Abstract
The purpose of this study was to develop a dynamic model to predict the risk of spontaneous preterm birth at < 32 weeks in twin pregnancy. A retrospective clinical study of consecutively asymptomatic women with twin pregnancies from January 2017 to December 2019 in two tertiary medical centres was performed. Data from one centre were used to construct the model, and data from the other were used to evaluate the model. Data on maternal demographic characteristics, transvaginal cervical length and funnelling during 20–24 weeks were extracted. The prediction model was constructed with independent variables determined by multivariate logistic regression analyses. After applying specified exclusion criteria, an algorithm with maternal and biophysical factors was developed based on 88 twin pregnancies with a preterm birth < 32 weeks and 639 twin pregnancies with a delivery ≥ 32 weeks. It was then evaluated among 34 pregnancies with a preterm birth < 32 weeks and 252 pregnancies with a delivery ≥ 32 weeks in a second tertiary centre without specific training. The model reached a sensitivity of 80.00%, specificity of 88.17%, positive predictive value of 50.33% and negative predictive value of 96.71%; ROC characteristics proved that the model was superior to any single parameter with an AUC of 0.848 (all P < 0.005). We developed and validated a dynamic nomogram model to predict the individual probability of early preterm birth to better represent the complex aetiology of twin pregnancies and hopefully improve the prediction and indication of interventions.
Similar content being viewed by others
Introduction
Complications of preterm birth (PTB) are the primary cause of death among children in the first 5 years of life, accounting for approximately 35% of deaths among newborns and 18% of all paediatric deaths1. Twin gestations have increased continuously over the past decades and currently account for 3% of all live births and approximately 15–20% of all PTBs, attributable in large part to the increased use of assisted reproductive technologies2,3. Compared with singletons, the neonatal mortality rate is more than fourfold higher in twins. Additionally, the risk of neurologic morbidity in very preterm infants was 4.1% for singleton infants and 15.4% for monochorionic twins4,5,6.
To date, strategies for the prevention of PTB in twin pregnancy, such as the use of vaginal progesterone, cervical pessary and cervical cerclage, remain controversial or are considered to have limited effects7,8,9,10,11,12,13,14,15. This is partly due to results from RCTs using inefficient models of risk assessment that lead to negative results for cervical cerclage, vaginal progesterone or cervical pessary. To address the growing desire for better guidance for clinical practice, it is necessary to distinguish asymptomatic patients who are at greater risk of early PTB from the whole twin-pregnancy population.
There are discrepant opinions on how precisely the risk of spontaneous preterm birth (SPTB) in twin pregnancies can be determined. More importantly, preterm birth is a complex syndrome with many causes and phenotypes. In twins, there is an additional pre-existing risk due to overdistension and the effect on the cervix but possibly also due to increased uterine irritation and subclinical inflammation after ART or physical and psychological maternal stress factors16,17. The great variety in PTB rates signifies that there are epigenetic transgenerational stress factors and determinants from the social environment and the health care system18,19,20. In addition, perinatal morbidity and mortality among twins vary by chorionicity, and monochorionicity is significantly associated with an increased risk of PTB21.
Previous studies have demonstrated the association between SPTB in twin pregnancies and specific clinical indicators, such as ethnic origin, age, nulliparity, chorionicity, body mass index (BMI), tobacco usage, history of previous preterm delivery, cervical length and funnelling21,22,23,24,25,26,27,28,29,30,31. Different combinations of clinical variables might indicate different likelihoods of SPTB. The purpose of this study is to synthesize an array of maternal demographic factors and clinical variables and develop a practical algorithm to calculate the risk of SPTB for twin pregnancies, similar to the first trimester genetic disease screening tools or the Framingham heart disease score32.
Results
Characteristics of the development and external validation groups
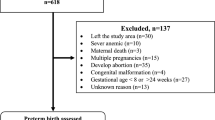
In total, 1013 asymptomatic twin pregnancies were eligible for the study, of which 727 collected from the Fujian Maternity and Child Health Hospital were assigned to the training group, while 286 from the Fujian Provincial Hospital were assigned to the external validation group (Fig. 1). In the whole study population, the numbers of positive cases of SPTB at < 28, 32, 34 and 37 weeks were 31 (3.06%), 122 (12.04%), 207 (20.43%) and 596 (58.84%), respectively.
There were no significant differences in maternal demographic and clinical characteristics between the training and validation groups (all P > 0.05), indicating that the features of the training and external validation groups were similar and that subsequent external validation would be representative (Table 1).
Predictive factors associated with SPTB at < 32 weeks
In the training group, we conducted univariate and multivariate regression analyses to detect the correlations between clinical variables and probabilities of preterm delivery before 28 weeks, 32 weeks, and 34 weeks by applying the AIC-based backward procedure (Table 2). Then, we constructed three ROC curves for predicting SPTB according to the results of multivariate analysis. By comparing the AUCs, we found that the predictive value for SPTB at < 32 weeks was the highest (Fig. 2). After comprehensively considering the predictive power and the number of positive cases of SPTB before the three gestational weeks, we chose to establish a predictive model for predicting PTB at < 32 weeks. Multivariate logistic regression analysis (< 32 weeks) showed that nulliparity, monochorionicity, lower prepregnancy BMI, previous preterm birth or late abortion, cervical funnelling and shorter cervical length were independent risk factors for SPTB at < 32 weeks.
Development and validation of a dynamic nomogram for SPTB at < 32 weeks
Based on meaningful independent factors in multivariate regression analysis, we developed a nomogram to predict SPTB probability at < 32 weeks (Fig. 3). Each point could be determined based on the intersection of the vertical line from the variable to the point axis. Then, the total risk score was calculated by adding each variable point. The possibility of twin SPTB at < 32 weeks could be read on the total point axis.
Nomogram for the prediction of PTB < 32 weeks based on six independent risk factors. To calculate the probability of PTB < 32 weeks in twin pregnancies, the point for each variable is assigned by the corresponding value of the "point" axis, and points are plotted on the total points axis. The comprehensive risk of PTB at < 32 weeks for twin pregnancy corresponds to the total points.
Furthermore, a user-friendly dynamic predicative nomogram was established and is available online (https://zhanwenqiang.shinyapps.io/DynNomapp/). The dynamic nomogram conveniently provided the individual probability of SPTB, which was calculated automatically by the input parameters of each subject (Fig. 4, PS: To facilitate readers’ understanding, we specifically recorded a video of how to use the model, which is in the attachment). Harrell's concordance index value of the nomogram model in the training group was 0.848 (95% CI 0.809–0.892). When applied to the external validation group, Harrell's concordance index value in the external group was 0.782 (95% CI 0.735–0.826). The calibration curves indicated that the probability predicted by the nomogram was in good agreement with the actual probabilities in both the internal cohort and external cohort (Fig. 5).
Screenshot of the online user-friendly model (https://zhanwenqiang.shinyapps.io/DynNomapp/) for the prediction of PTB. The users enter variables in the application tool on the left, and then the corresponding predicted probabilities and 95% confidence intervals (CIs) are displayed in the right figure. The table at the bottom right shows six examples of input variables and corresponding predicted probabilities.
Model performance test and risk stratification
Next, the restricted cubic spline curve showed that the risk escalated continuously with the increasing scores obtained from the nomogram, which proves the reliability of the model (Fig. 6). In the training group and external validation group, the AUCs of the nomogram predicting the probability of SPTB at < 32 weeks were 0.848 (95% CI 0.809–0.892) and 0.782 (95% CI 0.735–0.826), respectively. In both groups, the prediction accuracy of the nomogram was superior to that of any single predictor (all P < 0.005) (Fig. 7). With the ROC curve of the training group, the optimal cut-off value of the risk score (125.16) was calculated based on the maximum Youden index. Then, the cut-off value categorized the training population into the low-risk group (155 twin pregnancies with risk score ≤ 125.16) and the high-risk group (572 twin pregnancies with risk score > 125.16) (OR 17.09, 95% CI 10.28–28.62, P < 0.05). The model reached a sensitivity of 80.00%, specificity of 88.17%, positive predictive value (PPV) of 50.33% and negative predictive value (NPV) of 96.71%. By using the same cut-off value in the external validation group, the results also proved the predictive performance of the nomogram (Table 3). Thus, we observed that the probability of SPTB in the high-risk group was significantly higher than that in the low-risk group (HR 0.537, 95% CI (0.382–0.756), P < 0.001), and gestational age at delivery was significantly earlier in the high-risk group (Fig. 8).
Validation of the predictive accuracy of the nomogram and six individual predictors. ROC curves and AUCs were used to assess the predictive accuracy of the nomogram compared with either meaningful variable (prepregnancy BMI, nulliparity, chorionicity, previous preterm birth or late abortion, cervical funnelling, cervical length). P values show the AUC for the nomogram versus the AUC for other variables alone. AUC area under the curve, ROC receiver operating characteristic.
Discussion
In our retrospective analysis and external validation study, we developed a predictive model of SPTB at < 32 weeks based on maternal characteristics and sonographic cervical measurements to provide an accurate and comprehensive risk estimation, which can serve as an assessment tool to help physicians make decisions about further management of twin pregnancy.
The reason we comprehensively considered all the above factors when building the model was that the predictive performance of a single maternal factor or cervix geometry (including length) is not satisfactory, primarily due to poor sensitivity33,34,35,36,37. The mechanism of SPTB involves various mechanical stimuli (two continuously growing foetuses and the expanding uterus) and biochemical stimuli (inflammatory factors, fetoplacental signals and steroid hormones)38,39. Compared to that in singleton pregnancies, the mechanism of SPTB in twin pregnancies is predominantly determined by overdistension, whereas the role of inflammation and microbiologic invasion of the amniotic cavity (MIAC) is relatively minor16. Overdistension of the lower uterine segment and smooth muscle stretch in the human cervix provokes proinflammatory cytokine secretion, and research on changes in the cervical microstructure has been published by Vink et al.17,40,41. Jose Villar et al. proposed the use of a phenotypic classification system of PTB that does not force any PTB into a predefined phenotype but instead relies on a new conceptual framework in which a maternal clinical phenotype of PTB potentially related to a certain perinatal outcome is characterized by all relevant conditions observed during pregnancy18.
A series of common clinical characteristics, such as age, race, BMI, history of PTB, previous uterine surgeries, and tobacco usage, may indicate the initial states and variations in the structure and function of the cervix, which contributes to the risk of cervical insufficiency19,20,25,26,27,42,43. All these risk factors have interconnected effects and a computational framework for changing and remodelling the cervix. Our study is concordant with existing research indicating that nulliparity, lower prepregnancy BMI, history of PTB or late abortion, chorionicity, cervical funnelling and shorter cervical canal increase the possibility of SPTB in twin pregnancies. However, there is no risk calculation yet for SPTB before 32 weeks, which still represents a population with a tenfold increased risk for perinatal mortality compared to twins at term44. Our research incorporated maternal characteristics and biophysical tests of both cervical length and funnelling to develop a dynamic nomogram model that reached favourable PPV and NPV. Given that women with twin pregnancies are at high risk of preterm birth, better PPV and NPV indicate a higher rate of clinical diagnosis accuracy and a lower incidence of misdiagnosis. Therefore, our model may better guide clinical strategies, such as therapy decision-making and follow-up schedules, and could reduce complications for clinicians related to excessive monitoring and administration resulting from an undefined or inherently subjective risk assessment. Thus, the ability to generate a risk assessment and present it in the form of a percentage for each patient will enable caregivers to schedule more frequent follow-ups or administer targeted interventions, such as antenatal corticosteroids and tocolytic therapy, as well as transfer to a tertiary medical centre for patients at higher risk while reducing overtreatment and unnecessary hospitalization for those at lower risk. On the other hand, in the study design for the negative trials regarding PTB intervention, only a few researchers screened out and followed high-risk twin pregnancies, which may introduce confusion regarding indications for the interventions and result in bias when comparing outcomes7,10,11,45. To some extent, a lack of good care during surveillance frequently makes the difference in RCTs. It would be interesting in the future to determine whether the use of this tool to assess the indications for interventions and stratify patients according to risk could improve outcomes.
Our study has some limitations. Most importantly, it is limited by its retrospective design. There is a possibility of confounding bias: patients with unmeasured or unobservable factors who were excluded may represent patients at higher risk, so our study might ignore the most clinically interesting population. Second, the study population in the two centres is limited to our own population (Asian), which limits generalizability to people of different races. For example, in many high-resource countries, the risk of PTB is associated with obesity and is not underweight25,46. However, this potential limitation may also be considered a strength. All women included in the study were followed up and treated only in the two tertiary medical centres, which limits the confounding factors associated with the heterogeneity in provider bias, such as clinicians’ experience, and differences in the process of monitoring and management for offering the intervention. Based on the model, researchers in other countries can make use of their own data on demographic characteristics to justify the odds for their population. The last limitation is that because of the incomplete data for cervical length before 20 weeks, our model may poorly predict very early PTB since we adopted cervical measurements during 20–24 weeks and applied the system relatively late for the high-risk population47. In the future, we should concentrate on earlier evaluation of our algorithm to prevent early mortality and severe morbidity.
In summary, we developed and validated a dynamic nomogram model to predict the individual probability of early preterm birth; this nomogram better represents the complex aetiology of twin pregnancies and hopefully improves our understanding of the indications for interventions and, therefore, our ability to predict when they will be needed.
Materials and methods
Study population
We retrospectively collected data from 1461 consecutively asymptomatic women with twin pregnancies in the Fujian Maternity and Child Health Hospital (with an annual delivery number of more than 20,000 and a specified preterm birth clinic for ambulatory patients) and the Fujian Provincial Hospital (with 2398 beds and an annual delivery number of more than 5000) from January 2017 to December 2019. This retrospective study was performed with approval from the Ethics Committee of the Fujian Maternity and Child Health Hospital and the Fujian Provincial Hospital (Ethical approval number: 2019-014). The data were anonymous, and the requirement for informed consent was therefore waived. The completion and reporting of the study was in accordance with STROBE guidelines.
Subjects with any of the following conditions were excluded: incomplete records, genetic or structural abnormalities of either foetus, stillbirth of one or two foetuses, gestational age at birth < 20 weeks, twin birth weight < 500 g, monoamniotic or monochorionic twin pregnancy complicated by twin transfusion syndrome (TTTS) or twin anaemia–polycythaemia sequence (TAPS), placement of cervical cerclage, use of vaginal progesterone, maternal or foetal indications for iatrogenic PTB at < 32 weeks, or delivery at a medical centre other than ours. Women who gave birth before 20 weeks were excluded because in most cases, these women were likely to represent a unique subgroup of women whose cervical changes would have been detected very early and would be extremely obvious. Additionally, these women would not have had their cervical measurement at the indicated gestational stage in our study period, which was a major part of our research. As a result, we excluded 448 patients who met the exclusion criteria, and thus, 1013 patients met the inclusion criteria.
We assigned 727 samples collected from the Fujian Maternity and Child Health Hospital as the training group and 286 samples collected from the Fujian Provincial Hospital as the external validation group. All samples were reassessed by two obstetricians according to the inclusion and exclusion criteria (the flowchart showing the derivation of the development cohort and validation cohort is presented in Fig. 1).
Data collection
Medical records were surveyed retrospectively, and the following data were extracted from patients’ charts. Demographic characteristics included maternal age, prepregnancy body mass index (prepregnancy BMI), nulliparity, history of previous cervical surgery, history of tobacco usage, clinical data including validation of gestational age by first trimester ultrasound, chorionicity, history of previous preterm or late abortion (during 12–28 weeks), complications during pregnancy, use of assisted reproductive technology, cervical length (20–24 weeks) and cervical funnelling, and gestational age at delivery.
Gestational age was calculated from the last menstrual period (LMP) and confirmed by the foetal crown-rump length measurement at the first trimester ultrasonic scan. If a discrepancy of more than 7 days was observed, the sonographic gestational age was followed. Chorionicity was confirmed by identifying lambda and T signs with ultrasound imaging between 11+0 and 13+6 weeks of gestation48.
The ultrasound measurements were in accordance with a unified standard. All patients underwent transvaginal cervical length (TVCL) measurements between 20 and 24 weeks when the optimal image of the cervix was relatively easy to capture. The TVCL measurements of all subjects were performed by experienced sonographers at our ultrasound units. The ultrasound assessment was performed to measure the length of the cervical canal from the internal OS to the external OS and observe whether cervical funnelling appears with patients in the lithotomy position with an empty bladder. The measurement was repeated under gentle fundal pressure or the Valsalva maneuver unless severe cervical shortening was observed. Each examination was performed for at least 3 min as an evaluation period to detect the development of a “funnel”, which was defined as the protrusion of the amniotic membrane of 3 mm or more into the internal os as measured along the lateral border of the funnel (Fig. 9)49,50.
Statistical analysis
Model development
Quantitative data are expressed as the median (interquartile range, IQR), and qualitative data are expressed as the number (percentage). The Wilcoxon–Mann–Whitney test or Fisher’s exact test was performed to measure the distribution differences of variables between the development and external validation groups. Univariate and multivariate logistic regression analyses were used to detect the correlation between clinical variables and preterm birth at 28 weeks, 32 weeks, and 34 weeks by applying a backward procedure based on the Akaike information criterion (AIC). By drawing the ROC curve of the predicted probabilities of SPTB before three gestational weeks (28, 32, 34 weeks) with multivariate meaningful variables, the prediction power for SPTB before the three gestational weeks was compared. Based on these results, a nomogram model with higher predictive performance was established, and bootstrapping techniques were used for internal verification to improve the robustness of the model.
Model validation
The performance of the nomogram models in identification and calibration was evaluated. The discriminative ability and predictive ability of the model were evaluated through Harrell's C-index, and external crowds were introduced to further evaluate the predictive value of the model. The calibration curve was analysed by drawing the predicted probability of the nomogram and the actual occurrence of SPTB. Restricted cubic splines were used to evaluate the correlation between the model's predicted score and the risk of SPTB. Kaplan–Meier curves were generated to compare the pregnancy outcomes in the two groups with different risk stratifications. ROC curve analysis was used to evaluate the prediction performance of the nomogram model and that of each meaningful parameter.
Statistical analyses were all performed with R 3.6.0 software (R: A Language and Environment for Statistical Computing, {R Core Team}, R Foundation for Statistical Computing, Vienna, Austria, 2018, https://www.R-project.org). A two-sided P-value < 0.05 was considered to indicate statistical significance.
Ethics approval
All procedures performed in studies involving human participants were approved by the China Ethics Committee and the institutional ethical review boards of the Fujian Maternity and Child Health Hospital and the Fujian Provincial Hospital (Ethical approval number: 2019–014). Because the dataset contained no data enabling patient identification and all women received standard care, the study was exempt from informed consent requirements.
References
Oh, K. J. et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am. J. Obstet. Gynaecol. 221, e141–e140. https://doi.org/10.1016/j.ajog.2019.03.017 (2019).
Chauhan, S. P., Scardo, J. A., Hayes, E., Abuhamad, A. Z. & Berghella, V. Twins: Prevalence, problems, and preterm births. Am. J. Obstet. Gynaecol. 203, 305–315. https://doi.org/10.1016/j.ajog.2010.04.031 (2010).
Martin, J. A., Hamilton, B. E., Osterman, M. J., Curtin, S. C. & Matthews, T. J. Births: Final data for 2013. Natl. Vital Stat. Rep. 64, 1–65 (2015).
Zork, N., Biggio, J., Tita, A., Rouse, D. & Gyamfi-Bannerman, C. Decreasing prematurity in twin gestations: Predicaments and possibilities. Obstet. Gynaecol. 122, 375–379. https://doi.org/10.1097/AOG.0b013e31829b2dba (2013).
Rettwitz-Volk, W., Tran, T. M. & Veldman, A. Cerebral morbidity in preterm twins. J. Matern. Foetal. Neonatal. Med. 13, 218–223. https://doi.org/10.1080/jmf.13.4.218.223 (2003).
Taborda, A. & Oliveira, G. Neurodevelopmental outcomes of very preterm or very low birth weigth infants: Comparison of monochorionic and dichorionic twins with singletons. Acta Med. Port. 29, 702–710. https://doi.org/10.20344/amp.7079 (2016).
Rouse, D. J. et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N. Engl. J. Med. 357, 454–461. https://doi.org/10.1056/NEJMoa070641 (2007).
Committee on Practice Bulletins—Obstetrics, Society for Maternal–Foetal Medicine, Practice Bulletin No. 169: Multifoetal gestations: Twin, triplet, and higher-order multifoetal pregnancies. Obstet. Gynaecol. 128, e131–e146. https://doi.org/10.1097/AOG.0000000000001709 (2016).
Schuit, E. et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: An individual participant data meta-analysis. BJOG 122, 27–37. https://doi.org/10.1111/1471-0528.13032 (2015).
Norman, J. E. et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): A randomized, double-blind, placebo-controlled study and meta-analysis. Lancet 373, 2034–2040. https://doi.org/10.1016/s0140-6736(09)60947-8 (2009).
Liem, S. et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): A multicentre, open-label randomized controlled trial. Lancet 382, 1341–1349. https://doi.org/10.1016/s0140-6736(13)61408-7 (2013).
Merced, C. et al. Cervical pessary for preventing preterm birth in twin pregnancies with maternal short cervix after an episode of threatened preterm labour: Randomized controlled trial. Am. J. Obstet. Gynaecol. 221, e51-55. https://doi.org/10.1016/j.ajog.2019.02.035 (2019).
Li, C., Shen, J. & Hua, K. Cerclage for women with twin pregnancies: A systematic review and metaanalysis. Am. J. Obstet. Gynaecol. 220, 543–557. https://doi.org/10.1016/j.ajog.2018.11.1105 (2019).
Jarde, A. et al. Preterm birth prevention in twin pregnancies with progesterone, pessary, or cerclage: A systematic review and meta-analysis. BJOG 124, 1163–1173. https://doi.org/10.1111/1471-0528.14513 (2017).
Vincenzo, B. & Gabriele, S. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst. Rev. 9, 007235. https://doi.org/10.1002/14651858.CD007235.pub4 (2019).
Romero, R. et al. Infection and labour. Am. J. Obstet. Gynaecol. 163, 757–761. https://doi.org/10.1016/0002-9378(90)91063-i (1990).
AdamsWaldorf, K. M. et al. Uterine overdistention induces preterm labour mediated by inflammation: Observations in pregnant women and nonhuman primates. Am. J. Obstet. Gynaecol. 213, 830–831. https://doi.org/10.1016/j.ajog.2015.08.028 (2015).
Villar, J. et al. The preterm birth syndrome: A prototype phenotypic classification. Am. J. Obstet. Gynaecol. 206, 119–123. https://doi.org/10.1016/j.ajog.2011.10.866 (2012).
Culhane, J. F. & Goldenberg, R. L. Racial disparities in preterm birth. Semin. Perinatol. 35, 234–239. https://doi.org/10.1053/j.semperi.2011.02.020 (2011).
Souza, R. T. et al. Clinical and epidemiological factors associated with spontaneous preterm birth: A multicentre cohort of low risk nulliparous women. Sci. Rep. 10, 855. https://doi.org/10.1038/s41598-020-57810-4 (2020).
Marleen, S. et al. Association between chorionicity and preterm birth in twin pregnancies: A systematic review involving 29 864 twin pregnancies. BJOG https://doi.org/10.1111/1471-0528.16479 (2020).
Premru-Srsen, T., Verdenik, I., Steblovnik, L. & Ban-Frangez, H. Early prediction of spontaneous twin very preterm birth: A population based study 2002–2012. J. Matern. Foetal. Neonatal. Med. 28, 1784–1789. https://doi.org/10.3109/14767058.2014.968774 (2015).
Erez, O., Mayer, A., Shoham-Vardi, I., Dukler, D. & Mazor, M. Primiparity, assisted reproduction, and preterm birth in twin pregnancies: A population based study. Arch. Gynaecol. Obstet. 277, 311–317. https://doi.org/10.1007/s00404-007-0492-4 (2008).
Fuchs, F. & Senat, M. V. Multiple gestations and preterm birth. Semin. Foetal Neonatal Med. 21, 113–120. https://doi.org/10.1016/j.siny.2015.12.010 (2016).
Masho, S. W., Bishop, D. L. & Munn, M. Pre-pregnancy BMI and weight gain: where is the tipping point for preterm birth?. BMC Pregnancy Childbirth 13, 120. https://doi.org/10.1186/1471-2393-13-120 (2013).
McLennan, A. S. et al. The role of maternal age in twin pregnancy outcomes. Am. J. Obstet. Gynaecol. 217, e81–e80. https://doi.org/10.1016/j.ajog.2017.03.002 (2017).
Wisborg, K., Henriksen, T. B. & Secher, N. J. Maternal smoking and gestational age in twin pregnancies. Acta Obstet. Gynaecol. Scand. 80, 926–930. https://doi.org/10.1034/j.1600-0412.2001.801009.x (2001).
Easter, S. R., Little, S. E., Robinson, J. N., Mendez-Figueroa, H. & Chauhan, S. P. Obstetric history and likelihood of preterm birth of twins. Am. J. Perinatol. 35, 1023–1030. https://doi.org/10.1055/s-0037-1617758 (2018).
Yang, J. H., Kuhlman, K., Daly, S. & Berghella, V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet. Gynaecol. 15, 288–291. https://doi.org/10.1046/j.1469-0705.2000.00087.x (2000).
D’Antonio, F., Thilaganathan, B., Dias, T., Khalil, A. & Southwest Thames Obstetric Research. Influence of chorionicity and gestational age at single foetal loss on risk of preterm birth in twin pregnancy: Analysis of STORK multiple pregnancy cohort. Ultrasound Obstet. Gynaecol. 50, 723–727. https://doi.org/10.1002/uog.17426 (2017).
Saccone, G. et al. Risk of spontaneous preterm birth in IVF-conceived twin pregnancies. J. Matern. Foetal Neonatal Med. 32, 369–376. https://doi.org/10.1080/14767058.2017.1378339 (2019).
Lloyd-Jones, D. M. et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am. J. Cardiol. 94, 20–24. https://doi.org/10.1016/j.amjcard.2004.03.023 (2004).
Pagani, G., Stagnati, V., Fichera, A. & Prefumo, F. Cervical length at mid-gestation in screening for preterm birth in twin pregnancy. Ultrasound Obstet. Gynaecol. 48, 56–60. https://doi.org/10.1002/uog.15668 (2016).
Melamed, N. et al. Predictive value of cervical length in women with twin pregnancy presenting with threatened preterm labour. Ultrasound Obstet. Gynaecol. 46, 73–81. https://doi.org/10.1002/uog.14665 (2015).
Gyamfi, C., Lerner, V., Holzman, I. & Stone, J. L. Routine cervical length in twins and perinatal outcomes. Am. J. Perinatol. 24, 65–69. https://doi.org/10.1055/s-2006-958167 (2007).
Prodan, N. et al. Single and repeat cervical-length measurement in twin gestation with threatened preterm labour. Ultrasound Obstet. Gynaecol. 55, 496–501. https://doi.org/10.1002/uog.20306 (2020).
Honest, H. et al. The accuracy of maternal anthropometry measurements as predictor for spontaneous preterm birth: A systematic review. Eur. J. Obstet. Gynaecol. Reprod. Biol. 119, 11–20. https://doi.org/10.1016/j.ejogrb.2004.07.041 (2005).
Shynlova, O., Tsui, P., Jaffer, S. & Lye, S. J. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynaecol. Reprod. Biol. 144(Suppl 1), S2-10. https://doi.org/10.1016/j.ejogrb.2009.02.044 (2009).
Myers, K. M., Paskaleva, A. P., House, M. & Socrate, S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 4, 104–116. https://doi.org/10.1016/j.actbio.2007.04.009 (2008).
Vink, J. Y. et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynaecol. 215(478), e471-478. https://doi.org/10.1016/j.ajog.2016.04.053 (2016).
Vink, J. & Mourad, M. The pathophysiology of human premature cervical remodelling resulting in spontaneous preterm birth: Where are we now?. Semin. Perinatol. 41, 427–437. https://doi.org/10.1053/j.semperi.2017.07.014 (2017).
van der Ven, A. J. et al. Is cervical length associated with maternal characteristics?. Eur. J. Obstet. Gynaecol. Reprod. Biol. 188, 12–16. https://doi.org/10.1016/j.ejogrb.2015.02.032 (2015).
Dijkstra, K., Janssen, H. C., Kuczynski, E. & Lockwood, C. J. Cervical length in uncomplicated pregnancy: A study of sociodemographic predictors of cervical changes across gestation. Am. J. Obstet. Gynaecol. 180, 639–644. https://doi.org/10.1016/s0002-9378(99)70267-x (1999).
Gilbert William, M., Nesbitt Thomas, S. & Beate, D. The cost of prematurity: Quantification by gestational age and birth weight. Obstet Gynaecol. 102, 488–492. https://doi.org/10.1016/s0029-7844(03)00617-3 (2003).
Dang, V. Q. et al. Pessary compared with vaginal progesterone for the prevention of preterm birth in women with twin pregnancies and cervical length less than 38 mm: A randomized controlled trial. Obstet. Gynaecol. 133, 459–467. https://doi.org/10.1097/AOG.0000000000003136 (2019).
Pigatti Silva, F. et al. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci. Rep. https://doi.org/10.1038/s41598-019-49704-x (2019).
Honest, H. et al. Accuracy of cervical transvaginal sonography in predicting preterm birth: A systematic review. Ultrasound Obstet Gynaecol 22, 305–322. https://doi.org/10.1002/uog.202 (2003).
Khalil, A. et al. ISUOG practice guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynaecol. 47, 247–263. https://doi.org/10.1002/uog.15821 (2016).
Mancuso, M. S. et al. Cervical funneling: Effect on gestational length and ultrasound-indicated cerclage in high-risk women. Am. J. Obstet. Gynaecol. 203(259), e251-255. https://doi.org/10.1016/j.ajog.2010.07.002 (2010).
Iams, J. D. et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Foetal Medicine Unit Network. N. Engl. J. Med. 334, 567–572. https://doi.org/10.1056/NEJM199602293340904 (1996).
Acknowledgements
The study was supported by the Fujian Key Laboratory of Women and Children's Critical Diseases Research. We thank Haibo Li, PhD (Division of Birth Cohort Study, Fujian Maternity and Child Health Hospital) for his help in revising the manuscript.
Funding
This research was supported by the Fujian Key Laboratory of Women and Children's Critical Diseases Research [Fujian Maternity and Child Health Hospital], along with funds from the Fujian Maternity and Child Health Hospital Innovation Project under Contract No. YCXZ18-21.
Author information
Authors and Affiliations
Contributions
M.P., Z.L., J.Z. and W.Z. conceived the idea of this study. M.P., Z.L., J.Z. and W.Z. designed the search strategy and screened abstracts and full texts. Y.L., D.Y., L.L. and X.X. acquired the data. J.Z. and W.Z. performed the data analysis. All authors interpreted the data analysis and critically revised the manuscript. All authors had the opportunity to review the manuscript and provided their permission to publish the manuscript. All authors agree to take responsibility for the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Zhan, W., Lin, Y. et al. Development and external validation of a nomogram for predicting preterm birth at < 32 weeks in twin pregnancy. Sci Rep 11, 12430 (2021). https://doi.org/10.1038/s41598-021-91973-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-91973-y
This article is cited by
-
Development and validation of a prediction model on spontaneous preterm birth in twin pregnancy: a retrospective cohort study
Reproductive Health (2023)
-
Development and validation of a nomogram for individualized prediction of spontaneous extremely preterm birth at < 28 weeks in twin pregnancy: a retrospective cohort study
Archives of Gynecology and Obstetrics (2023)