Abstract
Glycosylation is critical for monoclonal antibody production because of its impact on pharmacokinetics and pharmacodynamics. Modulation of glycan profile is frequently needed in biosimilar development. However, glycosylation profile is not a single value like that of cell culture titer, hence making it challenging for the Design of Experiment (DoE) methodology to be directly applied. In this study, a Her2-binding antibody was developed as a biosimilar to Herceptin. Cluster analysis was introduced to demonstrate the similarity of glycan profiles between the samples and the reference with specific value—distance. The glycosylation was subsequently optimized with the DoE method. Basal medium and feed medium were found to be the significant factors to the glycosylation pattern. Moreover, a combination of medium and feed strategy was developed to attain the most similar glycoprotein molecule to that of the originator biologic drug. This study may provide an additional option to evaluate multivariable factors and assess biosimilarity and/or comparability in monoclonal antibody production.
Similar content being viewed by others
Introduction
Glycosylation is one of the most vital critical quality attributes (CQA) during therapeutics antibody development due to the fact that its impact on the pharmacokinetics (PK) and pharmacodynamics (PD) can be significant in most cases. Guidelines for biosimilar development from several regulatory agencies across the world including FDA and EMA all consider glycoform similarity as one of the most important requirements1,2,3,4,5,6. It follows that the glycosylation attribute would normally be under stringent control during Chemistry Manufacturing and Controls (CMC) development and manufacture phases.
There are two types of protein glycosylation: serine/threonine-linked glycosylation (O-linked) and asparagine-linked glycosylation (N-linked). For antibodies, O-linked glycosylation is seldom seen7,8. Only some Fc-fusion partner molecules such as etanercept and B cell activating factor receptor 3 (BR3)-Fc possess O-linked glycans9,10. The N-linked glycan is attached at the Asn297 position in the heavy chain of therapeutic antibodies or derivatives where an asparagine (Asn)-X-Ser/Thr (X denotes any amino acid except Pro) consensus sequence11 is present.
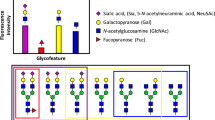
The N-linked glycans of human IgGs are typically biantennary complex structures. Two N-acetylglycosamine (GlcNAc), three mannose, and two GlcNAc residues compose the conserved core structure and two GlcNAc residues are β-1,2 linked to α-6 mannose and α-3 mannose, forming two antennae. Additional fucose (Fuc), galactose (Gal), bisecting GlcNAc, and sialic acid including N-acetylneuraminic acid (NANA) and N-glycolylneuraminic acid (NGNA) residues are added to the core, depending on the host glycosylation machinery. The major N-linked glycoforms of mAb therapeutics are shown in Fig. 1. The glycan structure showe considerable heterogeneity with more than several hundred glycoforms because of the random pairing of heavy-chain glycans with different structures11,12. Beside the expression hosts such as Chinese hamster ovary (CHO) cells and murine myeloma cells NS0 and SP2/0, cell culture conditions, medium and additives also affect antibody glycosylation as reported13 previously.
Glycosylation of therapeutic antibodies substantially affect PK profile, efficacy, and in some cases, antigenicity. Glycosylated antibodies with terminal high-mannose glycans exhibited fast clearance from the bloodstream14,15,16,17. Wright and coworkers revealed that IgG with glycans terminated with Man5 cleared significantly faster than those with more complex glycosylation such as G0F, G1F and G2F18,19. For Fc-fusion proteins, glycosylation played a more important role in determining the in-vivo clearance. Liu et al. demonstrated that the exposure of a humanized yeast-produced TNFαRII-Fc-fusion molecules were positively correlated to the quantity of the sialylation on the receptor molecule with higher sialic acid content resulting in higher exposure20. The glycosylation of humanized or fully human antibodies has critical impact on the Fc receptor-mediated effector functions. Many studies demonstrated defucosylated marketed mAbs such as rituximab and trastuzumab increased antibody-dependent cell-mediated cytotoxicity (ADCC) activities by at least two orders of magnitude in humans in vitro21,22,23. The first patent related to afucosylated IgG enhancing ADCC was published in 200024. An afucosylated IgG exhibited a 50-fold increase in binding to the Fcγ RIIIa receptor, and ADCC activity was greatly enhanced too22. The terminal Gal played an important role in complement-dependent cytotoxicity (CDC) activity. For rituximab, CDC activity and the affinity to C1q increased by two folds when Gal content increased in the heavy chain25. Furthermore, glycan composition could be the factor to cause immunogenicity. High-mannose-type N-glycans is highly immunogenic in human26. Antibodies produced in mouse myeloma cells may contain α-Gal epitope (Galα1-3Galβ1-4GlcNAc-R), which could be immunogenic in human. Considering these significant impacts of glycosylation, the glycan profile should be well-studied and controlled during the manufacturing process and the development of a biosimilar product.
Since the effects of cell line, culture mode, medium and manufacturing conditions on the antibody glycan profiles were reported extensively, the effects of basal/feed medium combination and galactose addition will be on the focus in this study. Mammalian cell culture medium are made up of either chemically defined or serum-free mixtures of at least 50–100 different components. The concentration of glucose, amino acid, vitamin, metal ion and lipids supplements can play a role in glycosylation control. 3 kinds of basal medium and 2 kinds of feed were tested in this study. Chee Furng et al. found that the glucose concentration below 0.7 mM led to decrease in sialylation levels and increase in both hybrid type and high mannose type glycans in CHO fed-batch cultures producing IFN-γ27. Amino acid feeding is another critical strategy for cell growth and productivity, whilst also potentially impact the glycan profile28. Thus, feed strategy is important to maintain glycan synthesis and should be optimized to produce an expected glycoform. Nucleotide-sugar precursors such as uridine, glucosamine and galactose modulate intracellular nucleotide-sugar pools and resulting sialylation and antennarity levels28. Galactose feeding can help facilitate a more fully galactosylated N-profile29. We chose galactose in this experiment because its effect on glycan profile was observed in work previously done on the development of this product.
In this study, a Her2-binding antibody was developed as a biosimilar to Herceptin. Although the amino acid sequence was the same as that of the originator, the glycan profile expressed by the candidate clone in the initial culture condition was different. So, in order to explore the optimum medium combination to attain a biosimilar antibody, we designed an optimization experiment by DoE in JMP and carried out the experiment in parallel micro-bioreactor platform AMBR 15. Due to the similarity of glycan profiles between expressed antibody and reference was difficult to identify with a specific response, a cluster analysis method was introduced to enable modeling and optimization.
Results
Cell growth and titer
Medium is a vital factor to affect cell growth and productivity. 3 kinds of basal medium and 2 kinds of feed medium were tested in this study. Including feed strategy and galactose addition time, a 24-run experiment was designed by JMP software and conducted in the Ambr system. We found that the cell growth variance was significant as shown in Fig. 2. The lowest integral of the viable cell density (IVC) was 93 × 106 cells·day/mL (TA-4), while the highest IVC was 154 × 106 cells·day/mL (TA-14). The IVC of TA-5 and TA-14 were extraordinarily high. The basal medium and feed medium for the two runs were both Dynamis and Feed B. This composition was the best to boost cell growth among the 24 runs. But the titer of TA-5 and TA-14 were not the highest (see Fig. 2), which indicated the Dynamis and Feed B composition did not enhance the specific productivity. Considering the cost of manufacturing, titer is very important. The highest titer was 2859 μg/ml from TA-1. By observing the scatter plot (see Fig. 2), most blue spots were higher titer, which indicated that the feed medium FEED B was a good feed medium to enhance titer. To analyze the effect on titer of all factors statistically, the factors and titer data were tabulated in JMP and the regress model was fitted (Table 1). According to the ANOVA analysis, the feed medium and feeding strategy were significant to productivity, while basal medium and galactose did not impact titer significantly. FEED B and feeding strategy 1 were the optimal factor for high productivity.
The scatter plotting of IVC vs. titer from 24-run experiment in Ambr. The IVC value indicated cell growth. Titer indicated the concentration of antibody when harvest. Basal medium CD14 is dot, basal medium CD 15 is square, Dynamis is triangle, FEED B is blue, PFF06 is red, galactose addition day 12 is light color and day 13 is dark color. The image was created by JMP V15 (http://www.jmp.com).
Glycosylation
The glycosylation profiles of antibodies from the 24 micro-bioreactors were analyzed by the HPLC as described in the methodology section. The typical chromatogram of glycan distribution was shown in Fig. 3 and the G0, G0F, G1F, G1′F and G2F can be qualified comparing to the glycan standards. Three minor unidentified peaks were named as PK1, PK4 and PK5. These undefined peaks were assumed to be the same glycans of samples and reference. For the reference, the glycan of PK4 is Man5 and PK 5 is G1 as reported by Xie et al.30. By summarizing all of the glycan peaks of the 24 samples and reference into the pie chart (see Fig. 4), the glycosylation distribution has a wide variation, and the TA-1, TA-5 and TA-14 were significant different from other samples and reference by visual inspection. However, it is hard to identify the antibody with the most similar glycan profile to reference from the other runs.
The HPLC chromatogram of glycan distribution. Compared to standards, peaks of G0, G0F, G1F, G1′F and G2F are indicated. PK1, PK4 and PK5 refers to the 3 minor unidentified peaks, respectively. The chromatogram was created by Agilent OpenLab CDS 2.0 (https://www.agilent.com/en/product/software-informatics/analytical-software-suite/chromatography-data-systems/openlab-cds).
The pie chart of glycan percentage of samples and reference. TA-1 to TA-24 refers to the samples obtained from the designed 24-run experiment in Ambr. One sector represents one sample’s glycan distribution. The shadowed sector represents reference. The image was created by JMP V15 (http://www.jmp.com).
The hierarchical cluster analysis method was applied to evaluate the glycan profile similarity between the samples and the originator. A dendrogram was generated with 25 leaves including 24 samples and 1 reference (see Fig. 5). Similar glycan profiles should be in one stem. The glycosylation pattern of TA-5 and TA-14 should be different from others because they were apart on the first folk. The G0F percentage was higher than the others and the cell growth was the highest, which was caused by the medium composition of Dynamis/Feed B. The samples TA-2, 3, 9, 13, 17, 18, 19, 22 were clustered to the same group to the reference, indicating that their glycan profiles were similar to that of the reference. And samples with same color were clustered together, which indicated that feed medium and galactose addition day should have effect on glycosylation. By further checking the similarity between the samples and reference, the distance value was generated based on cluster analysis (see Fig. 6). The distance value of TA-5 and TA-14 were the highest, which was agree with observation in pie chart and cluster true. The nearest distance was 5.7 from TA-22 which should have the most similar glycan profile to reference. All of the 8 samples in same trunk with reference had the short distance less than 10.
The dendrogram from hierarchical cluster analysis of samples and reference. TA-1 to TA-24 refers to the samples obtained from the designed 24-run experiment in Ambr. FEED B is blue, PFF06 is red, galactose addition day 12 is light color and day 13 is dark color. The image was created by JMP V15 (http://www.jmp.com).
The distance between samples and reference. TA-1 to TA-24 refers to the samples obtained from the designed 24-run experiment in Ambr. Basal medium CD14 is dot, basal medium CD 15 is square, Dynamis is triangle, FEED B is blue, PFF06 is red, galactose addition on day 12 is light color and day 13 is dark color. The image was created by JMP V15 (http://www.jmp.com).
Factors affecting glycosylation
To screen for factors that impact glycosylation statistically, the distance value from cluster analysis was set as the response in the model fit analysis. The statistical results were shown in Table 2. Among the four factors, base medium and feed medium had the most significant impact on similarity. On the graph of effect evaluation for factors (see Fig. 7), it is clear that the effect of CD14 and CD15 was almost the same and they were more appropriate for achieving biosimilarity for the antibody than Dynamis. For the feed medium, PFF06 can attain an antibody with more similar glycan profile to the reference compared to that with the Feed B. Consequently, the optimal medium composition was CD14 and PFF06. Considering the productivity, the feed strategy should be mode 1 and addition galactose on day 12. This condition is coincident with the run TA-19, that possessing high similarity and titer of 2605 μg/ml.
Effect evaluation of base medium, feed medium, strategy and galactose addition day on distance (Similarity). The image was created by JMP V15 (http://www.jmp.com).
Discussion and conclusions
In this study, the effect of base medium, feed medium, feeding strategy and galactose addition day on glycan profile were investigated. Medium combination was found to successfully modulate the glycosylation profile of an antibody to be similar to that of the reference. The Dynamis and Feed B combination could promote cell growth, but the glycosylation pattern was found to be different from the others. Thus, the metabolite pattern may be different from the other condition. Even though the base medium was the same, the feed medium played an important role in affecting the ratio of G0F to G1F. Andersen et al. found that galactose feeding can help to facilitate a more fully galactosylated N-glycan profile29. However, the galactose addition on day 12 and 13 made no significant difference to the final percentage of galactosylation. The reason may be the slow synthesis rate in the later phase and the galactosylation level reached a “plateau” at approximately 50–60%31.
The cluster analysis was found to be very useful in assessing the similarity between the biosimilars and the originator. Samples with similar quality could be grouped together to visualize the similarity. The specific value-distance denoting the similarity made the model fit possible, thereby the factors’ impact on similarity can be evaluated by ANOVA statistically.
Glycosylation is a vital CQA for monoclonal antibodies and recombinant proteins because of its impact on efficacy and pharmacokinetics. Modulation of glycan profile is often used in developing biosimilar products. Statistical method such as the DoE is very useful method to optimize or adjust process parameters for the purpose of increasing or decreasing a specific response. However, glycosylation profile is not a single value like a titer measurement, so it is hard to apply DoE method directly. In order to solve this problem, cluster analysis can be introduced. By using this method, the similarity of glycan between the samples and reference was demonstrated as the specific value-distance. In this study, base medium and feed medium were found to be the significant factors in impacting the glycosylation. A combination of medium and feed strategy was developed to attain the most similar glycoprotein to the originator. The best condition was found to be using CD14 as the base medium, PFF06 as the feed medium, feeding as strategy 1 and addition galactose on day 12.
Besides the glycosylation profile, other quality attributes such as cIEF, CEX and peptides mapping are of multi-parameter format. Thus, the cluster analysis method introduced here could also help to assess similarity of these quality attributes. Likewise, DoE can be used to evaluate factor effect and optimize inputs leading to reduced time and cost.
Material and methods
Cell line and reagents
A DG44 derived CHO cell producing Her2-binding antibody was studied in this article. The basal medium was CD14 (CD14, OPM Biosciences), CD15(CD15, OPM Biosciences) and Dynamis (A26615, Thermofisher) supplemented with 6 mM Glutamine (G8540, Sigma). Feed medium were EfficientFeed B (FEED B, A12456, Thermofisher) and PFF06 (PFF06, OPM Biosciences). The additive was galactose (G5388, Sigma). The compositions of all the used medium were developed by their vendors.
Cell culture condition and experimental design
The candidate cell line was cultured in Dynamis in clone screening phase. To test the new medium, the cells were cultured in CD14 and CD15 for 3 passages to allow the cell to adapt to the new medium. The fed-batch culture mode was applied in AMBR with temperature, pH, DO and agitation controlled. The temperature was set at 37 °C on the beginning culture and shifted to 35 °C on day 4. DO was maintained at 50% and pH was in the range of 6.8–7.2. The seeding density was 1 million cells/mL with 12 mL initial culture volume. Two feeding strategies was tested in the experiment. For strategy 1, feeding started from day 3 and the feeding volume was calculated through Eq. (1). For strategy 2, feeding volume was 4% of the initial volume on day 3 and 6% of the initial volume every other day till the harvest day. The glucose residual concentration was tested every day and glucose was supplemented to 3 g/L when viable cell density (VCD) was less than 10 million cells/mL and to 4 g/L otherwise. The feeding volume of glucose was calculated through Eq. (2). The base medium, feed medium, feeding strategy and the galactose addition day were designed by custom DoE function as four factors using JMP. 24 runs of experiment were conducted according to the design (Table 3). All the runs were harvested on day 14.
Vfeed: Feed volume of current day (mL), Vcurrent: Culture volume before feeding (mL), VGlc: Glucose solution addition volume (mL), Q feed: Feed rate factor, VCD1: Viable cell density of current day (106 cell/mL), VCD2: Calculated viable cell density of next day (106 cell/mL), Glcfeed: Glucose concentration in feed medium (g/L), Glctest: Glucose concentration in culture(g/L), Glctarget: Target glucose concentration after feeding (g/L), Glcsolution: Concentration of glucose solution (g/L).
Monitoring of cell growth and titer
VCD and viability were measured with CounterStar IC1000 every day. To compare cell growth for the 14-day culture in fed-batch, the IVC was calculated through Eq. (3). The antibody titer was tested by CEDEX Bio (Roche) after centrifugation of the sample for 5 min at 3000 rpm.
VCD(K) Viable cell density of day K, n Harvest day.
Purification and analysis of the glycosylation profile
The antibody was captured from the cell harvest supernatant using protein A column (Mabselect SuRe, GE) at first. After capture completed, the target protein was eluted by 20 mM citrate buffer (pH 3.60, Sinopharm). Elution was collected from 100 mAU and ended when the absorbance value come back to 100 mAU. The reference product was Trastuzumab purchased from Asian market. The N-glycans were released from 300 μg antibody by digestion with 0.5 μL PNGaseF (PO704L, Biolabs NEB) followed by labeling with 6 μL 2-AB (76,884, Sigma) at 65 °C for 3 h. Glycosylation patterns were analyzed by HPLC (1260, Agilent) with AdvanceBio glycan mapping column (4.6 mm × 150 mm, Agilent) and fluorescence detector (Ex: 260 nm, Em: 430 nm). The solvent A was 100 mM ammonium formate (17843, Sigma Fluka) and solvent B was acetonitrile (A998-4, Fisher). The gradient conditions of A:B used were 25:75 on 0 min, 40:60 on 25 min, 100:0 on 30 min, 25:75 on 35 min to 40 min. Elution rate was 0.5 mL/min and sample volume was 2 μL. The distribution of different major glycans such as G0F, G1F, G1′F and G2F can be shown in the chromatography map. Area under the curve analysis was done by integrating the peaks from 16 to 30 min followed by calculating the area percentage for each peak by area normalization method using the HPLC software.
Similarity assessment and ANOVA
The peak area percentages from the glycan analysis are used for assigning the similarity in this case. Peaks area percentages of samples and reference were entered to form a symmetrical matrix and Hierarchical Cluster Analysis was performed using JMP software (SAS Institute Inc.). A dendrogram can be generated and the distance matrix was saved to another data table. The distance denotes the similarity to originator. To analyze the effect of the 4 factors on glycan similarity, the distance was set as the response value and then fit model was run in JMP.
Data availability
All data generated or analyzed during this study are included in this published article.
References
FDA. Guidance for industry biosimilars: Questions and answers regarding implementation of the biologics price competition and innovation act of 2009. (2012).
FDA. Guidance for industry scientific considerations in demonstrating biosimilarity to a reference product. (2012).
FDA. Guidance for industry quality considerations in demonstrating biosimilarity to a reference protein product. (2012).
FDA. Guidance for industry clinical pharmacology data to support a demonstration of biosimilarity to a reference product. (2014).
EMEA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues. (2014).
EMEA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. (2014).
Hirayama, K. et al. Complete and rapid peptide and glycopeptide mapping of mouse monoclonal antibody by LC/MS/MS using ion trap mass spectrometry. Anal. Chem. 70, 2718–2725 (1998).
Leibiger, H., Wüstner, D., Stigler, R. D. & Marx, U. Variable domain-linked oligosaccharides of a human monoclonal IgG: Structure and influence on antigen binding. Biochem. J. 338, 529–538 (1999).
Pennica, D. et al. Biochemical characterization of the extracellular domain of the 75-kilodalton tumor necrosis factor receptor. Biochemistry 32, 3131–3138 (1993).
Stefanich, E. G. et al. Evidence for an asialoglycoprotein receptor on nonparenchymal cells for O-linked glycoproteins. J. Pharmacol. Exp. Ther. 327, 308–315 (2008).
Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 21, 11–16 (2005).
Jefferis, R. Recombinant antibody therapeutics: The impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 30, 356–362 (2009).
del Val, I. J., Kontoravdi, C. & Nagy, J. M. Towards the implementation of quality by design to the production of therapeutic monoclonal antibodies with desired glycosylation patterns. Biotechnol. Prog. 26, 1505–1527 (2010).
Goetze, A. M. et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 21, 949–959 (2011).
Liu, L. et al. Pharmacokinetics of IgG1 monoclonal antibodies produced in humanized Pichia Pastoris with specific glycoforms: A comparative study with CHO produced materials. Biologicals 39, 205–210 (2011).
Kanda, Y. et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: The high-mannose, hybrid, and complex types. Glycobiology 17, 104–118 (2007).
Yu, M. et al. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs 4, 475–487 (2012).
Wright, A. & Morrison, S. L. Effect of altered CH2-Associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J. Exp. Med. 180, 1087–1096 (1994).
Wright, A. et al. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 10, 1347–1355 (2000).
Liu, L. et al. The impact of glycosylation on the pharmacokinetics of a Tnfr2: Fc fusion protein expressed in glycoengineered Pichia Pastoris. Pharm. Res. 30, 803–812 (2013).
Li, H. et al. Optimization of humanized IgGs in glycoengineered Pichia Pastoris. Nat. Biotechnol. 24, 210–215 (2006).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Iida, S. et al. Nonfucosylated Therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγRIIIA. Clin. Cancer Res. 12, 2879–2887 (2006).
Hanai, N. et al. Method for controlling the activity of immunologically functional molecule. Espacenet https://worldwide.espacenet.com/patent/search/family/014346708/publication/EP1176195A1?q=EP1176195A1 (2002).
Raju, T. S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20, 471–478 (2008).
Durocher, Y. & Butler, M. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotech. 20, 700–707 (2009).
Wong, D. C. F., Wong, K. T. K., Goh, L. T., Heng, C. K. & Yap, M. G. S. Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO Cell Cultures. Biotechnol. Bioeng. 89, 164–177 (2005).
Hossler, P., Khattak, S. F. & Li, Z. J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 19, 936–949 (2009).
Andersen DC. Cell culture effects on the glycosylation of therapeutic proteins. Bioprocess International. (IBC Life Sciences, 2004)
Xie, H. et al. Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies. MAbs 2, 379–394 (2010).
Ehret, J., Zimmermann, M., Eichhorn, T. & Zimmer, A. Impact of cell culture media additives on IgG glycosylation produced in Chinese hamster ovary cells. Biotechnol. Bioeng. 116, 816–830 (2019).
Acknowledgements
The authors wish to acknowledge the Quality Analytical Science group in Dragonboat biopharmaceutical Co., for testing the glycosylation.
Author information
Authors and Affiliations
Contributions
J.X. wrote the main manuscript text. Z.S. and Z.W. analyzed the data by statistical software. Y.H., X.Z. and Y.S. provided the instructions for the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Shao, Z., Wang, Z. et al. Developing a medium combination to attain similar glycosylation profile to originator by DoE and cluster analysis method. Sci Rep 11, 7103 (2021). https://doi.org/10.1038/s41598-021-86447-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-86447-0