Abstract
We conducted a cross-sectional study to assess the likelihood of returning for routine breast cancer screening among women who have experienced a false-positive result (FPR) and to describe the possible individual and organizational factors that could influence subsequent attendance to the screening program. Several information were collected on demographic and clinical characteristics data. Electronic data from 2014 to 2016 related to breast screening program of the Local Health Authority (LHA) of Bologna (Italy) of women between 45 and 74 years old were reviewed. A total of 4847 women experienced an FPR during mammographic screening and were recalled to subsequent round; 80.2% adhered to the screening. Mean age was 54.2 ± 8.4 years old. Women resulted to be less likely to adhere to screening if they were not-Italian (p = 0.001), if they lived in the Bologna district (p < 0.001), if they had to wait more than 5 days from II level test to end of diagnostic procedures (p = 0.001), if the diagnostic tests were performed in a hospital with the less volume of activity and higher recall rate (RR) (p < 0.001) and if they had no previous participation to screening tests (p < 0.001). Our results are consistent with previous studies, and encourages the implementation and innovation of the organizational characteristics for breast cancer screening. The success of screening programs requires an efficient indicators monitoring strategy to develop and evaluate continuous improvement processes.
Similar content being viewed by others
Introduction
Breast cancer represents the most common cancer and the first cause of cancer death among women worldwide1. The mortality attributed to breast cancer has strongly decreased in recent decades, since the institution of mammography as a screening test2,3. Despite the reduction in breast cancer mortality and morbidity, mammographic screening is also known to be associated with potential negative effects on women who undergo the tests. Part of the potential harm coming from mammographic screening is related to the chance of obtaining false-positive results (FPR). An FPR is an abnormal screening mammogram requiring supplemental imaging or biopsies to eliminate the diagnosis of cancer. FPRs are quite common among women undergoing mammography. Around 5–10% of mammograms each year could result in an FPR, but the cumulative risk for a woman undergoing several rounds of screening could be higher. It has been estimated that over a 10 years period, 50–61% of women undergoing annual mammography will experience an FPR4,5. FPR can cause long lasting psychological consequences, such as fear and anxiety, that could re-occur in occasion of future rounds of screening6,7,8. It is known that prior negative experience with cancer screening may influence a patient’s behaviors and attitudes, including the willingness to obtain future screening, but evidence on this topic seems to be conflicting.
Some studies suggest that women with false-positive mammography (FPM) are more likely to return for subsequent screening9,10,11,12,13. Some other studies, on the contrary, found lower screening re-attendance following a FPM, delays in subsequent screening and higher risk of late stage at diagnosis14,15,16,17,18,19,20,21. Lastly, further studies found no differences in re-screening rates based on screening mammography outcome22,23,24,25,26. Secondary literature suggests that the effect could vary across countries and by population. Clinical and socio-demographic characteristics of women can affect future screening attendance, including education, income, frequency of contacts with a physician, ability to take time off work, age, personal or family history of breast cancer27,28,29,30. Since regular attendance is a main requirement for a successful screening program, all factors that could deter adherence should be known and minimized.
In order to limit the potential damage caused by bad management of the mammography screening program, maximum emphasis must be placed on quality control and quality assurance. In fact, the European guidelines for quality assurance in breast cancer screening and diagnosis include a summary table of key performance indicators that should be regularly monitored31.
In order to achieve the expected benefits and minimize negative outcomes, the Italian group for mammography screening (GISMa), together with the National centre for screening monitoring (ONS) created in 2002 by the Italian Ministry of Health, promotes the collection of data on the implementation of the process and impact indicators agreed on a national level, aiming at monitoring and promoting screening programs nationwide32. Until now little data is available about factors influencing attendance at the subsequent round of screening after an FPR in our country. The aim of this study is to assess the likelihood of returning for routine breast cancer screening among women who have experienced an FPR and to describe the possible individual and organizational factors that could influence subsequent attendance to the screening program.
Methods
Study design and setting
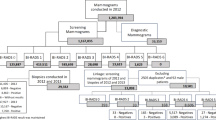
The study was carried out by retrospectively reviewing electronic data records from 2014 to 2016 related to breast screening program of the Local Health Authority (LHA) of Bologna (Italy). Women between 45 and 74 years old data were obtained; specifically, information included socio-demographic and clinical characteristics. In accordance with previous reports13,14,15,16,17,18,19,20, attendance rates at subsequent routine screening rounds were estimated after excluding women who were over the screening age range (i.e., 75 and older), had moved out of LHA of Bologna, had undergone earlier self- or general practitioner-referred private screening, or were deceased. Figure 1 details the steps of our sampling procedure.
Screening procedure
Breast screening program of the LHA of Bologna routinely screened annually women between 45 and 49 years, and each two years between 50 and 74 years. Eligible women were sent a personal letter inviting them to participate at I level screening test (mammography) at one of the two breast-unit in the area (Hospital 1 with volume of activity in the period 2014–2016 = 160,104 considering I level mammography and Hospital 2 with volume of activity in the period 2014–2016 = 22,171 considering I level mammography). Mammograms were performed by trained radiologist technician and were read independently by two board-certified radiologists with specific training in screening mammography. The evidence suggests that effectiveness of double reading is contingent on whether the two radiologists are blinded to one another's decisions, and how the decisions of the two radiologists are integrated33. If the opinions of two radiologists were conflicting, a third reading was requested; each mammogram was categorized as negative if at least 2 radiologists have defined it as negative; otherwise it was considered doubt/positive. If I level conclusion was negative, no further investigations were required; instead, if I level conclusion was doubt/positive, woman was invited to participate at II level screening tests, that provided a clinical breast examination and possible further diagnostic investigations (i.e., breast echography, further mammograms, tomosynthesis, mammotome, cytological and histological examinations). Women were considered to have undergone a false-positive cancer screening test if they had received a doubt/positive result at I level, but negative at the II one. We defined re-attendance in the screening program as participation at the next routine screening round following a screening invitation. Each round begins with the call to the I level mammography and ends with the end of the path ahead related to the invitation of interest.
Statistical analysis
The independent variables were chosen on the basis of the previous literature34,35,36,37,38 and on our hypotheses about the possible association with the dependent variable. Univariate analysis was performed using χ2 test for all categorical variables, and Student t-test for independent samples to compare all continuous variables. We have adjusted for potential counfounders using multivariable logistic regression model: independent variables for which p was 0.25 or less in univariate analysis were included in the multivariate logistic regression models. A two-sided p-value of 0.05 or less was considered as indicating a statistically significant difference. The following independent variables were included if they met the above mentioned criteria: age groups (45–49 = 0; 50–69 = 1; 70–74 = 2), nationality (Italian = 0; other = 1), district of residence (Appennino Bolognese = 1; Bologna = 2; Pianura Est = 3; Pianura Ovest = 4; Reno, Lavino, Samoggia = 5; San Lazzaro of Savena = 6), lead time between I level diagnostic mammography and I level diagnostic conclusion (≤ 10 = 1; 11–20 = 2; > 20 = 3), lead time between I level diagnostic conclusion and II level investigations (≤ 5 = 0; > 5 = 1), lead time between II level investigations and II level diagnostic conclusion (≤ 5 = 0; > 5 = 1), Hospital (Hospital 1 = 0; Hospital 2 = 1), invasive investigations (no = 0; yes = 1), previous screening tests (no = 0; yes = 1), age (continuous, in years). The results of the multivariable models are expressed as odds ratio (OR) with 95% confidence interval (95% CI) and p values. Statistical analysis was performed by using Stata Statistical Software (Version 14.0)39. We confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Ethical approval
The Ethics Committee of Local Health Authority (LHA) of Bologna (Italy) approved the protocol of the study (Prot.E.C.No. 50-2020-OSS-AUSLBO) in 23/01/2020.
Consent to participate
Considering the nature of the present study, which was based on reviewing medical records of discharged patients, no written consent was needed by the patients according to the ethics committee Area Vasta Emilia Centro della Regione Emilia-Romagna (CE-AVEC).
Results
Such as described in Fig. 1, during the study period a total of 7216 women (4% of 182,275 I level screening mammograms in 2014–2016) were invited for subsequent screening round; of these, 4847 experienced an FPR during mammographic screening and were recalled.
Overall, 3885 women (80.2%) adhered to the screening following an FPR. The characteristics of the population are shown in Table 1. Mean age was 54.2 ± 8.4 years old. The women were mostly Italian (92.8%), living in the district of Bologna (60%). Almost three quarters of the women had previous experiences with mammographic screening (72.3%). II level analyses were performed in two different settings: Hospital 1 (67.1%) and Hospital 2 (32.9%). 12% of the women underwent invasive tests, such as needle biopsy and fine needle aspiration. The time period required to complete all screening and diagnostic procedures could vary; for most women the time period from execution of I mammography screening and the availability of screening results was 10 days or less (73%) (lead time 1); lead time from I screening results to execution of II level tests was more than 5 days for 4258 women (88%) (lead time 2), in particular, we have found a significantly longer lead time 2 in Hospital 2 compared to Hospital 1 (94.4% vs 84.9%); lead time from the execution of the II level test to the conclusion of all diagnostic procedures was five or less days for 4189 women (86.6%) (lead time 3).
After the end of II level diagnostic procedures all of the women were invited to participate to the subsequent round of screening with the periodicity required by the protocol of the breast screening program.
Table 1 also shows the results from univariate analyses for factors influencing adherence to subsequent mammographic screening. Women resulted to be more likely to adhere to screening if they were Italian (χ2 = 12.08; p = 0.001), if they not lived in the Bologna district (χ2 = 36.70; p < 0.001), if they had to wait less than 5 days from the result of screening mammography to the execution of the II level test (χ2 = 8.52; p = 0.004), if they had to wait less than 5 days from II level test to end of diagnostic procedures (χ2 = 11.40; p = 0.001), if the diagnostic tests were performed at Hospital 1 (χ2 = 72.38; p < 0.001) and if they had previous participation to screening tests (χ2 = 19.76; p < 0.001). Multivariate stepwise logistic regression analysis results underlined those of the univariate analysis, except for lead time 2 (OR = 0.79; 95% CI 0.62–1.00; p = 0.057 ) (Table 2): women resulted to be less likely to adhere to screening if they were not-Italian compared to Italian (OR = 0.73; 95% CI 0.56–0.94; p = 0.014), if they lived in the Bologna district compared to who lived in San Lazzaro district (OR = 1.56; 95% CI 1.11–2.20; p = 0.011), if they had to wait more than 5 days from II level test to end of diagnostic procedures compared to who had to wait less than 5 days (OR = 0.46; 95% CI 0.29–0.74; p = 0.001), if the diagnostic tests were performed at Hospital 2 compared to Hospital 1 (OR = 0.56; 95% CI 0.47–0.67; p < 0.001) and if they had no previous participation to screening tests compared to who had did it (OR = 1.51; 95% CI 1.27–1.79; p < 0.001).
Discussion
Since the success of screening programs depends on high adherence rates, our study provides evidence about factors that can reduce adherence of women who have received an FPR. In U.S., women are more likely to re-screen after an FPR (RR 1.07, 95% CI 1.02–12), while in Europe and Canada the odds of future screening are lower (RR 0.97, 95% CI 0.93–1.01; RR 0.63, 95% CI 0.50–0.80, respectively)40,41. Consistent with other studies, we have found that 80.2% of women with an FPR returned for a subsequent screening mammogram; this result is in line with 78.3% highlighted by Alamo-Junquera et al. in 201234, and 82% by DeFrank et al. in 201242.
Other factors could influence the return for subsequent screening after an FPR, such as the nature and the extent of supplemental tests needed after mammography. Assessment can include non invasive tests as further mammograms, physical examination, ultrasound, and invasive tests as fine needle aspiration cytology (FNAC) or surgical biopsy. Furthermore, Alamo-Junquera et al. have found that women undergoing biopsy had lower re-attendance to future screenings compared to those who did not (OR = 0.56; 95% CI 0.53–0.59)34, whereas in our population it was not a significant difference. Other factors associated with high risk of failing to re-screen have been shown in literature: delays on invitation, longer waiting times in the mammography unit, difficulties in reaching the unit and absence of a formal reminder system35.
Our results show a lower adherence in not-Italian women compared to Italian (73% vs 80.7%). This result may depend on difficulties in communication and a poor understanding of the screening program. Some ethnic groups may also have a poor perception of risk due to low literacy levels about breast cancer risk and belonging to hard-to-reach populations36. In this view, health education and health literacy programs could have an impact in improving adherence to the screening program in this populations. Multi-lingual health service pathways should be improved, creating a bond between social and health professionals and cultural mediators; primary care team members may also play an important role in providing information and advice43.
Women living outside Bologna city area showed more adherence to the screening program than those who living in the city (from 82.7 to 87.2% vs 77.4%). In those contexts, it is much more difficult to turn towards private centers, outside of the Italian healthcare service, in order to reduce waiting times. In addition, the regional healthcare service created points of access using mobile clinics in territories far from the city center where it is much more difficult to find alternatives through private hospitals/centers.
Our findings, at the multivariate analysis, show less adherence in women who had to wait more than 5 days from the second level test to the final diagnostic result, compared to who wait less or equal than 5 days (75.2% vs 80.9%); this time was defined as the “Delay between the assessment and the assessment result” and included in the structural, logistic, organizational and functional indicators according to the GISMa32. Moreover, it was considered by the “European guidelines for quality assurance in breast cancer screening and diagnosis” as a cause of anxiety, which sometimes may be considerable; therefore, targets should be set in terms of working days (w.d.) at every stage where delay may arise33. Several studies in scientific literature reported negative consequences on screening attendance time in women who received an FPR; women who needed further investigation following a possibly positive or uncertain mammogram may be discouraged from adhering to the screening program14,15,16,17,18,19,20,21. Probably, the discomfort already encountered when undergoing a mammography, increased by an FPR, may result in a resilience in going on in further investigations.
Among the indicators and standards for the process evaluation of breast cancer screening, GISMa identifies a desirable “Recall rate (RR)—Further assessment rate” of less than 3% for the baseline test and less than 5% for follow-up tests32. The RR is defined as: “the percentage of women who have participated to the mammography screening program who have performed further investigation”. These analyses, which are performed to clarify the nature of an abnormality highlighted by the first level screening mammography can be performed after a woman's recall at a later screening session or at the same level session32. Our study shows a greater adherence to the breast screening program in Hospital 1 (RR 4.6%) compared to Hospital 2 (RR 17.8%) (83.6% vs 73.2%). European guidelines recommend that radiologists reporting screening mammograms should read at least 5,000 cases per year. Our results show longer wait time for second test at Hospital 2 (p < 0.001), evidencing that the volume of activity can influence the accuracy of cancer diagnosis and the efficiency of screening, as repeatedly observed in other European programs37,38. It has been shown that the volume of procedures or patients is a key determinant of quality and efficiency44. This indicator should be periodically monitored because a recall with a subsequent benignity outcome represents a negative effect of the screening program and results into additional costs. In addition, women who have experienced an FPRs are unnecessarily worried and some may feel distress which affects their ability to do their normal day-to-day activities. This personal feeling can have a negative impact also on the adherence for the subsequent screening rounds18. Hence, the optimal ratio between detection rate and RR must be improved, especially in case of FPR.
Our study has some limitations. The "lead time 2" may also be influenced by the woman's availability to carry out the diagnostic investigations. We have not studied the "delays on invitation" which could affect the adherence, possibly because women could enter into opportunistic screening, as shown in previous studies35. Moreover, the Bologna LHA does not have a “formal reminder system" which may reduce the probability that the woman will not perform the booked diagnostic examination.
As highlighted in other similar studies, our data are aggregated and anonymized; thus, we do not have access to detailed demographic risk factors beyond age. As a result, we cannot perform subgroup analyses on variables that are known to affect attendance45. Finally, we have not considered/excluded women who have developed cancer in the call range after the FPR.
Despite the mentioned weaknesses, our analysis encourages the implementation and innovation of the organizational characteristics for breast cancer screening; moreover, our study highlights the relevance of adhering to the RR indicator to avoid low participation by women. The success of screening programs requires an efficient indicators monitoring strategy to develop and evaluate continuous improvement processes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ghoncheh, M., Pournamdar, Z. & Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 17, 43–46 (2016).
Nelson, H. D. et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 151, 727–742 (2009).
Tabar, L. et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 361, 1405–1410 (2003).
Hubbard, R. A. et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann. Intern. Med. 155, 481–492 (2011).
Elmore, J. G. et al. Ten-year risk of false-positive screening mammograms and clinical breast examinations. N. Engl. J. Med. 338, 1089–1096 (1998).
Schwartz, L. M., Woloshin, S., Fowler, F. J. Jr. & Welch, H. G. Enthusiasm for cancer screening in the United States. JAMA 291, 71–78 (2004).
Brett, J., Austoker, J. & Ong, G. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last breast screening appointment. J. Public Health Med. 20, 396–403 (1998).
Health Quality Ontario. Women’s experiences of inaccurate breast cancer screening results: a systematic review and qualitative meta-synthesis. Ont. Health Technol. Assess. Ser. 16, 1–22 (2016).
Burman, M. L., Taplin, S. H., Herta, D. F. & Elmore, J. G. Effect of false-positive mammograms on interval breast cancer screening in a health maintenance organization. Ann. Intern. Med. 131, 1–6 (1999).
Taksler, G. B., Keating, N. L. & Rothberg, M. B. Implications of false-positive results for future cancer screenings. Cancer 124, 2390–2398 (2018).
El Hachem, Z., Zoghbi, M. & Hallit, S. Psychosocial consequences of false-positive results in screening mammography. J. Fam. Med. Prim. Care. 8, 419–425 (2019).
Lipkus, I. M., Halabi, S., Strigo, T. S. & Rimer, B. K. The impact of abnormal mammograms on psychosocial outcomes and subsequent screening. Psychooncology 9, 402–410 (2000).
Pinckney, R. G., Geller, B. M., Burman, M. & Littenberg, B. Effect of false-positive mammograms on return for subsequent screening mammography. Am. J. Med. 114, 120–125 (2003).
Román, R. et al. Effect of false-positives and women’s characteristics on long-term adherence to breast cancer screening. Breast Cancer Res. Treat. 130, 543–552 (2011).
Setz-Pels, W. et al. Re-attendance after false-positive screening mammography: a population-based study in the Netherlands. Br. J. Cancer 109, 2044–2050 (2013).
Nelson, H. D. et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. preventive services task force recommendation. Ann. Intern. Med. 164, 256–267 (2016).
McCann, J., Stockton, D. & Godward, S. Impact of false-positive mammography on subsequent screening attendance and risk of cancer. Breast Cancer Res. 4, R11 (2002).
Brett, J. & Austoker, J. Women who are recalled for further investigation for breast screening: psychological consequences 3 years after recall and factors affecting re-attendance. J. Public Health Med. 23, 292–300 (2001).
Dabbous, F. M. et al. Impact of a false-positive screening mammogram on subsequent screening behavior and stage at breast cancer diagnosis. Cancer Epidemiol. Biomark. Prev. 26, 397–403 (2017).
Hofvind, S. S., Wang, H. & Thoresen, S. The Norwegian Breast Cancer Screening Program: re-attendance related to the women’s experiences, intentions and previous screening result. Cancer Causes Control 14, 391–398 (2003).
Chiarelli, A. M. et al. False-positive result and reattendance in the Ontario Breast Screening Program. J. Med. Screen. 10, 129–133 (2003).
Hardesty, L. A., Lind, K. E. & Gutierrez, E. J. Compliance with screening mammography guidelines after a false-positive mammogram. J. Am. Coll. Radiol. 13, 1032–1038 (2016).
Lerman, C. et al. Psychological and behavioral implications of abnormal mammograms. Ann. Intern. Med. 114, 657–661 (1991).
Pisano, E. D., Earp, J., Schell, M., Vokaty, K. & Denham, A. Screening behavior of women after a false-positive mammogram. Radiology 208, 245–249 (1998).
O’Sullivan, I., Sutton, S., Dixon, S. & Perry, N. False-positive results do not have a negative effect on reattendance for subsequent breast screening. J. Med. Screen. 8, 145–148 (2001).
Andersen, S. B., Vejborg, I. & von Euler-Chelpin, M. Participation behaviour following a false-positive test in the Copenhagen mammography screening program. Acta Oncol. 47, 550–555 (2008).
Sheppard, V. B. et al. Are health-care relationships important for mammography adherence in Latinas?. J. Gen. Intern. Med. 23, 2024–2030 (2008).
François, G., Van Roosbroeck, S., Hoeck, S., Markovskaia, E. & Van Hal, G. A pivotal role for the general practitioner in a mixed mammographic screening model. Rev. Epidemiol. Sante Publique 60, 150–156 (2012).
Somkin, C. P. et al. The effect of access and satisfaction on regular mammogram and Papanicolaou test screening in a multiethnic population. Med. Care 42, 914–926 (2004).
Blanchard, K. et al. Mammographic screening: patterns of use and estimated impact on breast carcinoma survival. Cancer 101, 495–507 (2004).
Perry, N. et al. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis 4th edn. (European Commission, Office for Official Publications of the European Communities, 2006).
Giordano, L., Giorgi, D., Frigerio, A. & GISMa Group. Process indicators and standards for the evaluation of breast cancer screening programs. Epidemiol. Prev. 2, 1–48 (2006).
Taylor-Phillips, S. & Stinton, C. Double reading in breast cancer screening: considerations for policy-making. Br. J. Radiol. 93, 20190610 (2020).
Alamo-Junquera, D. et al. Effect of false-positive results on re-attendance at breast cancer screening programs in Spain. Eur. J. Public Health 22, 404–408 (2012).
Goossens, M. et al. Quantifying independent risk factors for failing to rescreen in a breast cancer screening program in Flanders, Belgium. Prev. Med. 69, 280–286 (2014).
Francovich, L. et al. Cervical and breast cancer screening among immigrant women resident in Italy. Epidemiol. Prev. 41, 18–25 (2017).
Blanks, R. G., Bennett, R. L., Wallis, M. & Moss, S. Does individual program size affect screening performance? Results from the United Kingdom NHS breast screening program. J. Med. Screen. 9, 11–14 (2002).
Giordano, L. et al. Breast cancer screening in Italy: evaluating key performance indicators for time trends and activity volumes. Epidemiol. Prev. 39, 30–39 (2015).
StataCorp. ,. Stata Statistical Software: Release 14 (StataCorp LP, 2015).
Brewer, N. T., Salz, T. & Lillie, S. E. Systematic review: the long-term effects of false-positive mammograms. Ann. Intern. Med. 146, 502–510 (2007).
Salz, T., Richman, A. R. & Brewer, N. T. Meta-analyses of the effect of false-positive mammograms on generic and specific psychosocial outcomes. Psychooncology 19, 1026–1034 (2010).
DeFrank, J. T. et al. Influence of false-positive mammography results on subsequent screening: do physician recommendations buffer negative effects?. J. Med. Screen. 19, 35–41 (2012).
Austoker, J. Breast cancer screening and the primary care team. BMJ 300, 1631–1634 (1990).
Esserman, L. et al. Improving the accuracy of mammography: Volume and outcome relationships. J. Natl. Cancer Inst. 94, 369–375 (2002).
Burnside, E. S., Vulkan, D., Blanks, R. G. & Duffy, S. W. Association between screening mammography recall rate and interval cancers in the UK Breast Cancer Service Screening Program: a cohort study. Radiology 288, 47–54 (2018).
Acknowledgements
We extend our sincere thanks to Dott. Andrea Ubiali, Dott.ssa Luciana Prete and to Screening Group of the LHA of Bologna, for their contribution to the realization and publication of the study.
Funding
No external funding for this manuscript.
Author information
Authors and Affiliations
Contributions
L.S. participated in the conception and design of the study, contributed to collected the data, to the data analysis and its interpretation, and wrote the first draft of the article. L.P. collected the data, contributed to the data analysis and interpretation. F.R. contributed to the data analysis, and wrote the first draft of the article. C.B., G.S. participated in the design of the study, and contributed to the data analysis. F.M. conceptualized and designed the study, was responsible for the data analysis and interpretation, and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Squillace, L., Pizzi, L., Rallo, F. et al. Subsequent attendance in a breast cancer screening program after a false-positive result in the Local Health Authority of Bologna (Italy). Sci Rep 11, 8530 (2021). https://doi.org/10.1038/s41598-021-87864-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-87864-x