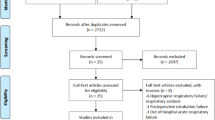
From September 2018 to July 2019, 142 patients were screened. After recruiting 59 patients, the lower incidence rate of complications in the helmet group met the early termination criteria for the study compared with the face mask group (P = 0.004) in the planned interim analysis after a study duration of 10 months on 1 July 2019 (half of the planned duration of the trial). We finally randomized 29 patients to the helmet group and 30 patients to the face mask group (Fig. 1). All patients completed the study, and their baseline characteristics are shown in Table 1.
Flow chart of inclusion participants. At the planned time of interim analysis, and the patients included were 29 in helmet group versus 30 in face mask group with P = 0.004, which met the early termination criterion (P < 0.005) and introduced the sample size in helmet group is an odd number and face mask group is an even number.
Effect of NIV with the helmet on the complications rate
The incidence rate of complications related to NIV was lower in the helmet group than in the face mask group [10% (3/29) vs 43% (13/30), respectively; P = 0.004] (Table 2). Complications in the helmet group were claustrophobia in two patients and neck skin redness in one patient. In the face mask group, complications were facial skin lesions, air leakage, eye irritation, and gastric distension in 13 patients. None of the patients with facial trauma developed complications in the helmet group, whereas one patient developed complications in the face mask group.
Effect of NIV with the helmet on secondary outcomes
PaO2/FiO2s were higher in the helmet group at 1 h, and at the end of NIV treatment compared with those in the face mask group (253.14 ± 64.74 mmHg vs 216.06 ± 43.86 mmHg, and 277.07 ± 84.89 mmHg vs 225.81 ± 63.64 mmHg, P = 0.013 and 0.012, respectively). Patients’ respiratory rates decreased obviously at 1 h, 4 h, and the end of NIV treatment in both groups, and the differences between the groups were statistically significant (18.10 ± 4.13 breaths/min vs 20.33 ± 2.54 breaths/min; 17.69 ± 3.09 breaths/min vs 20.07 ± 2.86 breaths/min; and 17.10 ± 2.97 breaths/minvs 19.30 ± 2.04 breaths/min; P = 0.016, P = 0.003, and P = 0.002, respectively). There were no significant effects on pH and partial pressure of arterial carbon dioxide (P > 0.05) (Table 3).
Patients tolerated the helmet better than the face mask at 4 h (proportion of excellent tolerance: 83% (24/29) vs 47% (14/30), respectively; P = 0.004) and at the end of NIV treatment (proportion of excellent tolerance: 69% (20/29) vs 30% (9/30), respectively; P = 0.03); the difference at 1 h was not significant (P > 0.05) (Table 4). One patient in the face mask group had to remove the mask and stopped NIV because of poor tolerance.
The rate of intubation was 3% (1/29) in the helmet group, which was slightly lower than the rate in the face mask group [3/30 (10%); P = 0.612]. There was no significant difference in the duration of NIV between the helmet and face mask group, respectively [median (IQR), 6 (4–12) h vs 6 (4–13) h; P = 0.802], or in the length of ICU stay [median (IQR), 7 (5–8) days vs 8 (6–10) days; P = 0.100] or ICU mortality between the two groups [3% (1/29) vs 3% (1/30); P = 1.000)] (Table 2).
Discussion
The results indicated that NIV with a helmet decreased complications related to NIV, increased oxygenation, decreased respiratory rate, and improved tolerance compared with NIV with a face mask in patients with chest trauma, while there was no evidence indicating that NIV with a helmet shortened ICU stay, and reduced intubation rates and ICU mortality.
In the past several decades, invasive mechanical ventilation was the prioritized support to improve gas exchange and facilitate chest stabilization in patients with chest trauma-related ARF35. However, complications, such as ventilator-associated pneumonia and barotrauma, were associated with prolonged ventilation and even led to higher mortality rates10,17,36. With the introduction of NIV, Antonelli et al. reported similar effects regarding improved gas exchange and fewer complications compared with invasive mechanical ventilation in a mixed population including 12% of patients with trauma17. Subsequently, studies indicated that early use of NIV promoted lung recruitment and reduced intubation and mortality rates3,11,12,37. However, complications related to NIV were still common and most were associated with using a face mask. The complication accounted for as high as 14.8% of NIV failure and caused a three-fold increase in hospital mortality19. Recently, a new NIV interface, a helmet, has been used to reduce complications related to NIV and improve tolerance in patients with ARF caused by severe pneumonia, exacerbation of chronic obstructive pulmonary disease, and acute cardiogenic pulmonary edema22,38. However, the benefits of NIV with a helmet were uncertain in patients with ARF caused by chest trauma.
This trial indicated that using the helmet significantly reduced the incidence of complications related to NIV. Skin lesions are a frequent problem with long-term NIV with face masks and have even led to NIV failure13,22. The helmet is made of a transparent plastic hood, which does not touch the patient’s face, nose bridge, or eyes, and in our study, the inflatable collar of the helmet caused redness of the neck skin in only one patient and prevented eye irritation38. None of the three patients with mild facial trauma in the helmet group developed complications. Four patients in the control group developed facial and nasal bridge skin lesions. As a result, the helmet was a good choice of interface, especially for patients with mild facial trauma31. Furthermore, the soft collar in the helmet is designed to seal the neck and minimize air leaks. In this study, the helmet was associated with fewer air leaks and better comfort than the face mask. Eye irritation resulted from direct contact between the upper edge of the oronasal mask and air leakage around the nasal bridge. For patients with clavicular or rib fractures, we selected the helmet without armpit braces, which further improved comfort and reduced the incidence of complications. However, claustrophobia occurred in the helmet group, which might have originated from the completely closed structure. The closed structure interfered mildly with the transmission of sound and may have caused claustrophobia, especially in those who were easily anxious, as can be seen with trauma patients in a stressed state. Claustrophobia could be mitigated by proactive communication from medical staff. It is necessary to explore whether wearing an in-ear earphone could reduce the incidence of claustrophobia, in future research.
In this trial, NIV with a helmet improved PaO2/FiO2 and decreased respiratory rate compared with NIV with a face mask, suggesting that the helmet was effective in relieving dyspnea. This might be attributed to the unique advantages of the helmet. First, the helmet did not require interrupting NIV treatment when patients drank, communicated, and cleared sputum31. Continuous NIV is crucial in the early phases of respiratory insufficiency and might reduce the need for intubation39. Second, the helmet was better tolerated in patients with chest trauma; therefore, patients did not require conversion to an invasive ventilator31. Our results showed that 83% of the patients in the helmet group showed excellent tolerance compared with 47% of the patients in the face mask group after 4 h of NIV treatment. Moreover, the helmet could be fit to any patient regardless of differences in their facial contour, such as edentulous teeth, facial deformity, or trauma22,40. Overall, patients with chest trauma-related ARF responded favorably to NIV with a helmet because of the comfort, and better tolerance and oxygenation; thus, helmet use might broaden the use of NIV.
In this trial, the incidence of complications in the face mask group was higher than that reported in other patient populations22,31,40. Currently, there are limited data describing complications related to NIV in patients with chest trauma. The only study of patients with thoracic trauma involved only 16% of the recruited patients, and reported a complication rate related to face masks of 33%26. The higher rate of complications in our trial might be attributed to pain caused by trauma, and stress and traumatic psychological reactions of patients with trauma, which decreased patients’ ability to tolerate the mask39.
The duration of noninvasive ventilation was relatively short in this study, although the duration was similar to a few related studies of NIV with a helmet21,23. This lower duration is because our hospital is located in an industrial area where many highways converge and construction sites abound, and which leads to multiple types of accidents. However, patients could be admitted to the hospital in the early stages of trauma. Early application of NIV alleviated pulmonary edema and prevented its development, and shortened the duration of ventilatory support18,41. This study serves to broaden the indications for helmet use and provides a new option for patients with chest trauma in addition to NIV with a face mask37 and high-flow nasal cannula therapy42,43.
Our study had several limitations. First, given the nature of the interface, the attending physicians could not be blinded during the analysis, and this may have introduced performance bias22. The helmet is a new interface, which may have affected medical staff regarding judging clinical outcomes despite the fact that we tried to keep the staff blind and to have the patients managed without discrimination. Second, the limited sample size does not present enough evidence for helmet use to reduce NIV failure and intubation rates, shorten the length of ICU stay, and decrease ICU mortality. Caution is needed regarding evaluating the clinical benefits of a helmet except for the lower incidence of complications, higher PaO2/FiO2, and better tolerance; large RCTs aiming to determine the long-term prognosis are needed. Third, to reduce invasive procedures in patients with trauma, we did not introduce extravascular lung water monitoring techniques, which somewhat limited the demonstration of pulmonary edema and quantitative measurement of the pathophysiological changes; however, these changes can be partly qualitatively judged with lung computed tomography imaging. Fourth, this study was terminated before reaching the planned sample size because of the prespecified interim analysis, which might have led to potential bias and exaggerated findings, particularly for the small number of events44. However, another study considered that overestimation was acceptable if the proportion of events in the interim analysis was > 50%, as in this study45.
Conclusions
NIV with a helmet decreased complications related to NIV, increased PaO2/FiO2, and improved tolerance compared with NIV with a face mask in patients with chest trauma. The new helmet may be a valid and optional interface for NIV in patients with chest trauma-related ARF.
Data availability
The data that underlie the findings of this trial are available from the corresponding authors, Q.L. or R.C.C., upon reasonable request.
Abbreviations
- NIV:
-
Noninvasive ventilation
- ARF:
-
Acute respiratory failure
- RCT:
-
Randomized controlled trial
- PaO2/FiO2 :
-
Arterial partial pressure of oxygen/fraction of inspired oxygen
- ICU:
-
Intensive care unit
- PS:
-
Pressure support
- SpO2 :
-
Saturation of pulse oxygen
- RR:
-
Respiratory rate
References
Vidhani, K., Kause, J. & Parr, M. Should we follow ATLS guidelines for the management of traumatic pulmonary contusion: The role of non-invasive ventilatory support. Resuscitation 52, 265–268. https://doi.org/10.1016/s0300-9572(01)00475-0 (2002).
Alisha, C., Gajanan, G. & Jyothi, H. Risk factors affecting the prognosis in patients with pulmonary contusion following chest trauma. J. Clin. Diagn. Res. 9, 17–19. https://doi.org/10.7860/JCDR/2015/13285.6375 (2015).
Schreiber, A. et al. Non-invasive mechanical ventilation in critically ill trauma patients: A systematic review. Turk. J. Anaesthesiol. Reanim. 46, 88–95. https://doi.org/10.5152/TJAR.2018.46762 (2018).
Wanek, S. & Mayberry, J. C. Blunt thoracic trauma: Flail chest, pulmonary contusion, and blast injury. Crit. Care Clin. 20, 71–81. https://doi.org/10.1016/s0749-0704(03)00098-8 (2004).
Ziegler, D. W. & Agarwal, N. N. The morbidity and mortality of rib fractures. J. Trauma 37, 975–979. https://doi.org/10.1097/00005373-199412000-00018 (1994).
Požgain, Z. et al. Pulmonary contusions after blunt chest trauma: Clinical significance and evaluation of patient management. Eur. J. Trauma Emerg. Surg. 44, 773–777. https://doi.org/10.1007/s00068-017-0876-5 (2018).
Martin, M. et al. The decreasing incidence and mortality of acute respiratory distress syndrome after injury: A 5-year observational study. J. Trauma 59, 1107–1113. https://doi.org/10.1097/01.ta.0000188633.94766.d0 (2005).
DuBose, J. J. et al. The relationship between post-traumatic ventilator-associated pneumonia outcomes and American College of Surgeons trauma centre designation. Injury 42, 40–43. https://doi.org/10.1016/j.injury.2009.08.026 (2011).
Tyburski, J. G., Collinge, J. D., Wilson, R. F. & Eachempati, S. R. Pulmonary contusions: Quantifying the lesions on chest X-ray films and the factors affecting prognosis. J. Trauma 46, 833–838. https://doi.org/10.1097/00005373-199905000-00011 (1999).
Gunduz, M., Unlugenc, H., Ozalevli, M., Inanoglu, K. & Akman, H. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg. Med. J. 22, 325–329. https://doi.org/10.1136/emj.2004.019786 (2005).
Mishra, S. P., Mishra, M., Bano, N. & Hakim, M. Z. Management of traumatic flail chest in intensive care unit: An experience from trauma center ICU. Saudi J. Anaesth. 13, 179–183. https://doi.org/10.4103/sja.SJA_699_18 (2019).
Hernandez, G. et al. Noninvasive ventilation reduces intubation in chest trauma-related hypoxemia: A randomized clinical trial. Chest 137, 74–80. https://doi.org/10.1378/chest.09-1114 (2010).
Antonelli, M. et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 27, 1718–1728. https://doi.org/10.1007/s00134-001-1114-4 (2001).
Non-invasive ventilation in acute respiratory failure. Thorax 57, 192–211. https://doi.org/10.1136/thorax.57.3.192 (2002).
Rochwerg, B. et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 50(2), 1602426. https://doi.org/10.1183/13993003.02426-2016 (2017).
Udekwu, P., Patel, S., Farrell, M. & Vincent, R. Favorable outcomes in blunt chest injury with noninvasive bi-level positive airway pressure ventilation. Am. Surg. 83, 687–695 (2017).
Antonelli, M. et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N. Engl. J. Med. 339, 429–435. https://doi.org/10.1056/NEJM199808133390703 (1998).
Duggal, A., Perez, P., Golan, E., Tremblay, L. & Sinuff, T. Safety and efficacy of noninvasive ventilation in patients with blunt chest trauma: A systematic review. Crit. Care 17, R142. https://doi.org/10.1186/cc12821 (2013).
Lemyze, M. et al. Rescue therapy by switching to total face mask after failure of face mask-delivered noninvasive ventilation in do-not-intubate patients in acute respiratory failure. Crit. Care Med. 41, 481–488. https://doi.org/10.1097/CCM.0b013e31826ab4af (2013).
Pisani, L. et al. Oronasal mask versus helmet in acute hypercapnic respiratory failure. Eur. Respir. J. 45, 691–699. https://doi.org/10.1183/09031936.00053814 (2015).
Özlem, Ç. G., Ali, A., Fatma, U., Mehtap, T. & Şaziye, Ş. Comparison of helmet and facial mask during noninvasive ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease: A randomized controlled study. Turk. J. Med. Sci. 45, 600–606. https://doi.org/10.3906/sag-1401-109 (2015).
Liu, Q., Gao, Y., Chen, R. & Cheng, Z. Noninvasive ventilation with helmet versus control strategy in patients with acute respiratory failure: A systematic review and meta-analysis of controlled studies. Crit. Care 20, 265. https://doi.org/10.1186/s13054-016-1449-4 (2016).
Patel, B. K., Wolfe, K. S., Pohlman, A. S., Hall, J. B. & Kress, J. P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 315, 2435–2441. https://doi.org/10.1001/jama.2016.6338 (2016).
Simon, B. et al. Management of pulmonary contusion and flail chest: An Eastern Association for the Surgery of Trauma practice management guideline. J. Trauma Acute Care Surg. 73, S351-361. https://doi.org/10.1097/TA.0b013e31827019fd (2012).
Galvagno, S. M. et al. Pain management for blunt thoracic trauma: A joint practice management guideline from the Eastern Association for the Surgery of Trauma and Trauma Anesthesiology Society. J. Trauma Acute Care Surg. 81, 936–951. https://doi.org/10.1097/TA.0000000000001209 (2016).
Ferrer, M. et al. Noninvasive ventilation in severe hypoxemic respiratory failure: A randomized clinical trial. Am. J. Respir. Crit. Care Med. 168, 1438–1444. https://doi.org/10.1164/rccm.200301-072OC (2003).
Xirouchaki, N. et al. Noninvasive bilevel positive pressure ventilation in patients with blunt thoracic trauma. Respiration 72, 517–522. https://doi.org/10.1159/000086501 (2005).
Kogo, M. et al. What is the impact of mildly altered consciousness on acute hypoxemic respiratory failure with non-invasive ventilation. Intern. Med. 57, 1689–1695. https://doi.org/10.2169/internalmedicine.9355-17 (2018).
Gregoretti, C. et al. Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: A multi-center study. Intensive Care Med. 28, 278–284. https://doi.org/10.1007/s00134-002-1208-7 (2002).
Schönhofer, B. & Sortor-Leger, S. Equipment needs for noninvasive mechanical ventilation. Eur. Respir. J. 20, 1029–1036. https://doi.org/10.1097/00003246-200203000-00019 (2002).
Antonelli, M. et al. New treatment of acute hypoxemic respiratory failure: Noninvasive pressure support ventilation delivered by helmet—A pilot controlled trial. Crit. Care Med. 30, 602–608 (2002).
Girault, C. et al. Interface strategy during noninvasive positive pressure ventilation for hypercapnic acute respiratory failure. Crit. Care Med. 37, 124–131. https://doi.org/10.1097/CCM.0b013e3181928706 (2009).
Chin, R., Lee, B.Y. Principles and practice of clinical trial medicine, analysis of data chapter. 15, 325–359. (2008).
Moher, D. et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 340, c869. https://doi.org/10.1136/bmj.c869 (2010).
Christensson, P., Gisselsson, L., Lecerof, H., Malm, A. J. & Ohlsson, N. M. Early and late results of controlled ventilation in flail chest. Chest 75, 456–460. https://doi.org/10.1378/chest.75.4.456 (1979).
Nava, S. & Hill, N. Non-invasive ventilation in acute respiratory failure. Lancet 374, 250–259. https://doi.org/10.1016/S0140-6736(09)60496-7 (2009).
Chiumello, D., Coppola, S., Froio, S., Gregoretti, C. & Consonni, D. Noninvasive ventilation in chest trauma: Systematic review and meta-analysis. Intensive Care Med. 39, 1171–1180. https://doi.org/10.1007/s00134-013-2901-4 (2013).
Liu, Q., Chen, R. C. & Cheng, Z. The advances of noninvasive ventilation with helmet. Zhonghua Jie He He Hu Xi Za Zhi 39, 723–726. https://doi.org/10.3760/cma.j.issn.1001-0939.2016.09.014 (2016).
Duggal, A., Sinuff, T. Noninvasive ventilation in patients with blunt chest trauma: What have we learned? Key response determinants and practical implications. In Noninvasive Mechanical Ventilation. (ed. Esquinas, A.) (Springer, Cham, 2016). https://doi.org/10.1007/978-3-319-21653-9_52.
Antonelli, M. et al. Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: A feasibility study. Anesthesiology 100, 16–24. https://doi.org/10.1097/00000542-200401000-00007 (2004).
Parry, N. G., Moffat, B. & Vogt, K. Blunt thoracic trauma: Recent advances and outstanding questions. Curr. Opin. Crit. Care 21, 544–548. https://doi.org/10.1097/MCC.0000000000000251 (2015).
Mu, G. H. et al. High-flow nasal cannula therapy for acute respiratory failure in patients with chest trauma: A single-center retrospective study. Injury https://doi.org/10.1016/j.injury.2020.07.064 (2020).
Halub, M. E. et al. High-flow nasal cannula therapy for patients with blunt thoracic injury: A retrospective study. Can. J. Respir. Ther. 52, 110–113 (2016).
Montori, V. M. et al. Randomized trials stopped early for benefit: A systematic review. JAMA 294, 2203–2209. https://doi.org/10.1001/jama.294.17.2203 (2005).
Shimura, M. Reducing overestimation of the treatment effect by interim analysis when designing clinical trials. J. Clin. Pharm. Ther. 44, 243–248. https://doi.org/10.1111/jcpt.12777 (2019).
Acknowledgements
We thank the doctors and nurses for the work in the research. We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 81400051) and the Key Project of Henan Higher Education (Grant number 19A320073).
Author information
Authors and Affiliations
Contributions
Q.L. conceived and designed the study, drafted the manuscript, explained the results and revised the manuscript. M.T.S. conducted the study, collected and analyzed the data. Drafted the manuscript, H.L.Z. and J.L.C. conducted the study, collected and analyzed the data. R.C.C. explained the results and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Q., Shan, M., Zhu, H. et al. Noninvasive ventilation with a helmet in patients with acute respiratory failure caused by chest trauma: a randomized controlled trial. Sci Rep 10, 21489 (2020). https://doi.org/10.1038/s41598-020-78607-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-78607-5
This article is cited by
-
Monitoring patients with acute respiratory failure during non-invasive respiratory support to minimize harm and identify treatment failure
Critical Care (2025)
-
A pilot crossover trial assessing the exercise performance patients with chronic obstructive pulmonary disease
Scientific Reports (2022)
-
The use of head helmets to deliver noninvasive ventilatory support: a comprehensive review of technical aspects and clinical findings
Critical Care (2021)