Abstract
The Brazilian Northern region registered a high incidence of COVID-19 cases, particularly in the state of Pará. The present study investigated the risk factors associated with the severity of COVID-19 in a Brazilian Amazon region of 100,819 cases. An epidemiological, cross-sectional, analytical and demographic study, analyzing data on confirmed cases for COVID-19 available at the Brazilian Ministry of Health's surveillance platform, was conducted. Variables such as, municipalities of residence, age, gender, signs and symptoms, comorbidities were included and associated with COVID-19 cases and outcomes. The spatial distribution was performed using the ArcGIS program. A total of 100,819 cases were evaluated. Overall, patients had the mean age of 42.3 years, were female (51.2%) and with lethality reaching 4.79% of cases. Main symptoms included fever (66.5%), cough (61.9%) and sore throat (39.8%). Regarding comorbidities, most of the patients presented cardiovascular disease (5.1%) and diabetes (4.2%). Neurological disease increased risk of death by nearly 15 times, followed by obesity (5.16 times) and immunodeficiency (5.09 time). The municipalities with the highest incidence rate were Parauapebas, Canaã dos Carajás and Jacareacanga. Similarity between the Lower Amazon, Marajó and Southwest mesoregions of Pará state were observed concerning the highest morbidity rates. The obtained data demonstrated that the majority of cases occurred among young adults, females, with the classic influenza symptoms and chronic diseases. Finally, data suggest that the highest incidences were no longer in the metropolitan region of the state. The higher lethality rate than in Brazil may be associated with the greater impacts of the disease in this Amazonian population, or factors associated with fragile epidemiological surveillance in the notification of cases of cure.
Similar content being viewed by others
Introduction
The Severe Acute Respiratory Syndrome (SARS) caused by coronavirus-2 (SARS-CoV-2) is an airborne and highly contagious infection, characterized by flu-like symptoms to severe respiratory symptoms, called COVID-19. After a rapidly spread worldwide, the SARS-CoV-2 pandemic has been considered a global emergency due to the significant social-economic and lives losses1, 2.
In average 5 days after exposure to the virus, individuals may go asymptomatic or present a wide range of symptoms, including mild to fulminant clinical manifestations, such as fever, dry cough, shortness of breath, pneumonia, muscle disorder characterized by myalgia and Guillain–Barré syndrome3. The diagnosis of COVID-19 is usually directly performed through detection of SARS-CoV-2 RNA by reverse-transcription polymerase chain reaction (RT-PCR), or indirectly by means of serologic tests. In addition, clinical abnormalities observed in computed tomography (CT) and laboratory tests, including ground-glass opacities, lymphopenia, elevated lactate dehydrogenase and alteration of the d-dimer may support diagnosis of individuals with a high clinical suspicion of infection4.
Mortality on COVID-19 setting is primarily associated with clinical complications, where specific groups more likely to evolve to severe cases, including elderly, pregnant women and those with chronic diseases; and often requiring intensive care, mechanical ventilation, use of antibiotics for secondary infections treatment and invasive maneuvers5,6,7. Deaths worldwide associated with SARS-CoV-2 reached the number of 2,244,213 by February, 2021, of which 1,062,191 registered in the Americas, followed by 759,122 in the European region. In the Americas, the United States and Brazil accounts for the highest number of deaths8. In Brazil, community transmission by SARS-CoV-2 was declared on March, 2020, and until February, 2021, 8,447,645 cases and 232,170 deaths were confirmed, with a lethality rate of 2.4%9. Among the Brazilian regions, the Northern region stood out with the highest mortality in the country, with a mortality rate of 128.1/100,000 inhabitants on February, 20219. In the region, the state of Pará presented the highest incidence, with 341,05 confirmed cases and 7854 deaths10.
The state of Pará have a great territorial extension and present a heterogeneous population composition, which may reflect on particular features and trends of distribution of COVID-19 in the region compared to other locations within the Brazilian territory and worldwide. The present study investigated the risk factors associated with the severity of COVID-19 in a Brazilian Amazon region of 100,819 cases.
Methods
Study design and geographical delineation
This is an epidemiological, cross-sectional, analytical and demographic study, which evaluated information on confirmed cases for COVID-19 available at OpenDataSUS (https://opendatasus.saude.gov.br/), a public data platform of the Brazilian Ministry of Health, from March 01 to July 29, 2020.
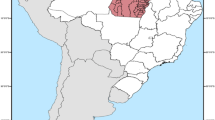
The study included data from the state of Pará, Northern Brazil, the second largest Brazilian state, with a land area of 1,245,870.798 km2. The state has six Mesoregions, comprised of 22 Microregions, in a total of 144 municipalities, with the city of Belém as its capital (Fig. 1)11. The state of Pará territory is composed by the largest tropical forest in the world, the Amazon Rainforest, with a low and flat relief, and with 58% of the territory is below 200 m, while altitudes above 500 m are in observed in Serra dos Carajás, Serra do Cachimbo and Serra do Acari12. The estimated population for 2020 was 8,690,745 inhabitants with a Human Development Index (HDI) of 0.646.

Source: OpenDataSUS, Ministry of Health. Software: ArcGis ((https://www.arcgis.com/).
Spatial location of the mesoregions and municipalities of the State of Pará-Amazon-Brazil.
Data collection and inclusion criteria
The included data was obtained from Brazilian surveillance platforms for COVID-19: The 'E-SUS notifies’, which is exclusive for the notification of suspected cases of respiratory syndrome by COVID-19, and the Epidemiological Surveillance Information System platform of Flu (SIVEP-GRIPE), which carries out the surveillance of all severe acute respiratory syndromes regardless of the etiology. Confirmed cases for COVID-19 and residents of the state of Pará were included, while those with lack of information and/or duplicated by notification register number, were excluded from analysis.
An interface of the two platforms was developed by filtering cases of flu-like illness confirmed to COVID-19 (E-SUS notifies) and cases of SARS confirmed to COVID-19 (SIVEP-GRIPE), generating a total of 89,891 and 12,681 cases from each platform, respectively. Of the 102,572 identified patients, 1753 were excluded due to lack of data, totaling 100,819 cases. Clinical-epidemiological data was extracted and evaluated, including: municipality of residence, age, sex, symptoms, comorbidities, confirmation criteria, cases outcome cases.
Statistical analysis
Database was constructed into a spreadsheet in Microsoft Excel software for Windows (2019) and statistically analyzed in SPSS 20.0 and MINITAB software. Descriptive analysis was performed using the absolute (Fa) and relative (Fr) frequency distributions of the study variables. Values of p ≤ 0.05 were considered statistically significant.
Association between outcome (dependent) and predictor (independent) variables was assessed by the Chi-square test or Fisher's exact test, when applicable. The lethality rate by age group and overall was performed. The Mann–Whitney test was applied to verify the age differences between sexes, and among survivors and those who evolved to death.
The multiple logistic regression test was applied to assess the association of death outcome and comorbidities, including: diabetes, obesity, asthma, immunodeficiency, heart, lung, neurological, kidney, hematological and liver diseases. Similarly, death outcome was associated with the presence of symptoms, such as: cough, nausea, headache, runny nose, nasal congestion, sore throat, myalgia/arthralgia, diarrhea, chills, adynamia and odynophagia. Receiver operating characteristic (ROC) curve and area under curve (AUC) were calculated and plotted based on statistically significant variables after binary logistic regression analysis, allowing evaluation of sensitivity and specificity of each symptoms/model in predicting the severity for COVID-19.
The morbidity index (MI) strategy was applied by transforming qualitative variables into decimal base numbers to assess the distribution and contribution of comorbidities to death outcome across the different mesoregions within State of Pará. An information grid arranging variables with a higher odds ratio for death outcome was constructed according to the following mathematical model Morbidity Index (IM) \(={\sum 2}^{n}.X;\) where n represents morbidity and X the presence (1) or absence (0)13. Kruskal–Wallis test was applied to verify the distribution of MIs among survivors and those who evolved to death, and also to verify their distribution among survivors and those who evolved to death within the mesoregions of State of Pará.
Pearson's correlation was applied to verify the association of age and clinical outcome, as well as with comorbidities and geographic mesoregions of State of Pará. Multivariate analysis using the simple Euclidean distance aggregation method was applied to assess similarities among mesoregions State of Pará according to the presence of comorbidities.
Spatial analysis
The cartographic bases used were obtained from the Brazilian Institute of Geography and Statistics (http://www.ibge.gov.br/). The study area and spatial distribution of cases in the municipalities of Pará were performed in ArcGIS software (https://www.arcgis.com/) and classified according to quartile into classes, based on the calculation of incidence (number of cases/population X 100,000): quartile 1 (124.3–623.5), quartile 2 (623.6–1142.6), quartile 3 (1142.7–2002.5), quartile 4 (2002.6–3608.6), quartile 5 (3608.7–5458.8). The municipalities with quartile 5 were named on the map.
Ethical considerations
The present study evaluated coded secondary data, without any health risk and possibility of identifying the respective patients, and was conducted in accordance to the Brazilian Law No. 12.527 of 18/11/2011, which regulates access to information14.
Results
From the total of 100,819 evaluated cases, 71,500 (70.9%) were confirmed by rapid test, 21,056 (20.9%) by RT-PCR, and 8263 (8.2%) by clinical-epidemiological criteria. Patients’ mean age was 42.30 ± 17.60 years, and those within the age range of 20 and 60 years, comprising 76.25% (76,877/100,819) presented higher susceptibility to the disease (χ2 = 43,878.19; p < 0.0001). Regarding gender, 51.24% were women (51,661/100,819) and 48.76% (49,158/100,819) were men, with the proportion of affected women significantly differing compared to men (χ2 = 62.141; p < 0.0001) (Fig. 2).
Table 1 presents the distribution of the clinical-epidemiological variables included in this analysis. The most frequent symptoms were fever (67,012/66.5%), cough (62,438/61.9%), sore throat (40,138/39.8%), dyspnea (34,862/34.6%), headache (24,671/24.5%) and myalgia/arthralgia (17,963/17.8%). Regarding the presence of comorbidities, 9.72% of patients presented at least one comorbidity, and of these, 2.1% had two or more associated conditions. The most common diseases included cardiovascular diseases and diabetes, representing 5.06% and 4.20% of the cases, respectively (Table 1). Considering all evaluated comorbidities, the obtained MI ranged from 0 to 1023 with a mean of 6.91 ± 34.37 among the 100,819 individuals. Among survivors, it ranged from 0 to 1023 with a mean of 5.17 ± 27.43, and among those who died, ranging from 0 to 872, with a mean of 41.44 ± 91.84.
Risk of death
Among the investigated cases, 4.79% (4835/100,819) had death as outcome, which mostly occurred among men (63.97%—3093/4835), indicating a twice higher relative risk of death when compared to than women (36.03%—1742/4835) (Odds Ratio 1.92; p < 0.001; 95% CI 1.82 ≤ µ ≤ 2.04) (Fig. 2). In the lethality rate by age group, > 80 accounted for (39.58%) lethality, followed by 60–79 (17.29%), 40–59 (2.87%), 20–39 (0.52%) and 1–19 years (0.58%) (Fig. 3). Risk of death was associated with age, as demonstrated by Pearson's test, which revealed a positive and significant correlation between death and advanced age (r = 0.323; p < 0.001) patients, and by Mann–Whitney test, which associated the median age of 70 years to death outcome (p < 0.0001).
The presence of comorbidities among survivors (8.1%—7762/95,984) and those who evolved to death (42.2%—2040/4835) significantly differed as verified by the binomial Chi-square test (95% CI 40.8–43.6; p < 0.0001). All comorbidities were associated with mortality of patients, except asthma (0.8%) ([95% CI 0.6–1.1]; p = 0.7366) (Tables 1, 2).
In addition, the binary logistic regression model with death as the dependent variable and symptoms as predictors was significant (χ2(12) = 14,646.45; p < 0.001; R2 Nagelkerke = 0.423). Variables were associated with death, included age, sex, fever, cough, dyspnea, nausea, and nasal congestion; while headache, myalgia, runny nose, adynamia and sore throat were associated with survival (Table 2). According to this model, death among patients who reported dyspnea increased by 676.6%; in the same way as the presence of nasal congestion, nausea and being male and feeling nausea, the increase in death was around 85.9%, 61.1% and 78.4%, respectively.
The ROC curves are presented in Fig. 4. Since the platelet was a protective factor, all patients’ platelet values were multiplied by − 1 to make its ROC curve above the reference line. The above parameters were all valuable for predicting the severity of COVID-19 (p < 0.05). The AUC of the logistic regression model was 0.774 (95% CI 0.722–0.827). The AUC and optimal thresholds of each independent risk or protection factors are presented in Table 3.
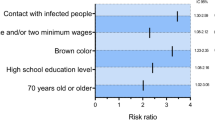
In further analysis, the presence of comorbidities was associated with risk of death among patients, including neurological disease, obesity, immunodeficiency, diabetes, cardiovascular diseases, Pneumopathy, Nephropathy, asthma e liver diseases in the binary logistic regression model. Hematologic disease was excluded from the model due to its high collinearity, as well as, liver disease and asthma due to lack of significancy in the model. Thus, logistic regression was significant (χ2(7) = 3834,89; p < 0.0001; R2 Nagelkerke = 0.117), revealing that the risk of death among patients reporting neurological diseases increased 14.48-fold, followed by obesity (odds ratio 5.16) and immunodeficiency (odds ratio 5.09) (Table 2). Death increased by 1341.9% among patients with neurological diseases, followed by obesity (413.9%) and immunodeficiency (402.3%).
Finally, the distribution of the mean MIs among survivors and those who died revealed a highly significant difference by the Kruskal–Wallis test (H = 6351.263; GL = 1; p-value < 0.001) (Fig. 5).
Spatial analysis
The presence of comorbidities was not associated with any of the geographic mesoregions of the State of Pará, as verified Pearson's test. The distribution of MIs between the different geographic mesoregions did not differ by the Kruskal–Wallis independence test among those who progressed to death (χ2 = 7.967; gl = 5; p = 0.158), however, when considering survivors, the MIs significantly differed (χ2 = 779.546076; gl = 5; p-value < 0.0001) (Table 4). Given the statistical significance of the test applied, we followed the test of comparison between the morbidity rates of the mesoregions by the Pairwise Method with Bonferroni correction, revealing statistically significant differences, as presented in Table 4. In this aspect, it was verified that the mean ranks of the MI in the Southeast mesoregion were lower than in the Southwest, but higher compared to Lower Amazon, Marajó, and Northeast mesoregions. Regarding Southwest mesoregion, the mean ranks of the MI were higher than Marajó, Belém metropolitan region and Northeast mesoregions. In Lower Amazon mesoregions, mean ranks of the MI were lower concerning that of observed in Belém Metropolitan region, Marajó, and Northeast mesoregions.
Also, considering the Kruskal–Wallis statistical differences between groups, this study tested whether the presence of the comorbidities clustered with the mesoregions. Thus, multivariate analysis by the simple clustering method using the Euclidean distance without standardization revealed similarity, which ranged from 54.00 to 95.77% among three different mesoregions groups, where similarity was observed between the mesoregions of Lower Amazon, Marajó and Southwest, as presented in Fig. 6.
The spatial distribution of COVID-19 cases was heterogenous within Pará state, being possible to observe areas concentrating higher incidence rates, compared to lower rates in other areas (Fig. 7). The incidence of the disease ranged from 124.3 to 5458.8, with an average of 1091.89. The highest incidence was registered in the Southwest mesoregion, with 1488.49, ranging from 530.84 to 5256.92, with a mean of 1756.62 and standard deviation of 1344.89. Metropolitan region of Belém registered most of infection cases (Fig. 8), with an incidence of 1059.86, ranging from 396.26 to 2002.57, mean 913.75 and standard deviation 465.47. Most cases diagnosed in Pará State, about 87.90% (88,618/100,819), were due to local transmission.

Source: OpenDataSUS, Ministry of Health. Software: ArcGis ((https://www.arcgis.com/).
Spatial distribution of the incidence of cases of COVID-19 by municipalities of the State of Pará-Amazon-Brazil, in the period from March to June/2020.
Discussion
The present study evaluated the clinical-epidemiological and spatial factors associated with 100,819 cases confirmed for COVID-19 in the State of Pará, the second-largest Brazilian, located in the Brazilian Amazon region. In this study, the case fatality rate was 4.8%, which is higher than the rate reported in Brazil for the same study period, which was (3.1%)15.
AUC revealed that age, dyspnea, cough, gender and fever were considered predictors to the severity of COVID-19. Salzberger et al.16 describe that 5–10% of patients with SARS poorly progress, leading to a mortality rates up to 1.4%, especially among those with 60 years of age or more. The obtained data showed that the mean age of patients was 42.3 years and mostly females (51.2%), but with most of death cases occurring among men, and with and a median age of 70 years, similarly as observed in Beijing, China17, 18.
Ambrosino et al.19 highlight that age above 60 years is a risk factor for mortality and can be aggravated by the presence of chronic diseases such as hypertension, diabetes, cardiovascular diseases, hypercholesterolemia and obesity conditions. In addition, elderly patients become more susceptible to the severity of SARS-CoV-2 infection due to use of polypharmacy and deficient immune response associated with immunosenescence20. On the other hand, a dysregulated immune response may lead to atypical clinical presentations, such as the absence of fever, which is the main sign of infection, impairing adequate screening for the disease in this age group21. Finally, the most reported symptoms among this population are in line with observations from several studies22,23,24,25.
Regarding comorbidities, 9.7% of patients presented at least one comorbidity, while 2.1% had two or more comorbidities. Among survivors, 8.1% had at least one comorbidity, and 42.2% among those who died also had one, the most common being cardiovascular diseases (5.1%) and diabetes (4.2%). However, when it comes to the odds ratio of dying, neurological diseases represented the comorbidity as the main predictor of mortality by (14.48) times, followed by obesity (5.16) and immunodeficiency (5.09).
As highlighted by several studies, conditions such as smoking, diabetes, and hypertension, which are even related to disease severity, increase ACE2 expressions, enzyme which its higher expression may increase vulnerability to SARS-CoV-2. Additionally, ACE2 expression is higher in cardiac and pulmonary tissues, which contributes to severe pulmonary and cardiovascular complications. Finally, a hyperinflammatory response leads to a drop in O2 saturation and complications in myocardial stability, leading to cardiopulmonary failures, such as acute cardiac injury and ground-glass lung lesions on CT scans, which are serious complications directly associated with mortality26,27,28,29.
Studies from New York and the United Kingdom show a higher prevalence of comorbidities than this study30, 31 but are similar to the results on comorbidities in patients with COVID-19 in a meta-analysis and the COVID-19 Brazil Bulletin17, 32. The presence of comorbidities, especially cardiovascular disease, diabetes, and kidney disease, is a risk factor for mortality, so specific strategies should be directed to this group to reduce deaths33.
Regarding neurological diseases as a high predictor for mortality, García-Azorín et al.34 showed in a retrospective cohort of those hospitalized for COVID-19, that the presence of neurological diseases is the main independent predictor for COVID-19 mortality among older people and those with other risk factors, such as cardiovascular.
In the present study, obesity increased the risk of death by 5 times. A study conducted in three hospitals in China, with obese patients (75) and non-obese controls (75), showed that obese patients were admitted to hospital with high C-reactive protein and low lymphocytes compared to controls, leading to a longer hospital stay and a three times higher risk of severe COVID-19. Authors suggest that the underlying chronic low-grade inflammation and suppression of innate and adaptive immune responses are associated with poor outcomes among obese patients, as well as that obesity may affect mechanical dysfunction, which is a factor for lower respiratory tract infection severity and secondary infections35.
In line with previous reports, immunodeficiency was a major risk factor for death in the present study, increasing its chances by five times. The meta-analysis of Gao et al.36 showed that immunosuppressed patients had 3.39 times higher chances of poor clinical evolution and mortality. To Babaha e Rezaei37, unfavorable outcomes in COVID-19 setting are related to opportunistic and/or secondary infections, specially by bacterial and fungal agents. By contrast, immunosuppression causing B-lymphocyte deficiency may minimize the effects of the inflammatory cytokines storm, leading to mild symptoms cases. Thus, B-lymphocyte deficiency may prevent the hyper-inflammation process, however, predisposes patients to other potentially fatal infections.
Our data evidenced that all comorbidities, except asthma, were significantly related to patients’ mortality. Although the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention (CDC) include asthma patients as risk groups for COVID-19, asthma patients accounted only for 12% of COVID-19 hospitalizations, a smaller number than the current 20% of asthma patients hospitalized due to influenza in the US. Although inaccuracy on incidence data, studies suggests that asthmatics do not appear to be as affected as other COVID-19 risk groups28, 38. Differently from asthma, lung disease was a comorbidity significantly associated with death cases. Early reports also demonstrated that this condition is associated with increased ACE2 expression in lung tissue and small airways, increasing risk for severe clinical manifestations among hospitalized patients for was found in other studies, in which, having lung disease increased the risk of severe COVID-1928, 38,39,40,41.
In the spatial distribution, the high incidences of COVID-19 in the municipalities of Parauapebas, Canaã dos Carajás and Jacareacanga were concerning. Emergency situation was declared in Jacareacanga city due to lack of health professionals, equipment and medications for treatment of indigenous people in nearby villages, causing high morbimortality among this population and death of at least six indigenous leaders. Additionally, Jacareacanga was among the 10 cities in Brazil with the highest incidence and mortality by COVID-19, with 15.7% of all city population been infected by SARS-CoV-2 by august, 202042.
Regarding the MIs by mesoregion, similarities between the Lower Amazon, Marajó, Southwest mesoregions were observed. The Metropolitan region of Belém presented similarities with the Northeast mesoregion, but with lower MIs compared to others regions. Thus, the higher presence of morbidity in a mesoregion may be associated with distinct characteristics of that population, as well as exposure to environmental factors, resulting in higher rates of infection and mortality due to COVID-19. The clustering similarities between Marajó, Lower Amazon and Southwest mesoregions may be explained by the fact these communities are characterized by a relatively small and fragmented population, strong indigenous component, and poor socioeconomic conditions, factors which could enhance probability of poor prognosis of patients. Finally, the impact of infectious diseases on these groups is not necessarily due to the absence of specific genes related to immune response capacity, but to the fact that these populations, especially the indigenous ones, are biologically very homogeneous from the genetic point of view and lack a social structure capable of providing basic healthcare43.
Several mesoregions of Pará suffer from environmental contamination by mercury, with riverside and traditional communities being the most affected. Most of these communities are based along the Amazon River Basin, which permanently receive tons of mercury from illegal gold mining, specially Tapajós River, causing damage to agriculture and fishing actives44 A systematic review by Castro and Lima45 showed that the riverside populations along the Tapajós River presented Hg levels above the WHO permitted limit. Mercury reaches individuals through local food, the main one being the consumption of fish, which are contaminated during the flow of matter in the food chain. This exposure and intoxication can directly affect the central nervous system and immune system, thus these exposed populations would have more comorbidities than other populations from regions without mining44, and this would corroborate the unfavorable outcome of SARS-CoV-2 infection observed in this study, particularly among people with neurological morbidities.
A limitation of this study was the lack of proper filling out the notification forms, causing several variables to be excluded. It is also noteworthy that the epidemiological situation of COVID-19 in this region may be more incident due to several municipalities lacking mass testing capacity or due to underreporting. Another important point to highlight in the limitation is the priority that municipalities perform and investigate the deaths by COVID-19, consequently being the first to be entered into the surveillance system when compared to cases that evolved to the cure, thus many cases of healing have not yet been to the surveillance system which may be associated with higher lethality in this study, the data are subject to change because the cases and deaths are under constant investigations to qualify the data. This limitation is directly associated with the quality of epidemiological surveillance and was experienced by the main author of this study, by several factors, lack of trained professionals and high demand for service.
Conclusion
The clinical and epidemiological features of COVID-19 in the state of Pará were similar to those in Brazil and other countries. Most infected were female, young adults, with the classic clinical picture of fever, cough, sore throat, myalgia/arthralgia, headache. Most common comorbidities were cardiovascular diseases and diabetes in the study population. However, the characteristics of the deaths identified a higher lethality rate than in Brazil, being male and elderly, with one or more chronic diseases, who presented with dyspnea, nasal congestion, fever, nausea and cough. All comorbidities were associated with deaths, except asthma, which proved not to be a risk factor for complications or deaths for COVID-19. Besides, neurological diseases, obesity, and immunodeficiency stood out in the increased chance ratio of progression to death. The higher lethality rate than in Brazil may be associated with the greater impacts of the disease in this Amazonian population, or factors associated with fragile epidemiological surveillance in the notification of cases of cure.
Furthermore, it was shown that the mesoregions with the highest incidences in the study period were the Southwest and Southeast, which have similar characteristics, such as indigenous populations and mining regions, which require attention from public health policies for these vulnerable populations. Also, we verified being elderly or suffering from chronic diseases and living in certain geographical areas of the state of Pará, such as the Southwest, Southeast and Marajó mesoregions, seem to be predictors for complications and mortality. Finally, we highlight the need of further studies to clarify which risk factors/markers contribute to the severity of COVID-19, which may aid on the development and implementation of surveillance strategies, and reducing mortality within this region.
References
Park, M., Cook, A. R., Lim, J. T., Sun, Y. & Dickens, B. L. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 9, 967 (2020).
Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S. C. & Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19) (StatPearls Publishing, 2020).
Mao, L. et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77, 683 (2020).
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). JAMA 324, 782 (2020).
Bulut, C. & Kato, Y. Epidemiology of COVID-19. Turk. J. Med. Sci. 50, 563–570 (2020).
Ministério da Saúde (BR). Definição de Caso e Notificação. Portal do Governo Brasileiro 1–9 (Accessed 4 July 2020); https://coronavirus.saude.gov.br/definicao-de-caso-e-notificacao (2020).
Bastos, L. S. et al. COVID-19 e hospitalizações por SRAG no Brasil: uma comparação até a 12a semana epidemiológica de 2020. Cad. Saude Publica 36, e00070120 (2020).
OPAS. Folha informativa COVID-19—Escritório da OPAS e da OMS no Brasil—OPAS/OMS|Organização Pan-Americana da Saúde. (Accessed 9 Feb 2021); https://www.paho.org/pt/covid19 (2020).
Ministério da Saúde (BR). Coronavírus Brasil. (Accessed 9 Feb 2021); https://covid.saude.gov.br/ (2021)
SESPA. Coronavírus no Estado do Pará. PRODEPA (Accessed 9 Feb 2021); https://www.covid-19.pa.gov.br/public/dashboard/41777953-93bf-4a46-b9c2-3cf4ccefb3c9 (2021).
IBGE, I. B. de G. e E. Pará|Cidades e Estados|IBGE. (Accessed 7 July 2020); https://www.ibge.gov.br/cidades-e-estados/pa/ (2020).
Pará, G. do E. do. O Pará—Governo do Pará. (Accessed 25 July 2020); https://www.pa.gov.br/pagina/47/para (2020).
Mendes, H. D. L. Os Números Binários: do Saber Escolar ao Saber Científico. J. Int. Estud. em Educ. Matemática 10, 41 (2017).
Planalto do brasil. LEI No 12.527, DE 18 DE NOVEMBRO DE 2011. (Accessed 25 July 2020); http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2011/lei/l12527.htm (2011).
Ministério da Saúde (BR). Coronavírus Brasil 10-09-2020. covid.saude.gov.br (Accessed 11 Sep 2020); https://covid.saude.gov.br/ (2020).
Salzberger, B. et al. Epidemiology of SARS-CoV-2 infection and COVID-19. Internist (Berl). 61, 782–788 (2020).
Ministério da Saúde (BR). BOLETIM EPIDEMIOLÓGICO ESPECIAL Doença pelo Coronavírus COVID-19, vol. 30. (Secretaria de Vigilância em Saúde, 2020).
Tian, S. et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 80, 401–406 (2020).
Ambrosino, I. et al. Gender differences in patients with COVID-19: A narrative review. Monaldi Arch. Chest Dis. Arch. Monaldi per le Mal. del torace 90, 318–324 (2020).
Pfister, G. Naive T cells in the elderly: Are they still there?. Ann. N. Y. Acad. Sci. 1067, 152–157 (2006).
Nikolich-Zugich, J. et al. SARS-CoV-2 and COVID-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience 42, 505–514 (2020).
Li, L. et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 92, 577–583 (2020).
Li, R. et al. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J. Clin. Virol. 127, 104363 (2020).
Zhang, J.-J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75, 1730–1741 (2020).
Qian, G.-Q. et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM 113, 474–481 (2020).
Mitrani, R. D., Dabas, N. & Goldberger, J. J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Hear. Rhythm https://doi.org/10.1016/j.hrthm.2020.06.026 (2020).
Ng, M.-Y. et al. Imaging profile of the COVID-19 infection: Radiologic findings and literature review. Radiol. Cardiothorac. Imaging 2, e200034 (2020).
Jackson, D. J. et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 146, 203-206.e3 (2020).
Liu, S., Zhi, Y. & Ying, S. COVID-19 and asthma: Reflection during the pandemic. Clin. Rev. Allergy Immunol. 59, 78–88 (2020).
Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323, 2052 (2020).
Atkins, J. L. et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J. Gerontol. Ser. A https://doi.org/10.1093/gerona/glaa183 (2020).
Yang, J. et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 94, 91–95 (2020).
Ssentongo, P., Ssentongo, A. E., Heilbrunn, E. S., Ba, D. M. & Chinchilli, V. M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One 15, e0238215 (2020).
García-Azorín, D. et al. Neurological comorbidity is a predictor of death in Covid-19 disease: A cohort study on 576 patients. Front. Neurol. 11, 781 (2020).
Gao, F. et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 43, e72–e74 (2020).
Gao, Y., Chen, Y., Liu, M., Shi, S. & Tian, J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J. Infect. 81, e93–e95 (2020).
Babaha, F. & Rezaei, N. Primary immunodeficiency diseases in COVID-19 pandemic: A predisposing or protective factor?. Am. J. Med. Sci. 360, 740–741 (2020).
Broadhurst, R. et al. Asthma in COVID-19 hospitalizations: An overestimated risk factor? Ann. Am. Thorac. Soc. 0, 1–13 (2020).
Argenzian, M. G. et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 369, (2020).
Arentz, M. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA https://doi.org/10.1001/jama.2020.4326 (2020).
Chhiba, K. D. et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J. Allergy Clin. Immunol. 146, 307-314.e4 (2020).
G1 Pará, R. L. Aldeias indígenas do Pará reclamam da falta de estrutura para tratar Covid-19 em Jacareacanga | Pará | G1. g1.globo.com (. Accessed 11 Oct 2020); https://g1.globo.com/pa/para/noticia/2020/08/08/aldeias-indigenas-do-para-reclamam-da-falta-de-estrutura-para-tratar-covid-19-em-jacareacanga.ghtml (2020).
Santos, R. V & Coimbra Jr., C. Ε. A. Saúde e Povos Indígenas. (Editora FIOCRUZ, 1994). https://doi.org/10.7476/9788575412770.
BerzasNevado, J. J. et al. Mercury in the Tapajós River basin, Brazilian Amazon: A review. Environ. Int. 36, 593–608 (2010).
de Castro, N. S. S. & de Lima, M. O. Hair as a biomarker of long term mercury exposure in Brazilian Amazon: A systematic review. Int. J. Environ. Res. Public Health 15, 500 (2018).
Author information
Authors and Affiliations
Contributions
D.M.S. and R.S.P.L. performed the study design, literature review, data collection, data analysis, interpretation of results. A.L.S.F., C.A.F.S., and R.J.P.S.G. performed the spatial distribution analysis. Y.C.R. performed research review, contribution to discussion and translation. K.V.B.L. and L.N.G.C.L. performed research supervision, guidance, and approval of final version. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sardinha, D.M., do Socorro Pompeu de Loiola, R., Ferreira, A.L.d.S. et al. Risk factors associated with the severity of COVID-19 in a region of the Brazilian Amazon. Sci Rep 11, 20569 (2021). https://doi.org/10.1038/s41598-021-00009-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-00009-y
This article is cited by
-
Non-rigorous versus rigorous home confinement differently impacts mental health, quality of life and behaviors. Which one was better? A cross-sectional study with older Brazilian adults during covid-19 first wave
Archives of Public Health (2023)
-
COVID-19 contagion across remote communities in tropical forests
Scientific Reports (2022)
-
Characteristics and outcomes of severe COVID-19 in hospitalized patients with cardiovascular diseases in the Amazonian region of Brazil: a retrospective cohort
Scientific Reports (2022)