Abstract
Hepatorenal dysfunction is a strong risk factor in patients with heart failure (HF). We investigated the prognostic significance of hepatorenal dysfunction in 172 consecutive patients undergoing transcatheter tricuspid valve repair (TTVR). The model for end-stage liver disease excluding international normalized ratio (MELD-XI) score was calculated as 5.11 × ln(serum total bilirubin [mg/dl]) + 11.76 × ln(serum creatinine [mg/dl]) + 9.44. Patients were stratified into two groups: high (≥ 14) or low (< 14) MELD-XI score, according to the best cut-off value to predict a one-year composite outcome consisting of all-cause mortality and HF hospitalization. Compared to patients with low MELD-XI score (n = 121), patients with high MELD-XI score (n = 51) had a higher incidence of the composite outcome (47.1% vs. 17.4%; p < 0.0001). In the multivariable analysis, the MELD-XI score was an independent predictor of the composite outcome (adjusted hazard ratio: 1.12; 95% confidence interval [CI] 1.05–1.19; p = 0.0003). In addition, post-procedural TR < 3 + after TTVR was independently associated with a reduction in MELD-XI score six months after TTVR (adjusted odds ratio: 3.37; 95% CI 1.09–10.40; p = 0.03). Thus, the MELD-XI score was associated with the risk of one-year composite outcome, consisting of mortality and HF hospitalization, after TTVR and may help the risk stratification in patients undergoing TTVR.
Similar content being viewed by others
Introduction
Tricuspid regurgitation (TR) is a common valvular disease in patients with heart failure (HF) and is associated with impaired functional capacity and reduced long-term survival1,2,3. The treatment options for TR are still limited, and surgical repair for isolated TR is still controversial because of the high risk associated with the surgery4,5. TR patients often have multiple comorbidities that contribute to an elevated surgical risk. Therefore, minimally invasive catheter-based procedures are thought to be a promising alternative to reduce TR with lower procedural risks6,7. Recently, encouraging results with several different approaches of transcatheter tricuspid valve repair (TTVR) have been reported8,9,10,11. Although TTVR is alternative to surgical TV repair with a comparably low level of invasiveness, approximately 40% of the TTVR patients still experience adverse clinical events, including mortality or hospitalization due to heart failure12. Data regarding the characteristics of patients who are more likely to benefit from such interventions are still lacking. Thus, it is crucial to identify simple and useful tools for risk stratification, to support clinical decision-making in TTVR.
Multi-organ dysfunction, including hepatic and renal dysfunction, affects the prognosis and complicates the management of heart failure13,14,15. Cardiac dysfunction can lead to the renal or hepatic dysfunction and vice versa, the so-called cardiorenal and cardiohepatic syndromes. TR is associated with right-heart venous congestion and reduced forward stroke volume16,17, which contributes to hepatorenal dysfunction. The presence of hepatorenal dysfunction is associated with impaired clinical prognosis in patients with HF and TR18,19,20. However, the prognostic value of hepatorenal dysfunction on TTVR has not been well studied.
The Model for End-stage Liver Disease eXcluding International normalized (MELD-XI) score is one of the scoring models that have been widely used for the assessment of renal and hepatic function21. The MELD-XI score reflects liver and renal function and is calculated based on serum total bilirubin and creatinine levels. Previous reports have shown the prognostically predictive value of the MELD-XI score in patients with HF19,20,22,23. In the present study, we investigated the association between the MELD-XI score and clinical outcome after TTVR.
Results
Clinical characteristics of the study population
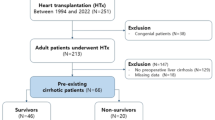
Of 195 patients who underwent their first TTVR, 23 patients were excluded from the present analysis, including 4 patients with hemodialysis, 11 patients with concomitant transcatheter mitral valve repair, and 8 patients without sufficient laboratory data for assessments of the MELD-XI score. Consecutively, a total of 172 patients were analyzed. The mean age of the patients was 77.3 ± 7.3 years and 39% were of male gender (Table 1). Eighty-seven percent of the patients were in the New York Heart Association (NYHA) functional class III or IV. The expected risk for surgical mortality was elevated, 15.1% [IQR 8.2% to 26.6%], as assessed by the median logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE). The pre-procedural TR severity was graded as 3 + , 4 + , or 5 + in 49%, 40% and 11% of the study participants, respectively (Table 2). The mean left ventricular ejection fraction (LVEF) was 55.6 ± 10.2%, the mean tricuspid annular plane systolic excursion (TAPSE) was 17.7 ± 5.2 mm, and the secondary etiology of TR was observed in 96% of the patients.
Procedural success was achieved in 156 patients (91%) (Table 3). Edge-to-edge repair was performed in 81% of patients with the MitraClip/TriClip or PASCAL systems, whereas annuloplasty was performed in 18% with the Cardioband or Trialign systems. One patient was simultaneously treated with PASCAL and Cardioband systems.
Analysis of MELD-XI score
The mean MELD-XI score was 11.0 ± 5.5 (Table 4), and the distribution of the MELD-XI score and its components, total bilirubin and creatinine levels, are presented in Fig. 1. In addition, the mean estimated glomerular filtration rate (eGFR) was 48.7 ± 22.2 ml/min/1.73 m2, and the mean total bilirubin was 0.74 mg/dl [IQR 0.53 mg/dl to 1.00 mg/dl]. The MELD-XI score was correlated to age, sex, LVEF, LV end-diastolic volume, and right ventricular dimension (Supplemental Table 1). In the multivariable analysis, age had the strongest correlation (standardized β: 0.32; 95% CI 0.17–0.46; p < 0.0001), followed by LVEF (standardized β: − 0.29; 95% CI − 0.49 to − 0.17; p = 0.0007).
Within one year after TTVR, 45 patients (26.2%) experienced the composite outcome, including 22 patients (12.8%) who died, 14 patients (8.1%) who died due to cardiovascular causes, and 32 patients (18.6%) who were re-hospitalized due to worsening HF (Table 3). The ROC analysis showed that the best MELD-XI score to discern the one-year composite outcome was 14 (Supplemental Figure 1). The Harrel’s C-statistic of MELD-XI score for the one-year composite outcome was 0.68 (95% CI 0.60–0.77; p < 0.0001), and those of eGFR and total bilirubin were 0.66 (95% CI 0.58–0.74; p < 0.0001) and 0.56 (95% CI 0.58–0.74; p < 0.0001), respectively (Supplemental Table 2).
According to the cut-off value, 51 patients (29.7%) had a high MELD-XI score (≥ 14). Patient characteristics and echocardiographic findings for each group are shown in Tables 1 and 2. Patients with a high MELD-XI score (≥ 14) were older and a higher proportion were male than those with a low MELD-XI score (< 14) (79.1 ± 6.3 years vs. 76.6 ± 7.5 years; p = 0.04, and 52.9% vs. 33.1%; p = 0.02, respectively). LVEF was lower in patients with a high MELD-XI score (≥ 14) than those with a low MELD-XI score (< 14) (52.9 ± 12.5% vs. 56.8 ± 8.9%; p = 0.02), whereas TAPSE, TR severity, and procedural findings were comparable between the two groups.
Association between the MELD-XI score and clinical outcome after TTVR
Device type, post-procedural echocardiographic findings, and the rate of procedural success were comparable between the high MELD-XI score (≥ 14) and low MELD-XI score (< 14) groups. The incidence of AKI after TTVR was higher in patients with a high MELD-XI score (≥ 14) than those with low MELD-XI score (< 14) (19.6% vs. 7.4%; p = 0.02).
Patients with a high MELD-XI score (≥ 14) had a higher incidence of the one-year composite outcome compared to those with a low MELD-XI score (< 14) (47.1% vs. 17.4%; p < 0.0001). Moreover, the incidences of all-cause mortality and HF hospitalization were higher in patients with a high MELD-XI score (≥ 14) than in those with a low MELD-XI score (< 14) (25.5% vs. 7.4%; p = 0.001, and 33.3% vs. 12.4%; p = 0.001, respectively). The incidence of cardiovascular mortality was numerically higher in patients with a high MELD-XI score (≥ 14) than in those with a low MELD-XI score (< 14) (13.7% vs. 5.8%; p = 0.08). The Kaplan–Meier curves for each of the clinical outcomes are shown in Fig. 2.
Clinical outcome according to the MELD-XI score within one year after TTVR. Kaplan–Meier curves demonstrating clinical outcomes within one year after TTVR, including the composite outcome (A), all-cause mortality (B), cardiovascular mortality (C), and HF hospitalization (D), according to the MELD-XI score.
The MELD-XI score was associated with an incidence of the one-year composite outcome after TTVR (HR 1.12; 95% CI 1.07–1.20; p < 0.0001) (Table 5 and Supplemental Table 3). In a multivariable analysis, the MELD-XI score as a continuous variable remained an independent predictor of the composite outcome (adjusted HR in model 1: 1.13; 95% CI 1.05–1.19; p = 0.0003, and adjusted HR in model 2: 1.13; 95% CI 1.06–1.20; p = 0.0001). In addition, a high MELD-XI score (≥ 14) was also an independent predictor of the composite outcome (adjusted HR in model 1: 3.39; 95% CI 1.83–6.29; p = 0.0001, and adjusted HR in model 2: 3.83; 95% CI 2.07–7.13; p < 0.0001).
Improvement of MELD-XI score after TTVR
In 62 patients who at six months after TTVR had available total bilirubin and creatinine levels, the serial changes in the MELD-XI score were evaluated. The mean MELD-XI score at the follow-up was 11.0 ± 7.4, and 26 patients (64.2%) had a reduction in the MELD-XI score (Supplemental Table 4). In a multivariable logistic regression analysis, post-procedural TR < 3 + was associated with a MELD-XI reduction at six-month follow-up (adjusted OR: 3.37; 95% CI 1.09–10.40; p = 0.03), while baseline LV end-diastolic volume was inversely related to a MELD-XI reduction (adjusted OR for a 10 ml increase: 0.98; 95% CI 0.95–0.99; p = 0.03) (Supplemental Table 5).
Discussion
This is the first study assessing the prognostic impact of hepatorenal function in HF-patients undergoing TTVR due to advanced TR. The main findings are summarized as follows: (1) the MELD-XI score, as both a continuous and dichotomous variable, was independently associated with the risk of the composite outcome of mortality and HF hospitalization within one year after TTVR; and (2) a sufficient TR reduction by TTVR with post-procedural TR < 3 + was independently associated with an improvement in MELD-XI score at six-month follow-up.
Hepatic and renal dysfunctions are associated with a higher surgical risk for TR. Accordingly, the current guidelines recommend TV interventions for severe symptomatic isolated TR before the onset of multi-organ damage to the liver and kidney24. However, the appropriate tool for pre-procedural assessment of multi-organ damage has not yet been identified. Since both renal and hepatic dysfunction are strong predictors of adverse clinical outcomes in patients with HF13,15, the combination of both can increase their ability for risk stratification. The MELD-XI score is one of the most established scoring systems for hepatorenal dysfunction, which is an alternative to the MELD scoring system that excludes the international normalized ratio from the calculation. The predictive value of the score has recently been proven in patients with HF20,22,23. In patients undergoing surgical tricuspid valve repair, the association of the MELD-XI score with the incidence of adverse events has been reported in previous studies20,25. In the present study, we revealed the association of the MELD-XI score, as both a dichotomous and continuous variable, with the risk of adverse events within one year after TTVR, such as all-cause mortality, cardiovascular mortality, and HF hospitalization. Additionally, patients with a high MELD-XI score had a higher incidence of AKI during the hospitalization for TTVR. These findings underline the prognostic impact of hepatorenal function and the utility of the MELD-XI score as a simple tool for risk stratification in patients undergoing TTVR.
Hepatorenal function can reflect altered hemodynamics and thus, improve after TV interventions20,25. Improvement of the MELD-XI score after surgical tricuspid valve repair was associated with better prognosis. Reduction of the regurgitant volume load by the TV interventions can relieve venous congestion and multi-organ hypoperfusion and improve hepatorenal function. In contrast, an insufficient reduction of TR can prolong venous congestion and hepatorenal hypoperfusion, which can lead to a further progression of hepatorenal dysfunction. In the present study, a reduction in the MELD-XI score was observed in approximately 40% of the patients who underwent serial assessments for 6-months follow-up after TTVR. Post-procedural TR of less than 3 + upon discharge was an independent predictor of a reduction in the MELD-XI score, while baseline LV end-diastolic volume was inversely related to MELD-XI improvement. These findings are in accordance with the previous studies in surgical TV repair25. Thus, it might be necessary, especially for patients with renal or hepatic dysfunction, to achieve a sufficient reduction of TR by TTVR for the sake of improving their hepatorenal function.
Recently, the post-procedural changes in multi-organ function, including hepatorenal function, after TTVR has attracted attention amongst clinician scientists. Karam et al. showed significant reductions in total bilirubin and aspartate transaminase levels at six months after TTVR, while the renal function did not significantly change26. However, the number of patients in the study who were evaluated at the follow-up was small, and patients with concomitant transcatheter mitral valve repair were also included. Besler et al. found that nutritional status improved after TTVR in patients with symptomatic TR27. Malnutrition is one of the multi-organ damages that are attributed to venous congestion and hypoperfusion due to TR as well as hepatorenal dysfunction. Furthermore, a reduction in TR by TTVR was a predictor of the improvement in nutritional status, which was associated with a better outcome. Our findings were in agreement with previous reports. However, the underlying mechanisms of the improvement in hepatorenal function after TTVR are still insufficiently understood. Larger studies are needed to assess predictors of the improvement in hepatorenal function after TTVR.
There are several limitations to this study that should be acknowledged. Although the present study included a comparably high number of patients for this novel field, the single-centric and observational characteristics of the present study might have impacted our results owing to patient selection bias. Furthermore, the possibility remains that confounders were insufficiently considered in the multivariable analysis. Nevertheless, we conducted several multivariable models, adjusting for various clinical or echocardiographic covariates, which may at least partially address the issue. Second, we could not distinguish acute hepatorenal injury from chronic dysfunction, because we only evaluated the MELD-XI score from the latest laboratory data before TTVR. Finally, we evaluated serial measurements of the MELD-XI score after TTVR. However, approximately 60% of the patients could not be evaluated because of death within six months or a lack of the laboratory data at the follow-up.
In conclusion, hepatorenal dysfunction, represented by the MELD-XI score, was independently associated with the composite outcome of all-cause mortality and hospitalization due to heart failure after TTVR, irrespective of other clinically important variables such as LVEF, TAPSE, and coronary artery disease. Moreover, HF patients with a high MELD-XI score (≥ 14) had a higher incidence of all-cause mortality and HF hospitalization within one year after TTVR and had higher rates of post-procedural AKI. Post-procedural TR < 3 + was associated with a reduction in the MELD-XI score within the six-month follow-up period. The MELD-XI score is a simple and objective scoring system to assess renal and hepatic functions, which are both aggravated by the progression of TR. Therefore, the MELD-XI score can aid in pre-procedural risk-stratification, patient selection, and decision-making for the timing of TTVR in patients with TR.
Methods
Study population
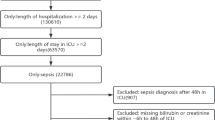
This study was a retrospective analysis of data from a local registry, which is a single-center, prospective, consecutive database of patients treated at the University of Bonn28,29. We analyzed consecutive patients who underwent TTVR from June 2015 to September 2020 and had available the pre-procedural serum creatinine and total bilirubin results. We excluded patients with hemodialysis and those who underwent concomitant transcatheter mitral valve repair.
Procedure
The indication for TTVR was severe or greater TR accompanied by symptomatic HF according to the NYHA functional classification in patients considered as inoperable or at a high surgical risk. After a standardized diagnostic workup, including transesophageal echocardiography (TEE), the decision to perform the intervention was taken by the interdisciplinary heart team. Procedures were performed using the MitraClip/TriClip (Abbott Vascular, Santa Clara, California), PASCAL (Edwards Lifesciences, Irvine, California), Cardioband (Edwards Lifesciences), or Trialign (Edwards Lifesciences) devices under general anesthesia with three-dimensional TEE and fluoroscopic guidance. Details of the device system and procedure have previously been well described8,10,30,31. The discretion of whether a second or third device needed to be used was left up to the treating physicians. Procedural success was defined as successful device implantation and a reduction of TR by ≥ 1 grade, as assessed by transthoracic echocardiography upon discharge after TTVR.
Assessment of hepatorenal function
The hepatorenal function was assessed using the MELD-XI score, which was calculated as 5.11 × ln(serum total bilirubin in mg/dl) + 11.76 × ln(serum creatinine in mg/dl) + 9.4421. Serum creatinine and total bilirubin results that were taken within the one week prior to TTVR were included. In addition, postprocedural measurements of these values were collected at six months after TTVR, and a reduction in MELD-XI score was defined as: baseline MELD-XI score was higher than post-procedural MELD-XI score at follow-up. Acute kidney injury (AKI) was defined according to the Acute Kidney Injury Network criteria as an absolute increase in serum creatinine of ≥ 0.3 mg/dl or a relative increase of ≥ 50% from baseline to 48 h after the procedure32.
Echocardiographic parameters
We assessed echocardiographic parameters that were collected at baseline and discharge, according to the current guidelines33. TEE was performed at baseline and during the procedure with a Vivid E95 ultrasound system (GE Healthcare, Illinois, USA). According to a combination of semiquantitative and quantitative assessments, the severity of TR was graded as follows: grade 0, none; 1 + , mild; 2 + , moderate; 3 + , severe; 4 + , massive; 5 + , torrential34. All measurements were reviewed by two independent cardiologists that were dedicated to echocardiographic evaluation.
Clinical follow-up
The primary endpoint was a composite outcome, consisting of all-cause mortality and hospitalization due to worsening HF within one year after TTVR. As secondary endpoints, all-cause mortality, cardiovascular mortality, and hospitalization due to worsening HF within one year after TTVR were examined separately. All suspected adverse events were independently adjudicated by the local heart team according to the criteria of the Valve Academic Research Consortium 235. The need for hospitalization due to worsening HF was determined based on the attending physicians’ discretion, without any prespecified criteria. The occurrence of clinical events was recorded from the admission records and outpatient medical records. In addition, HF medication, including beta-blockers, renin-angiotensin system (RAS) inhibitors, and aldosterone antagonists, or dosage with a standardized furosemide equivalent were recorded at baseline36.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed variables are presented as the mean ± standard deviation and compared using t-tests. In contrast, non-normally distributed variables were expressed as medians with an interquartile range (IQR) and compared between groups using the Mann–Whitney U-test. Categorical data were presented as numbers and percentages, and the differences between groups were evaluated using the chi-square test. Logistic regression analysis was performed to detect parameters that were related to the MELD-XI score and those that were related to the reduction of MELD-XI score. The variables with p < 0.05 in the univariate analysis were incorporated into a multivariable regression model. The receiver-operating characteristic (ROC) analysis was used to investigate the cut-off value of the MELD-XI score, to predict the composite outcome within one year after TTVR. According to the cut-off value, patients were categorized into two groups: high MELD-XI and low MELD-XI. In addition, Harrell’s C-statistic was used to compare the predictive ability of hepatorenal functional markers for the composite outcome by the area-under-the-curve analysis, accounting for censoring. Kaplan–Meier cumulative event curves for the composite outcome, all-cause mortality, cardiovascular mortality, and HF hospitalization were generated by using two groups according to the high or low MELD-XI score. Differences between the groups were compared using the log-rank test. Univariate and multivariable Cox-proportional hazard models were used to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) for the MELD-XI score for the composite outcome within one year after TTVR. In a univariate analysis, we analyzed the HRs of clinical parameters (model 1) and cardiac and procedural parameters (model 2) that were determined, considering the number of end points and multicollinearity. In the multivariable analyses, covariates were included that showed significance (p < 0.05) in the univariate analyses. Statistical significance was set at p < 0.05. All analyses were conducted using Stata 15.1 (StataCorp, College Station, TX, USA) or JMP version 14.0 for Mac (SAS Institute Inc., Cary, NC, USA).
Ethical statement
Our registry was approved by the local Ethical Committee at the University of Bonn in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants after receiving a full written and oral explanation of the purpose of our registry.
References
Chorin, E. et al. Tricuspid regurgitation and long-term clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 21, 157–165. https://doi.org/10.1093/ehjci/jez216 (2020).
Topilsky, Y. et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc. Imaging 12, 433–442. https://doi.org/10.1016/j.jcmg.2018.06.014 (2019).
Topilsky, Y. et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc. Imaging 7, 1185–1194. https://doi.org/10.1016/j.jcmg.2014.07.018 (2014).
Dreyfus, J. et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur. Heart J. 41, 4304–4317. https://doi.org/10.1093/eurheartj/ehaa643 (2020).
Axtell, A. L. et al. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J. Am. Coll. Cardiol. 74, 715–725. https://doi.org/10.1016/j.jacc.2019.04.028 (2019).
Taramasso, M. et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J. Am. Coll. Cardiol. 74, 2998–3008. https://doi.org/10.1016/j.jacc.2019.09.028 (2019).
Taramasso, M. et al. The international multicenter trivalve registry: which patients are undergoing transcatheter tricuspid repair?. JACC Cardiovasc. Interv. 10, 1982–1990. https://doi.org/10.1016/j.jcin.2017.08.011 (2017).
Nickenig, G. et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 394, 2002–2011. https://doi.org/10.1016/S0140-6736(19)32600-5 (2019).
Fam, N. P. et al. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc. Interv. 12, 2488–2495. https://doi.org/10.1016/j.jcin.2019.09.046 (2019).
Nickenig, G. et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge mitraclip technique. Circulation 135, 1802–1814. https://doi.org/10.1161/CIRCULATIONAHA.116.024848 (2017).
Asmarats, L. et al. Long-term outcomes of the FORMA transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation: insights from the first-in-human experience. JACC Cardiovasc. Interv. 12, 1438–1447. https://doi.org/10.1016/j.jcin.2019.04.038 (2019).
Mehr, M. et al. 1-year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation: results from the trivalve registry. JACC Cardiovasc. Interv. 12, 1451–1461. https://doi.org/10.1016/j.jcin.2019.04.019 (2019).
Rangaswami, J. et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the american heart association. Circulation 139, e840–e878. https://doi.org/10.1161/CIR.0000000000000664 (2019).
Samsky, M. D. et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J. Am. Coll. Cardiol. 61, 2397–2405. https://doi.org/10.1016/j.jacc.2013.03.042 (2013).
Xanthopoulos, A., Starling, R. C., Kitai, T. & Triposkiadis, F. Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail. 7, 87–97. https://doi.org/10.1016/j.jchf.2018.10.007 (2019).
Maeder, M. T., Holst, D. P. & Kaye, D. M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 14, 824–830. https://doi.org/10.1016/j.cardfail.2008.07.236 (2008).
Lau, G. T., Tan, H. C. & Kritharides, L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am. J. Cardiol. 90, 1405–1409. https://doi.org/10.1016/s0002-9149(02)02886-2 (2002).
Allen, L. A. et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Eur. J. Heart Fail. 11, 170–177. https://doi.org/10.1093/eurjhf/hfn031 (2009).
Suzuki, K. et al. Liver function and prognosis, and influence of sacubitril/valsartan in patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 22, 1662–1671. https://doi.org/10.1002/ejhf.1853 (2020).
Egbe, A. C., Miranda, W. R., Dearani, J., Kamath, P. S. & Connolly, H. M. Prognostic role of hepatorenal function indexes in patients with ebstein anomaly. J. Am. Coll. Cardiol. 76, 2968–2976. https://doi.org/10.1016/j.jacc.2020.10.035 (2020).
Malinchoc, M. et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31, 864–871. https://doi.org/10.1053/he.2000.5852 (2000).
Kawahira, M. et al. Prognostic value of impaired hepato-renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Fail. https://doi.org/10.1002/ehf2.13195 (2021).
Kim, M. S. et al. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J. Am. Coll. Cardiol. 61, 2253–2261. https://doi.org/10.1016/j.jacc.2012.12.056 (2013).
Otto, C. M. et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143, e72–e227. https://doi.org/10.1161/CIR.0000000000000923 (2021).
Chen, Y. et al. Prognostic value of hepatorenal function by modified model for end-stage liver disease (MELD) score in patients undergoing tricuspid annuloplasty. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.118.009020 (2018).
Karam, N. et al. Impact of transcatheter tricuspid valve repair for severe tricuspid regurgitation on kidney and liver function. JACC Cardiovasc. Interv. 12, 1413–1420. https://doi.org/10.1016/j.jcin.2019.04.018 (2019).
Besler, C. et al. Nutritional status in tricuspid regurgitation: implications of transcatheter repair. Eur. J. Heart Fail. 22, 1826–1836. https://doi.org/10.1002/ejhf.1752 (2020).
Kavsur, R. et al. Pulmonary capillary wedge pressure (PCWP) as prognostic indicator in patients undergoing transcatheter valve repair (TTVR) of severe tricuspid regurgitation. Int. J. Cardiol. 318, 32–38. https://doi.org/10.1016/j.ijcard.2020.06.031 (2020).
Kavsur, R. et al. Prognostic significance of the get with the guidelines-heart failure (GWTG-HF) risk score in patients undergoing trans-catheter tricuspid valve repair (TTVR). Heart Vessels https://doi.org/10.1007/s00380-021-01874-3 (2021).
Nickenig, G. et al. Two-year outcomes with the cardioband tricuspid system from the multicentre, prospective TRI-REPAIR study. EuroIntervention https://doi.org/10.4244/EIJ-D-20-01107 (2020).
Hahn, R. T. et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J. Am. Coll. Cardiol. 69, 1795–1806. https://doi.org/10.1016/j.jacc.2017.01.054 (2017).
Mehta, R. L. et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31. https://doi.org/10.1186/cc5713 (2007).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Hahn, R. T. et al. Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc. Imaging 12, 469–490. https://doi.org/10.1016/j.jcmg.2018.07.033 (2019).
Kappetein, A. P. et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 60, 1438–1454. https://doi.org/10.1016/j.jacc.2012.09.001 (2012).
Felker, G. M. et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 364, 797–805. https://doi.org/10.1056/NEJMoa1005419 (2011).
Acknowledgements
We thank Dr. Meghan Lucas (scientific coordinator for the Heart Center Bonn, Bonn, Germany) for proofreading the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study concept and design: T.T., R.K., and M.U.B. Acquisition of data: T.T., R.K., A.S., J.V., and C.Ö. Analysis and interpretation of data: T.T. and R.K. Statistical analysis: T.T. Drafting of manuscript: T.T., R.K., M.W., V.T., S.Z., G.N., and M.U.B.
Corresponding author
Ethics declarations
Competing interests
Georg Nickenig has received research funding from the Deutsche Forschungsgemeinschaft, the German Federal Ministry of Education and Research, the EU, Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St Jude Medical, and has received honoraria for lectures or advisory boards from Abbott, AGA Medical, AstraZeneca, Bayer, Berlin, Cardiovalve, Berlin Chemie, Biosensus, Biotronic, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St Jude Medical. Marcel Weber has received lecture or proctoring fees from Abbott, Boehringer-Ingelheim, Edwards Lifesciences, Janssen, Neochord, Pfizer, and Servier. Tetsu Tanaka was financially supported in part by a Fellowship from the Japanese College of Cardiology. All other authors report no relationship with industry and other entities.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, T., Kavsur, R., Sugiura, A. et al. Prognostic impact of hepatorenal function in patients undergoing transcatheter tricuspid valve repair. Sci Rep 11, 14420 (2021). https://doi.org/10.1038/s41598-021-93952-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-93952-9
This article is cited by
-
Combining MELD-XI score and hemodynamic parameters enhances short-term prognostic value in patients undergoing transcatheter tricuspid valve interventions
Wiener klinische Wochenschrift (2026)
-
Refining accuracy of RV–PA coupling in patients undergoing transcatheter tricuspid valve treatment
Clinical Research in Cardiology (2024)