Abstract
In this study, a bacterial strain Serratia sp. was employed for the reduction of synthetically prepared different concentration of Cr(VI) solution (10, 25, 40, 50 and 100 mg/L). Cometabolism study have been carried out in the binary substrate system as well as in the tertiary substrate system. The results revealed that when glucose was added as a co-substrate, at low Cr(VI) concentration, complete reduction was achieved followed by increased biomass growth, but when Cr(VI) concentration was increased to 100 mg/L, the reduction decline to 93%. But in presence of high carbon iron filings (HCIF) as co-substrate even at higher Cr(VI) concentration i.e. 100 mg/L, 100% reduction was achieved and the cell growth continued till 124 h. The study was illustrated via Monod growth kinetic model for tertiary substrate system and the kinetic parameters revealed that the HCIF and glucose combination showed least inhibition to hexavalent chromium reduction by Serratia sp.
Similar content being viewed by others
Introduction
A prevalent environmental pollutant chromium, is widely used in different industries such as cement, tannery, smelting industries and in electroplating1 for various purposes2,3. The presence of chromium mostly occurs in two different oxidation form i.e. hexavalent and trivalent one that signifies their toxicity. Amongst the two, the former one is highly toxic because of its higher solubility4 penetrable capability through biological membranes, highly mobile and have carcinogenic, mutagenic property5 and also intramolecular nucleic acid interaction, whereas, the latter one is less toxic, act as an important element in trace amount5 and important in metabolism of carbohydrate and lipids6. Having mutagenic and carcinogenic effects on humans such as internal hemorrhage, nausea, dermatitis, asthma, liver and kidney damage7 based on this, the powerful pollutant has been recognized as type I carcinogen and been further assigned the limit of total chromium presence in drinking water to less than 0.1 mg L−1 by the International Agency for Research on Cancer (IARC) and the United States Environmental Protection Agency (USEPA) respectively8. Cr(VI) reduction through conventional methods like chemical reduction, reverse osmosis, ion exchange, precipitation, adsorption and coagulation have major drawbacks including generation of secondary pollutants and high operating cost9,10,11. High carbon iron filings (HCIF) may offer the best choice as a reactive media due to its non-toxicity, low cost and availability. HCIF is capable of adsorbing, reducing and transforming many chlorinated hydrocarbons, heavy metals, and nitroaromatic compounds. A study by10 demonstrated the use of HCIF in Cr(VI) reduction and the results revealed that Cr(VI) adsorption to graphite inclusions that are present on HCIF had a great role in reduction of aqueous concentration. An experiment conducted by12 for effective removal of Cr(VI) from wastewater using ultrasonic pretreated sludge derived stable magnetic active carbon. The synthesized UMC had a high proportion of ZVI. Both the ZVI and carbon in the UMC was disclosed to be the domain electron donors for treating Cr(VI)-containing wastewater having concentration 2 mg/L. Another method of Cr(VI) removal was adopted by13 using Fluorine and nitrogen co-doped magnetic carbons (FN-MCs) which showed good removal efficiency. But the above mentioned methods have certain shortcomings like generation of secondary pollutants and high operational cost. An eco-friendly approach towards treatment of hexavalent chromium is biological reduction just before the disposal of wastewater into the environment by industries14. There are a number of microorganisms involved in the process of reduction and has been proved to be effective in this context15,16,17,18. But some of the forces hinders the overall process of microbial reduction. Focus should be given on reducing the hindrance such as adequate amount of nitrogen and carbon source, suitable pH and temperature suitability of wastewater, and the other toxic heavy metals presence in wastewater. These problems create difficulty for the non-indigenous microorganisms to effectively treat the wastewater.
Cometabolic degradation has been found to be eco-friendly and economically feasible in the process of treating recalcitrant compounds19, and coking wastewater (CWW) treatment20. Many studies stated that when phenol was used as the cometabolic substrate, the degradation efficiency of p-nitrophenol21, carbazole22 and 4-chlorophenol23 increased. In cometabolism, metabolic substrates such as glucose, methyl alcohol are sufficient source of carbon and energy for microbes to grow. These substrates induces enzymes synthesis that accelerates metabolism of growth and non-growth substrates, producing NADH as electron donor in metabolic pathway and subsequently these metabolites take part in degradation of various compounds24. Little attention has been given to cometabolic reduction of hexavalent chromium.
Earlier in our previous work, Serratia sp. was able to reduce different range of hexavalent chromium. But the inhibition effect of hexavalent chromium was higher for Serratia sp. As a result, the growth of the bacterial species was hindered after few hr25. To reduce the inhibitory effect of Cr(VI) on the bacteria, this study demonstrates the use of glucose and high carbon iron filings (HCIF) as cometabolic substrates for microbial reduction of Cr(VI).
Materials and methods
Chemicals
The chemicals, reagents and microbial media were of pure grade. Cr(VI) standard solution of 1000 mg L−1 have been prepared by mixing K2CrO7 in Milli Q water. For measuring hexavalent chromium concentration, Diphenyl carbazide (DPC) solution (0.25%) was prepared by dissolving it in 95%. Microbes were grown in broth media of Lureia Bertani (Hi-Media India). Glucose (Rankem, purity 99.0%) were used as cometabolic substrates.
Experimental process
Bacterial strain was isolated from iron ore mines as discussed in the work done previously by25. Commercially available cast iron rod was chipped on a lathe machine and was brought into iron filings in a dough-sized ball mill. HCIF thus obtained were washed with N2 sparged 1 N HCl for 4–5 times so that the organic material adsorbed on HCIF surface during chipping process may be removed as well as the reactivity of HCIF may be enhanced. Afterwards, HCIF was washed again with N2 sparged milli Q water for 10–12 times to remove excess HCl. Later, moisture content of HCIF was removed by washing with 95% acetone and HCIF were dried in N2 atmosphere in a vacuum desiccator and then used for the experimental purpose. Cometabolism studies were conducted for enhancing the reduction capacity of the HCIF-bacteria system. Batch experiments were conducted with two different combination (i) Binary system containing bacteria and glucose (1 g/250 mL) and (ii) Tertiary system with bacteria, HCIF (1 g/250 mL) and glucose (1 g/250 mL) having different Cr(VI) concentration (10, 25, 40, 50, and 100 mg/L) in an incubator shaker at 35 °C and 120 rpm. Samples were taken at every 12 h and analysis of Cr(VI), biomass, and glucose concentration was measured by Uv–Vis spectrophotometer at 540, 600 and 510 nm, respectively. Samples were taken at regular time and concentration of Cr(VI), bacterial growth, and glucose was measured by Uv–Vis spectrophotometer at wavelength of 540, 600 and 510 nm respectively.
Rate kinetics model
The pseudo first and second order was applied to define the kinetic degradation of microbe’s kinetics of hexavalent chromium which is given below:
where, C is the concentration at any time, initial concentration is denoted as CO, k is the pseudo first-order rate constant (day−1), and reaction time is denoted by t.
Kinetics of microbial growth in system containing single substrate
Growth kinetics in single system comprises of bacteria and glucose and chromium exposure. In a batch system, the specific growth rate of a cell, µ (h−1) is defined as
where, µ is calculated at the exponential phase of the growth curve. X is the concentration of cell in either cell number at time ‘t’ based on the viable counts (CFU/mL) or in either g/L (dry basis). The specific growth rate of cell on single substrate is expressed in terms of µ in Eq. (2), which is a function of concentration of resource. The Haldane Andrew model was applied here in the single substrate system as it has its wide use in representing the growth kinetics of single substrate:
where, µmax is the maximum specific growth rate (day−1), substrate concentration is defined by the term ‘S’ and Ks is the substrate affinity constant (mg/L).
Growth kinetics in tertiary substrate system
To stimulate Co-metabolism, for the cell growth on mixtures of substrate, various models have been proposed. In this study, Monod model used in binary substrate system19 was modified to tertiary substrate system for the analysis of growth kinetic parameters. The equation for the specific growth rate on tertiary inhibitory substrates was as follows:
where the subscripts C, P, and h represents glucose as a carbon source, Cr(VI) and HCIF respectively. The physical meaning of Ks and µmax is basically the same as described in Eq. (4) and obtained in single system. µmaxCSC, denotes maximum specific growth rate in presence of glucose which was obtained from the results of growth kinetics in binary substrate system, Similarly, µmaxPSP, is denoted by maximum specific growth rate in presence of chromium and µmaxhSh, is denoted by maximum specific growth rate in presence of HCIF which were obtained from the model fitting of growth kinetics in binary substrate system. Equation (4) implies that there are kinetic interactions between all the three substrates if all K2i, K3i, and K4i (i = C, P and h) are not equal to zero. Here, the literal meaning of K2, K3 and K4 is described below:
-
\({K}_{2 C }{S}_{P}\) = Inhibition of glucose in the presence of Cr(VI)
-
\({K}_{3 C}{S}_{h}\) = Inhibition of glucose in the presence of HCIF
-
\({K}_{4C}{S}_{P}{S}_{h}\) = Inhibition of glucose in the presence of Cr(VI) and HCIF
-
\({K}_{2P}{S}_{C}\) = Inhibition of Cr(VI) in the presence of glucose
-
\({K}_{3P}{S}_{h}\) = Inhibition of Cr(VI) in the presence of HCIF
-
\({K}_{4P}{S}_{C}{S}_{h}\) = Inhibition of Cr(VI) in the presence of glucose and HCIF
-
\({K}_{2h}{S}_{C}\) = Inhibition of HCIF in the presence of glucose
-
\({K}_{3h}{S}_{P}\) = Inhibition of HCIF in the presence of Cr(VI)
-
\({K}_{4h}{S}_{C}{S}_{P}\) = Inhibition of HCIF in the presence of glucose and Cr(VI).
Statistical analysis
The experiments have been performed in triplicates and mean of three samples were taken. To determine the mean and standard deviation of the data sets, XLSTAT package of Microsoft excel 2013 was used. One way ANOVA followed by Duncan’s post hoc test was performed to determine the biomass concentration of the test isolate in absence and presence of chromium.
Results and discussion
Reduction of Cr(VI) in batch reactors in the presence of cometabolic substrate (HCIF and glucose)
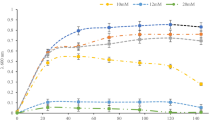
The strain isolated was identified as Serratia sp.25. It was used in our previous study for reduction of different concentration of Cr(VI) in two different ways, i.e. one by the strain alone and the other by co-assistance of HCIF with the strain. This study was performed to demonstrate the cometabolic effect of substrate on reduction of hexavalent chromium. Cometabolism study was conducted to evaluate the cometabolic activity of Serratia sp. in two different sets i.e. when glucose was added (1 g/250 mL) to batch reactors containing Cr(VI) and bacteria (Set 1) and when glucose was added to batch reactors containing Cr(VI), bacteria and HCIF (Set 2) for the reduction of hexavalent chromium by Serratia sp. The biomass concentration, Cr(VI) concentration and glucose concentration was measured in the two sets. Figure 1A,B, shows the biomass abundance at different concentration of Cr(VI) (10, 25, 40, 50, and 100 mg/L) when glucose was added as cometabolic substrate in both the sets. The results of Set 1 indicated that when glucose was added to batch reactors containing only bacteria and Cr(VI), the bacterial growth was highest for 10 mg/L Cr(VI) solution, the growth increased till 50 h and after which it started declining. The lesser the concentration of Cr(VI), the higher was the bacterial growth. For 25 mg/L Cr(VI) concentration, the growth was highest at 24 h and then it declined and lasted till 60 h. In case of 50 mg/L of Cr(VI) concentration, the biomass growth was very low in comparison to other concentrations. However, slight increase in growth was observed at 24 h. In case of 100 mg/L of Cr(VI) concentration, the bacteria attained its log phase at 50 h and after that the stationary phase was observed. In this case the lag phase increased with increase in initial Cr(VI) concentration which may be due to the inhibitory effect of Cr(VI) on the growth of microorganism. At high concentrations, the inhibitory effect increased due to the fact that a fixed amount of inoculum was used for all different concentration of Cr(VI). In the second experimental set (Set 2), it was found out that at all the different concentration of Cr(VI) (10, 25, 40, 50 and 100 mg/L) the log phase started after 50 h and the cell doubling continues till 85 h. After this the decline in biomass concentration was observed. The results from the study indicated that when only glucose acted as co-metabolic substrate, the biomass growth lasted till 60 h but when glucose was added to the HCIF and bacterial set, the bacterial growth continues till 120 h. This may be due to the reason that, HCIF may be acting as a co-metabolic substrate in enhancing the bacterial growth along with the glucose. HCIF too acts as a source of growth to bacteria like glucose, when supplied with low dosing26.
Experiments were conducted for evaluating the reduction in Cr(VI) concentration with respect to time for the same experimental set as discussed above (Set 1 and Set 2). For Set 1, the results indicated that complete reduction of 10 mg/L and 25 mg/L Cr(VI) concentration was achieved at 40–50 h and 75–76 h, respectively, and similarly for 40 mg/L and 50 mg/L Cr(VI) concentration, the complete reduction was achieved at 80 h. But in case of 100 mg/L Cr(VI) concentration, complete reduction was not achieved till 90 h of experiment (Fig. 2A). At lower concentration of Cr(VI) (10 and 25 mg/L) the maximum reduction was achieved in less time. The reason may be due to the presence of high concentration gradient of Cr in the solution, makes it more difficult to reduce completely27. Although glucose was added as a co-metabolic substrate for enhancing the activity of microbes, complete reduction efficiency was not achieved.
The results from the second experimental set (Set 2) showed that for 10 and 25 mg/L Cr(VI) concentration, the reduction was initiated after 15 h and complete reduction was achieved between 30 and 40 h. In case of 40 and 50 mg/L Cr(VI) concentration, the complete reduction was achieved at 60 h. For 100 mg/L Cr(VI) concentration, the reduction was initially slow but after 70 h the reduction was faster and it was completely achieved at 120 h (Fig. 2B). This result was better in comparison to the first experimental set. The results clearly indicated that HCIF was also acting as a co-metabolic substrate in enhancing the reduction efficiency of Cr(VI). The possible mechanism behind improved Cr(VI) reduction is due to coexistence of Serratia sp. with HCIF which helped in reduction of Cr(VI) as well as helped in maintaining the longevity of HCIF. Secondly the strain reduces Fe(III) to Fe(II), resulting in higher dissolved Fe(II) and thus maintaining the structured morphology of HCIF by removing the passivated ferric precipitates on iron surface. It has been confirmed by28 also that in the presence of NZVI, the Cr(VI) reduction efficiency by strain DIRB HS01could be improved. Similar type of cometabolic study has been performed by29 in which cometabolic degradation of blended biodiesel by a fungal strain Monilliella wahieum Y12T was carried out. The results showed that degradation of petroleum diesel (ULSD) was enhanced when the fungal strain was used with biodiesel. Another experiment conducted by30 demonstrated the effect of hydroxypropyl-β-cyclodextrin on cometabolism of phenol and phenanthrene by Chryseobacterium sp. The results stated that cometabolic activity of phenol and hydroxypropyl-β-cyclodextrin accelerated the degradation of phenol and had great phenanthrene removal rate. Addition of hydroxypropyl-β-cyclodextrin led to increased solubility and phenanthrene toxicity was also reduced thus had improved cometabolic degradation.
Glucose concentration was also determined at different Cr(VI) concentration at different time interval. Glucose is highly consumed by microbes for their growth and in enhancement of any kind of bacterial activity. Bacterial growth and glucose consumption are inversely proportional to each other. As bacterial growth tends to increase, the glucose consumption also increases because glucose is needed for growth purpose. The similar trend was observed in this study. For Set 1, with doubling of cell number, the glucose consumption increased and was highest at 24 h when the bacterial growth attended its log phase, as discussed above. The glucose concentration started declining after 24 h and it was negligible at 60 h (Fig. 3A). With decrease in glucose concentration, the bacterial growth also declined. The limited amount of glucose available for consumption will automatically destroy the population due to competition for limited amount of food source.
In Set 2, the results indicated that glucose concentration declined slowly with respect to time and the complete decline was observed at 120 h (Fig. 3B).This may be due to the presence of HCIF which also served as a source of growth for microbes, so the glucose consumption in this case was less as compared to set 1 where only glucose was present. As there is presence of two co-metabolic substrate, the burden on substrates may get reduced19. The rate kinetics model followed pseudo first order in the case when only glucose was present as substrate (Set 1) and in Set 2 when glucose and HCIF was present as substrates, it followed pseudo second order (Table 1). The rate constant was higher in case of Set 2, when both the substrates were present due to which the Cr(VI) reduced completely. The rate decreased with increase in Cr(VI) concentration in both the Sets 1 and 2. One way ANOVA followed by Duncan’s post hoc test results confirmed that the results were statistically different (Table 2).
Kinetics of microbial growth in single and tertiary substrate system
The experimentally acquired data for the specific growth rate of the strain at its growth in single substrate system consisting of different chromium concentration as substrate 1, different glucose concentration as substrate 2 and different HCIF concentration as substrate 3 were used to fit the stated kinetic models using the nonlinear regression analysis in GraphPad Prism 6 software for evaluating the kinetic parameters. Among other inhibitory growth kinetic models, Haldane Andrews’s model gave the best fit for the experimentally acquired data for all substrates (Fig. 4A–C). The R2 value for only chromium as a substrate was found to be 0.977, µmax value 0.002346 and the inhibition coefficient value to be 134.12. When only glucose was added as a substrate, the µmax and inhibition coefficient were found to be 0.1129 and 1.01 respectively with R2 0.9916. In case of HCIF, the µmax and the inhibition coefficient were 0.1923 and 11.99 respectively and R2 value was 0.9876. The values of specific growth rate obtained from fitting the values of single substrate system was later applied to the various inhibitory growth models to check for the cometabolic activity in tertiary substrate system.
Out of the other inhibitory models used for fitting, Monod model was best fitted for the growth kinetics in tertiary substrate system in order to evaluate the interaction between the three co-metabolic substrate (i.e. HCIF and glucose and chromium) in enhancing Cr(VI) reduction. Previous studies31 showed that addition of some co-metabolic substrate to the system was effective in dealing the degradation of refractory compounds. To understand the co-metabolism between glucose, Cr(VI) and HCIF in the tertiary system, Eq. (3) was used for the cell growth kinetic analysis. The relative kinetic parameters obtained from the analysis are presented in Table 3.The model was best fitted with the R2 value of 0.988 (Fig. 5).
The values of K2CSP (Inhibition of glucose in the presence of Cr(VI)), K3C Sh (Inhibition of glucose in the presence of HCIF) and K4C SP Sh (Inhibition of glucose in the presence of Cr(VI) and HCIF) increased with increasing Cr(VI) concentration. Amongst the three kinetic constants, the value of K3C Sh is lower as compared to K4C SP Sh and K2C SP. Lower values of K3C Sh, indicates that the inhibition of glucose consumption is low in presence of HCIF due to the fact that HCIF was also providing food source for the microbial growth other than glucose. The values of K2C SP and K4C SP Sh revealed that when only Cr was present (K2C SP) the inhibition on glucose consumption was higher as compared to when both Cr and HCIF (K4C SP Sh) were present. Similarly, amongst the other three kinetic constant values K2P SC (Inhibition of Cr(VI) reduction in the presence of glucose), K3P Sh (Inhibition of Cr(VI) reduction in the presence of HCIF) and K4P SPSC (Inhibition of Cr(VI) reduction in the presence of glucose and HCIF), K2P SC values were higher which indicates that when only glucose was present (K2P SC) inhibition on Cr reduction was higher followed by inhibition on Cr reduction when only HCIF was present (K3P Sh). But when both substrates (glucose and HCIF) were present (K4P SPSC), the inhibition on Cr reduction was lowest. Inhibition of HCIF consumption due to glucose (K2h SC) is lowest as compared to inhibition of HCIF consumption in presence of both Cr and glucose (K4h SPSC) and when only Cr was present (K3h SP). When HCIF and glucose were present, microbes were utilizing both glucose and HCIF for their growth. The higher values of K2P and K3P suggested high degree of inhibition of glucose and HCIF on Cr(VI) reduction and diauxic growth of the strain. Similarly lower values of K2C and K3C indicated the negligible impact of Cr(VI) imposed to glucose and HCIF consumption. It has been studied by32 that for the growth of cell on mixed substrates, there are categorization of the interaction between the growth substrates which is defined as competitive, noncompetitive and uncompetitive inhibition of growth. On the basis of these observations, the results showed that glucose and Cr(VI) were not structurally similar, so uncompetitive inhibition was the best interpretation to the interaction between glucose and Cr(VI). The interaction constants K2h and K3h (inhibition of iron due to glucose and Cr(VI) respectively) were one to threefold larger than the corresponding K2C and K3C. Thus it can be inferred that another possible mixed pattern of interaction exists involving both competitive and uncompetitive inhibition. The values of kinetic constants K4C, K4P and K4h indicated that K4P and K4h values are lower as compared to the value of K4C. This may be due to the reason that the inhibition factor was more in case when there were presence of Cr and HCIF which poses competitive inhibition. The results confirmed that both glucose and HCIF as a co-metabolic substrate had a great role in Cr(VI) reduction, but when HCIF was added as another substrate, it increased the reduction efficiency of the system. The strain utilized glucose as well as HCIF for maintaining their activity and for their growth. It has been reported by33,34 that two substrates (glucose and biphenyl) had great role in bioremediation of PBDEs. However, a large number of reports35 indicated that optimum dose of co-substrates is essential to use because excessive dose of any of the co-substrates hampers the degradation efficiency and was attributed for the inhibition between the substrates. Excess dosage of substrates would cause self-inhibition on growth of the cell, thus decrease reduction efficiency. Therefore, it is extremely important to choose appropriate co-substrate and optimum dosage of substrate.
Conclusion
The efficiency of Cr(VI) reduction by Serratia sp. was improved by cometabolism activity. Meanwhile, it is known that presence of diversity of carbon sources impose effects on the interactive activity of metabolic pathways and substrates. Glucose had great role as anti-competitive inhibitor in Cr(VI) reduction. However, on the other side, another substrate HCIF had both competitive and uncompetitive inhibition on Cr(VI) reduction by enhancing the strain ability to help in complete reduction. Both glucose and HCIF as a co-metabolic substrate had a great role in Cr(VI) reduction, but when HCIF was added as another substrate, it increased the reduction efficiency of the system and combination of HCIF and glucose showed least inhibition to hexavalent chromium reduction by Serratia sp. The outcomes of the current work enlightens a noteworthy approach to carry out treatment of other compounds as well and a great finding for enhancing long term performance of ZVI PRBs in combination with Serratia Sp. for remediation of Cr(VI) contaminated sites.
References
Sathvikaa, T. et al. A co-operative endeavor by nitrifying bacteria Nitrosomonas and Zirconium based metal organic framework to remove hexavalent chromium. Chem. Eng. J. 360, 879–889 (2019).
Pradhan, D., Sukla, L. B., Sawyer, M. & Rahman, P. K. S. M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 55, 1–20 (2017).
Wua, M. et al. Bioreduction of hexavalent chromium using a novel strain CRB-7 immobilized on multiple materials. J. Hazard. Mater. 368, 412–420 (2019).
Di Palma, L., Verdone, N. & Vilardi, G. Kinetic modeling of Cr(VI) reduction by nZVI in soil: The influence of organic matter and manganese oxide. Bull. Environ. Contam. Toxicol. 101(6), 692–697 (2018).
Brasili, E. et al. Remediation of hexavalent chromium contaminated water through zero-valent iron nanoparticles and effects on tomato plant growth performance. Sci. Rep. 10(1), 1–11 (2020).
Lin, C. J. et al. Chromate reduction by zero-valent Al metal as catalyzed by polyoxometalate. Water Res. 43, 5015–5022 (2009).
Mpouras, T., Polydera, A., Dermatas, D., Verdone, N. & Vilardi, G. Multi wall carbon nanotubes application for treatment of Cr (VI)-contaminated groundwater; Modeling of batch & column experiments. Chemosphere 269, 128749 (2020).
Scharf, B. et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 4, 5729 (2014).
Desai, C., Jain, K. & Madamwar, D. Evaluation of in vitro Cr(VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresour. Technol. 99, 6059–6069 (2008).
Srivastava, S., Yadav, G. K., Sinha, A. & Mishra, B. K. Comparative study for reduction of hexavalent chromium by high carbon iron filings (HCIF) and electrolytic Iron:mass transfer limitations. Asian. J. Chem. 27(4), 1398–1402 (2014).
Banerjeea, S., Misraa, A., Chaudhury, S. & Dama, B. A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. J. Hazard. Mater. 367, 215–223 (2019).
Gong, K. et al. Ultrasonic pretreated sludge derived stable magnetic active carbon for Cr (VI) removal from wastewater. ACS Sustain. Chem. Eng. 6(6), 7283–7291 (2018).
Huang, J. et al. Hexavalent chromium removal over magnetic carbon nanoadsorbents: Synergistic effect of fluorine and nitrogen co-doping. J. Mater. Chem. A 6(27), 13062–13074 (2018).
Singh, R., Kumar, M. & Bishnoi, N. R. Development of biomaterial for chromium (VI) detoxification using Aspergillus flavus system supported with iron. Ecol. Eng. 91, 31–40 (2016).
Arévalo-Rangel, D. L., Cárdenas-González, J. F., Martínez-Juárez, V. M. & Acosta-Rodríguez, I. Hexavalent chromate reductase activity in cell free extracts of Penicillium sp. Bioinorg. Chem. Appl. https://doi.org/10.1155/2013/909412 (2013).
Robins, K. J., Hooks, D. O., Rehm, B. H. & Ackerley, D. F. Escherichia coli NemA is an efficient chromate reductase that can be biologically immobilized to provide a cell free system for remediation of hexavalent chromium. PLoS ONE 8(3), 59–200 (2013).
Ge, S., Ge, S., Zhou, M. & Dong, X. Bioremediation of hexavalent chromate using permeabilized Brevibacterium sp. and Stenotrophomonas sp. cells. J. Environ. Manag. 157, 54–59 (2015).
Qu, M. et al. Bioremediation of hexavalent chromium contaminated soil by a bioleaching system with weak magnetic fields. Int. Biodeterior. Biodegrad. 128, 41–47 (2018).
Lv, Y. C., Li, L. H., Chen, Y. C., Tang, Z. H. & Hu, Y. Y. Effects of glucose and biphenyl on aerobic cometabolism of polybrominated diphenylethers by Pseudomonas putida: Kinetics and degradation mechanism. Int. Biodeterior. Biodegrad. 108, 76–84 (2016).
Zhoua, J. et al. Cometabolic degradation of low-strength coking wastewater and the bacterial community revealed by high-throughput sequencing. Bioresour. Technol. 245, 379–385 (2017).
Jamshidian, H., Khatami, S., Mogharei, A., Vahabzadeha, F. & Nickzad, A. Cometabolic degradation of para-nitrophenol and phenol by Ralstonia eutropha in a Kissiris immobilized cell bioreactor. Korean J. Chem. Eng. 30(11), 2052–2058 (2013).
Shi, S., Qu, Y., Zhou, H., Ma, Q. & Ma, F. Characterization of a novel cometabolic degradation carbazole pathway by a phenol-cultivated Arthrobacter sp. W1. Bioresour. Technol. 193, 281–287 (2015).
Heidari, H., Sedighi, M., Zamir, S. M. & Shojaosadati, S. A. Bisphenol A degradation by Ralstonia eutropha in the absence and presence of phenol. Int. Biodeterior. Biodegrad. 119, 37–42 (2017).
Wang, W. et al. Performance robustness of the UASB reactors treating saline phenolic wastewater and analysis of microbial community structure. J. Hazard. Mater. 331, 21–27 (2017).
Upadhyay, S., Tarafdar, A. & Sinha, A. Assessment of Serratia sp. isolated from iron ore mine in hexavalent chromium reduction: kinetics, fate and variation in cellular morphology. Environ. Technol. 41(9), 1117–1126. https://doi.org/10.1080/09593330.2018.1521875 (2020).
Jiang, C., Xu, X., Megharaj, M., Naidu, R. & Chen, Z. Inhibition or promotion of biodegradation of nitrate by Paracoccus sp. in the presence of nanoscale zero-valent iron. Sci. Total Environ. 530, 241–246 (2015).
Das, A. P. & Mishra, S. Biodegradation of the metallic carcinogen hexavalent chromium Cr (VI) by an indigenously isolated bacterial strain. J. Carcinog. 9(6). https://doi.org/10.4103/1477-3163.63584 (2010).
Shi, Z. et al. Hexavalent chromium removal by a new composite system of dissimilatory iron reduction bacteria Aeromonas hydrophila and nanoscale zero-valent iron. Chem. Eng. J. 362, 63–70 (2019).
Ye, C., Ching, T. H., Yoza, B. A., Masutani, S. & Li, Q. X. Cometabolic degradation of blended biodiesel by Moniliella wahieum Y12T and Byssochlamys nivea M1. Int. Biodeterior. Biodegrad. 125, 166–169 (2017).
Xiao, M. et al. Effect of hydroxypropyl-β-cyclodextrin on the cometabolism of phenol and phenanthrene by a novel Chryseobacterium sp. Bioresour. Technol. 273, 56–62 (2019).
Field, J. A. & Sierra-Alvarez, R. Microbial transformation and degradation of polychlorinated biphenyls. Environ. Pollut. 155(1), 1–12 (2008).
Kargi, F. & Shuler, M. L. Bioprocess Engineering: Basic Concepts (Prentice-Hall PTR, 1992).
Lu, M. et al. Biodegradation of decabromodiphenyl ether (BDE-209) by a metal resistant strain, Bacillus cereus JP12. Bioresour. Technol. 149, 8–15 (2013).
Shi, G. et al. Aerobic biotransformation of decabromodiphenyl ether (PBDE-209) by Pseudomonas aeruginosa. Chemosphere 93(8), 1487–1493 (2013).
Xin, J., Liu, X., Liu, W. & Zheng, X. L. Aerobic transformation of BDE-47 by a Pseudomonas putida sp. strain TZ-1 isolated from PBDEs-contaminated sediment. Bull. Environ. Contam. Toxicol. 93(4), 483–488 (2014).
Author information
Authors and Affiliations
Contributions
S.U. did the experimentation, modeling and wrote the manuscript. A.S. conceptualized and guided the whole work and edited final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Upadhyay, S., Sinha, A. Modeling cometabolism of hexavalent chromium by iron reducing bacteria in tertiary substrate system. Sci Rep 11, 10864 (2021). https://doi.org/10.1038/s41598-021-90137-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-90137-2