Abstract
There are currently heavy metals in most industrial effluents which are among the most significant environmental pollutants. Hexavalent chromium is one of the most significant heavy metals. In this research for the first time, eliminating the hexavalent chromium from the aqueous medium/aquedia applying bee carcasses and corpses modified with polyethylene was examined. Adsorption experiments were conducted discontinuously on laboratory solutions, including hexavalent chromium. The optimal adsorption conditions such as different pH factors, contact time, initial chromium concentration, and adsorbent value on the adsorption rate were examined at different levels, and adsorption isotherms were plotted. Some adsorbent properties were examined using Field Emission Scanning Electron Microscopy, XRD analysis, Fourier Transform Infrared Spectroscopy, and BET test to study the properties of the synthesized adsorbent. This study indicated that the highest percentage of removal related to polyethylene composite and bee carcasses in the presence of polyethylene glycol was 50.56% among the bee carcasses composites. The parameters effective on the adsorption process for polyethylene composite and bee carcasses and losses in the presence of polyethylene glycol suggested that the adsorption percentage increased for this composite by decreasing the pH, increasing the contact time, and increasing the adsorbent. The highest percentage of adsorption was obtained when the pH was 2, the contact time was 120 min and the adsorbent value was 8 g/L and the initial concentration of chromium was 100 ppm. The most optimal removal percentage was achieved at the pH = 2, the contact time was 30 min, and the adsorbent value was 2 g/L, and the initial chromium concentration was 100 ppm. The results of drawing adsorption isotherms also indicated that higher R2 had a better fit than Langmuir for polyethylene composite and bee carcasses in the polyethylene glycol Freundlich equation.
Similar content being viewed by others
Introduction
Progressing the science and technology and the mechanization of human life and providing welfare and comfort for him/her have caused to enter dangerously and polluting substances into the environment1,2. Surface and groundwater are the main sources of clean water. Notwithstanding, many water resources have become polluted due to the rapid population growth and expanding industrial development in the world3. This can be due to the constant discharge of organic and mineral wastes from human activities in natural water resources4. We can refer to industrial effluents, including heavy metals, as one of the main and most significant environmental pollutants. Heavy metals are elements with a density higher than 3 g/cm3. Metal ions concentrated in the living body provide health and economic problems due to the biological toxicity of these contaminated ions5,6,7,8,9. Human resources to produce chromium include paints10, mining11, plating12, automobile manufacturing13, metal processing14, leather tannery15, and textiles manufacturing16. Chromium (VI) is more deadly than other metal ions and causes serious health problems at very high concentrations of 1000 mg/L while entering the body17. Consequently, it is required and essential to apply appropriate methods and develop new methods instead of the old chemical and physical methods to separate and remove them and purify water polluted with these heavy metals in today's society18,19,20,21. Repulsive forces between negatively charged materials and chromium (VI) ions or negative loads transfer chromium (VI) in the aquatic system and absorb chromium ions in the environment in soil and water environments. The concentration of chromium (VI) in groundwater and chromium-polluted wastewater worldwide is 30–200 mg/L22. Considering the higher toxicity of chromium (VI), the US-EPA23 institute has determined a safe chromium concentration of 0.1 mg/L in drinking water sources and 0.05 mg/L for home usages24. It can be absorbed through the respiratory system, skin, and gastrointestinal tract and has been recognized as one of the most significant human carcinogens, including lung cancer25,26. Chromium (VI) provides some side effects for humans such as asthma, irritation, and inflammation in the nose, contact dermatitis, deep wounds, piercing of the nasal septum, irritation of the lungs, skin allergies, and damage to the kidneys and liver27,28,29,30. At present, several methods are applied to remove and separate chromium from aqueous media31,32,33,34. Each one owns its advantages and disadvantages. For instance, it is not easy to recover the chromium in the chemical precipitation method because of producing a high amount of effluent35. The reverse osmosis method consumes a lot of energy and electricity and produces effluent36. Adsorption as a simple, environmentally friendly, and cost-effective method can eliminate different water pollutants than other techniques37,38,39,40,41,42. This adsorption process is a surface phenomenon43,44,45. Researchers and scientists currently seek to explore new attractions to separate and remove chromium and other heavy metals. It is possible to use wastes and other materials to achieve this objective in this regard46,47,48. The conductive polymers of polypyrrole and polyaniline and their composites are among the materials that have lately been recognized as adsorbents49. In such polymers, sensitivity to heat and air, conductivity, ease to moldability, solubility, and processability are highly different depending on the type of monomer and its synthesis method50,51,52. Polyaniline, a distinguished binding polymer, has been highly considered due to its ease of preparation, low price, and environmental stability53. Also, synthetic Polyaniline Composites (PANI) has multifunctional groups (active sites) and show high adsorption capacity for organic and inorganic pollutants54,55,56. Furthermore, this polymer was highly considered due to its electrical conductivity in the proper range57, many applications have been considered in various fields, and low cost, high environmental stability58. Much research has been conducted in this field around the world28. Other studies were examined the removal of the heavy metals using Paratoluene sulfonic/polyaniline nanocomposite59, polyaniline/sawdust/polyethylene glycol60, polyaniline/walnut shell61, polyaniline/zeolite nanocomposite62, Bacterial cellulose/polyaniline nanocomposite aerogels63. The results of this research have explained that various types of polyaniline and its composites can be synthesized by changing the synthesis conditions with absolutely different advantages and properties. This study, with a new idea and for the first time, aims to synthesize bee carcass composite and polyethylene modified bee wastes and apply them to separate chromium from aqueous media.
Materials and methods
All applied materials did not require additional purification because of their high purity, except for monomer aniline, distilled and stored in the refrigerator before using until thoroughly discolored (because it is volatile and quickly destroyed and oxidized by air). All experiment stages used distilled water and were performed at 25 °C (PEG = 35,000 MW). All materials, including polyethylene glycol, aniline, potassium iodate, potassium dichromate, activated carbon, sulfuric acid, were employed, which were made by the German company Merck.
Devices
Experiments were carried out using a pH meter PMT-Model 2002, XRD (PANALYTICAL X'PERT), a shaker machine, Field Emission Scanning Electron Microscope Machine (QUANTA FEG 450), Fourier transform spectrophotometer or Infrared spectrometer (FTIR) model TENSOR 27, UV–Vis Spectrometer device model GES30, BET device model Belsorp II made by BEL company.
Synthesis methods
Preparing bee carcass and waste and polyaniline in the presence of polyethylene glycol
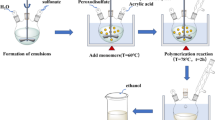
0.9 g of an oxidizing substance, including potassium iodate as an oxidant and 0.7 g of Polyethylene Glycol (PEG), was mixed in 150 mL of 1 M sulfuric acid solution obtained by combining 53.3 mL of pure sulfuric acid with 947 mL of distilled water in each experiment using a shaker. In another container, 1 g of bee carcasses and wastes were mixed with 1.5 mL of distilled aniline. It was transferred to the solution container and mixed on a shaker at room temperature for 5 h after soaking the contents of this container. The solutions were then removed from the shaker and passed through filter paper. The product was then washed several times with distilled water and dried in an oven at 50 °C for 48 h.
The method to separate the Chromium using composites synthesized with bee carcasses and wastes
We provided a standard solution of 50 ppm chromium after providing the synthesized composites and adsorbents. A complete discontinuous mixing system was adopted to adsorb the heavy metal. Accordingly, 0.1 g of adsorbent (composites synthesized with bee carcasses and wastes) was added to 50 mL of 50 ppm chromium solution, and a shaker was used to mix it for 30 min with a shaker (magnetic stirrer) at a specified speed of 120 rpm. Then, we passed the solution through filter paper and the chromium concentration in aqueous media was determined by using the UV–Vis spectrophotometer28. The parameters regarded in this study include the amount of used adsorbent, contact time for perfect mixing, pH, and initial concentration of chromium.
Freundlich and Langmuir adsorption isotherms
Freundlich and Langmuir's isotherms are proposed as follows:
and:
Ce is the concentration of metal in the liquid phase after mixing time (mg/L), n and k are Freundlich constants, X is the amount of adsorbed material per unit mass of adsorbent (mg/g), which indicated the intensity and capacity of adsorption, respectively. b is the Langmuir constant, which indicates the absorption energy, and Xm is the maximum amount of adsorbed material per unit mass of adsorbent (mg/g). In this study, different amounts of adsorbent were added to a solution of 50 mg/L chromium (VI) at ambient temperature.
Investigating the chromium recovered by Polyaniline
0.1 g of synthesized polymer (composites synthesized with bee carcasses and wastes) was added to 150 mL of chromium-containing solution at a concentration of 50 ppm, and a shaker (magnetic stirrer) was used to mix it for 30 min at 120 rpm. After mixing, the solution was passed through a sieve, and 0.1 M sulfuric acid of the total adsorbent left on the filter paper was added to 50 mL and mixed on a magnetic stirrer for 30 min. Presently, after mixing, we filtered the solution through a filter paper and measured the concentration of chromium ion in the solution. The total adsorbent left on the filter was then added to 150 mL of 50 ppm chromium in the next step and mixed for 30 min in a shaker (magnetic stirrer). After final mixing, we measure chromium ion as follows.
C0 is the initial concentration of chromium, Ce is the final concentration of chromium after separation, and Ct is the chromium concentration in sulfuric acid solution 0.1 M in this equation.
Results and discussion
Investigating the chromium separation using bee carcasses and wastes and their composite with polyaniline
Table 1 shows the results of chromium separation using bee carcasses and wastes and their composites with polyaniline. As Table 1 explains, polyaniline coating has been empowered to affect the removal efficiency of all materials. The highest percentage of removal was related to bee-carcass composites with polyaniline in the presence of polyethylene glycol (4.6 g/L) with a removal percentage of about twice the activated carbon and 50.56% of removal among bee carcasses and wastes provided by polyaniline. Polyaniline coating on bee carcasses and wastes could increase the percentage of chromium removal. Earlier research and studies have explained that polyaniline owns the regenerative property of hexavalent chromium, and the presence of nitrogen (-NH) sites in polyaniline has absorbed chromium64. Figure 1 shows the results of field emission scanning electron microscopy for bee carcasses and wastes, and Fig. 2 (parts a and b) explains the results of bee carcasses and wastes with polyaniline. As this figure (parts a and b) show, Aniline is well coated on the surface of bee carcasses and wastes in polyaniline composite and bee carcasses and wastes, and polyaniline particles have been placed in the form of thin, irregular scales on the surface of bee carcasses and wastes.
FESEM image of: (a) bee carcasses/polyaniline composite with 50000X magnification, (b) bee carcasses/polyaniline composite with 100,000 X magnification, (c) bee carcasses/polyaniline/polyethylene glycol composite with 50,000 X magnification, (d) FESEM images of bee carcasses/polyaniline/polyethylene glycol composite with 100,000 X magnification.
According to Fig. 2c and d, the polyaniline particles are more uniformly and regularly and spherically covered on the surface of bee carcasses in the composite of polyaniline and bee carcasses and wastes in the presence of polyethylene glycol. Polyethylene glycol is a stabilizing material, and the adsorption of aniline particles has increased and has affected the shape and arrangement of the particles during the polymerization process. The achieved composite is more regular with a higher surface area, and a higher adsorption percentage has occurred. Table 2 shows the results achieved by analyzing the pore size and BET specific surface area for bee composite and wastes and polyaniline, bee composite and wastes, and polyaniline and polyethylene glycol. The results explain that polyaniline and polyethylene glycol have been increased the lateral surface of the composite in the carcass composite and bee wastes. Consequently, the removal efficiency has been increased.
Figure 3 shows the FTIR spectrum of bee carcasses and wastes, and Fig. 4 shows the bee carcasses and composites and their polyaniline and polyethylene glycols. The deformation and location of the bands of the FTIR spectrum, with the different molecular environments, can be a helpful guide to the molecular structure of a compound65. As Figs. 3 and 4 show, the peaks recognized in 3400,2925,2110,1630,1193,1116,634 cm−1 belongs to functional groups such as tensile aromatic CH−, aromatic C=C−, alkyne C=C−, alkyl tensile CH−, or amine NH− and alcohol OH−.
Figure 5 shows the XRD results for bee carcasses and wastes. Figure 6 shows the polyaniline composite and bee carcasses and wastes in polyethylene glycol to determine the tested mineral properties of the adsorbents. As the analysis results accomplished on the synthesized composite explained, benzene compounds were observed.
Examining the adsorption test conditions for bee carcass composite and waste/polyaniline/polyethylene glycol
Investigating the influence of pH of chromium solution on the efficiency of separation by bee carcass and wastes/polyaniline/polyethylene glycol
pH is a factor that can enhance the adsorption efficiency and is one of the most significant and main parameters that determine the adsorption operation59,66. The pH influences biomass active site load and heavy metals behavior in solution67. Solution pH commonly impacts adsorbent ionization and adsorbent surface properties68. Nature of physicochemical interactions between adsorption sites and heavy metal ions to the pH of solution utilized in adsorption approaches, to a great extent69,70. in this research, five pHs (2, 4, 6, 8, and 10) were surveyed for chromium isolation and the results were indicated in Fig. 7. Alkaline pHs were avoided to prevent chromium sequestration.
The results reveal that the highest adsorption of chromium (VI) occurs at pH = 2 at 78.98%, and the lowest percentage of chromium (VI) adsorption happens at pH = 10 at 9.46%. The study results explained that the pH of the solution highly affects the amount of adsorption so that in most cases, increasing the pH decreases the amount of chromium adsorption. Changes in pH influence chromium adsorption because it determines the type of ionic species of chromium and the charge of the adsorbent surface. The predominant form at pH is 2 for Chromium + 6, and less than it, is as HCrO4− and Cr2O72−, its most value is HCrO4−, which is easily absorbed due to its low free energy. On the other hand, at high pH, the charge on the adsorbent surface is negative. Hence, the inclination to adsorb its anions decreases through the electrostatic process. According to increasing the adsorption at low pH and adding the potassium dichromate salt, chromium ions will be available in the form of Cr2O72− in the environment. Consequently, we can explain the decrease in adsorption by increasing pH because the –OH groups on the adsorbent based on Le Chatelier's principle tend to be transferred to the medium at low pH due to the low concentration of –OH ions in solution. Consequently, positive groups are formed on the adsorbent, which leads to adsorption, and –OH groups will no longer tend to be released on the adsorbent. The concentration pH will be increased in the solution by increasing the value of pH. Accordingly, fewer positive environments are created, and consequently, the absorption process occurs to a lower extent.
Examining the contact time
The adsorption rate is frequently very high in the first stage of adsorption processes71. Figure 8 shows the effect of contact time of polyethylene composite sorbent and bee wastes in the presence of polyethylene glycol with solutions including chromium (VI) pH = 6. The metal concentration was 50 mg/L. The adsorbent was 2 g/L. Studies and researches conducted in this field explain that the amount and percentage of removal of the ascending path have been increased by increasing the contact time because many active and empty places in the adsorbent surface will be occupied over time59,72. And since, even after 120 min, the removal efficiency is still high, and there is no desorption process of the metal ions from the adsorbent at this time; therefore, the lowest amount of chromium removal was 21.16% in 5 min, and the highest amount of chromium removal was 61.64% in 120 min. Presently, the results achieved in the diagram show that the optimal contact time is 30 min, which is 50.56% for the removal rate, and from this time on, there is no significant removal rate.
Examining the amount of adsorbent
The amount of adsorbent applied in removing metal ions is highly significant73,74,75,76,77. At this stage, we added different amounts of adsorbent (1, 2,4,6,8 g/L) to the chromium solution at pH = 6 and optimal contact time (30 min). As the results of Fig. 9 show, the removal efficiency was increased by increasing the amount of adsorbent. Polyaniline composite with bee carcass and waste in the presence of polyethylene-glycol could purify up to 81.1% of the solution, including 50 mg/L chromium in an adsorbent amount of 8 g/L. It is because of an increase in available places for the adsorption of metals, which has been performed by increasing the amount of adsorbent46. The results achieved in the diagram show that the optimal amount of adsorbent is 2 g per liter, which is a 50.56% removal rate, and the removal rate is then increased with a lower slope.
Examining the effect of initial chromium concentration
A driving force is created in an aqueous solution with the initial concentration of sorbate, which helps to transfer Cr (VI) ions to the adsorbent surface78. At this stage, we examined the effect of initial concentrations of chromium (at four levels of 5, 10, 50, and 100 ppm) on the separation process by the selected composite. As the results of Fig. 10 show, polyethylene composite and bee carcasses and wastes in the presence of polyethylene glycol could remove the chromium optimally in the range of various concentrations of water and wastewater. And increasing the initial concentration of chromium from 5 to 100 ppm, the percentage of chromium removal from 45.63 to 57.59%, explains that the amount of adsorption was increased by increasing the initial concentration of chromium in the effluent. As the concentration of the solution increases, the density of ions in the solution increases. As a result, as the ions approach the surface of the particles, the adsorption percentage increases, consequently, the adsorption percentage is increased as the ions approach the surface of the particles, and consequently, the possibility of adsorption saturation of the metal ions increases23, 79.
Langmuir and Freundlich adsorption isotherms for bee carcass composites and wastes/polyaniline/polyethylene glycol
The Langmuir and Freundlich adsorption models are usually used for absorption data80. We tested absorption data by Langmuir81 and Freundlich82 equations. At this stage, the adsorption isotherms were examined according to the values obtained before. Accordingly, Figs. 11 and 12 show the fit of the results with Langmuir and Freundlich adsorption models for chromium (VI). Table 3 also shows the parameters and constant coefficients of these models. Chromium metal fitted with both models due to the proximity of two R2s to each other, but it had higher compatibility in the Langmuir model due to higher R2 (R2 = 0.9598). The coefficient n of the Freundlich model, which is an indicator of adsorption intensity, was calculated at 1.19 for the adsorption of chromium by the adsorbent. The slope is increased by increasing the adsorbent concentration in this isothermal model but is eventually decreased to zero by filling the empty adsorption sites. The coefficient K is a measure of the absorption power in the Freundlich model, which is equal to 0.739 L per gram. In the Langmuir model, the RL constant defines an important characteristic of this isotherm, named the equilibrium parameter. We calculated RL values achieved for chromium adsorption by 0.611, which is between 0 and 1, and indicates the optimal adsorption to interpret metal adsorption by adsorbent83. The maximum adsorption capacity for chromium metal (Xm) was 45.25 mg/g of adsorbent copper in the Langmuir model, and b (constant of adsorption tendency) was determined by 0.0127 L/mg.
Recreation and reuse of bee composite carcass and waste/polyaniline/polyethylene glycol
Reutilizing multiple adsorbents to remove water contaminants is ordinarily a great improvement because it can decrease the cost of synthesizing and purchasing the chemicals required to make or modify some adsorbents. Moreover, reusability restricts the disposal of adsorbents after a single-use84. As previous research explains, we can reuse some chromium adsorbents after a simple treatment with dilute solutions of sodium hydroxide, hydrochloric acid, or sulfuric acid85,86,87,88. According to the obtained results, for the initial solution of 50 ppm chromium, after the recovery of 10.58 ppm chromium was returned, which means about 41.85% desorption occurred. The recycled adsorbent was then re-used to remove chromium and increased the initial concentration of 50 mg/L chromium to 34.22 in similar conditions, and about 31.56% was removed.
Conclusion
This study tested bee carcass composites and wastes and polyaniline with polyethylene glycol additive to remove hexavalent chromium from an aqueous medium. Ultimately, we recognized that the highest percentage of removal related to the polyethylene composite and bee carcass and waste in the presence of polyethylene glycol was 50.56% among the carcass and bee waste composites, which we then selected as the best adsorbent. The parameters influencing the adsorption process for polyethylene composite and bee carcass and waste in the presence of polyethylene glycol in the next step, which had the adsorption percentage increased for this composite by decreasing the pH, increasing the contact time, and increasing the adsorbent, and increasing the initial concentration of chromium. The highest percentage of adsorption was obtained when the pH was 2, the contact time was 120 min and the adsorbent value was 8 g/L and the initial concentration of chromium was 100 ppm. The most optimal removal percentage was achieved at the pH = 2, the contact time was 30 min, and the adsorbent value was 2 g /L, and the initial chromium concentration was 100 ppm. The outlining of the adsorption isotherms also revealed that the Langmuir equation had a better fit than Freundlich because of the higher R2. Ultimately, the results were achieved by reduction and desorption affirmed that the adsorbent could absorb continuously, and recreation and recovery performed.
References
Akinterinwa, A. et al. Synthesis of Cross-Linked Carboxymethyl Legume Starch for Adsorption of Selected Heavy Metals from Aqueous Solutions. Adv. J. Chem. A. 3(5), 594–611 (2020).
Kumar, S., Shahnaz, T., Selvaraju, N. & Rajaraman, P. V. Kinetic and thermodynamic studies on biosorption of Cr (VI) on raw and chemically modified Datura stramonium fruit. Environ. Monit. Assess. 192(4), 1–24 (2020).
Mustafa, H.M., Hayder, G., Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: A review article. Ain Shams Eng. J., In Press, Corrected Proof, (2020).
Safauldeen, S. H. et al. Phytoremediation efficiency of water hyacinth for batik textile effluent treatment. J. Ecol. Eng. 20(9), 177–187 (2019).
Bozorgian, A., Zarinabadi, S., Samimi, A. Preparation of Xanthan Magnetic Biocompatible Nano-Composite for Removal of Ni2+ from Aqueous Solution. Chem. Methodol. 4(4), 477–493 (2020).
Qadeer ul, H., Munir, A., Bashir, A., Ali, F., Muhammad Shahid, R. Mitigation of Cu(Ii) from Aqueous Solution by Using Sandal Santalum Album Distillation Biomass. Adv. J. Chem. A. 3(4), 473–484 (2020).
Jin, Y. et al. Assessment of sources of heavy metals in soil and dust at children’s playgrounds in Beijing using GIS and multivariate statistical analysis. Environ. Int. 124, 320–328 (2019).
Ameri Akhtiar Abadi, M., Masrournia, M., Abedi, M.R. Simultaneous Extraction and Preconcentration of Benzene, Toluene, Ethylbenzene and Xylenes from Aqueous Solutions Using Magnetite–Graphene Oxide Composites. Chem. Methodol. 5(1), 11–20 (2021).
Shahnaz, T., Sharma, V., Subbiah, S. & Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 36, 101283 (2020).
Ghosh, A., Dastidar, M.G., Sreekrishnan, T., Bioremediation of chromium complex dye by growing aspergillus flavus. Water quality management. Springer, pp. 81–92 (2018).
Coetzee, J.J., Bansal, N., Chirwa, E.M., Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Exposure Health, pp. 1–12 (2018).
Wang, G. et al. Novel multi-metal containing MnCr catalyst made from manganese slag and chromium wastewater for effective selective catalytic reduction of nitric oxide at low temperature. J. Clean. Prod. 183, 917–924 (2018).
Rodríguez, R., Espada, J., Gallardo, M., Molina, R. & López-Muñoz, M. Life cycle assessment and techno-economic evaluation of alternatives for the treatment of wastewater in a chrome-plating industry. J. Clean. Prod. 172, 2351–2362 (2018).
Lee, C.-G. et al. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 166, 203–211 (2017).
Angelucci, D. M., Stazi, V., Daugulis, A. J. & Tomei, M. C. Treatment of synthetic tannery wastewater in a continuous two-phase partitioning bioreactor: Biodegradation of the organic fraction and chromium separation. J. Clean. Prod. 152, 321–329 (2017).
Kuppusamy, S., Jayaraman, N., Jagannathan, M., Kadarkarai, M. & Aruliah, R. Electrochemical decolorization and biodegradation of tannery effluent for reduction of chemical oxygen demand and hexavalent chromium. J. Water Process Eng. 20, 22–28 (2017).
Gupta, B., Mishra, A., Singh, R. & Thakur, I. S. Fabrication of calcite based biocomposites for catalytic removal of heavy metals from electroplating industrial effluent. Environ. Technol. Innovat. 21, 101278 (2021).
Thakare, S. R. & Pal M. R. Hydrothermal Synthesis of Poly (Aniline-Co-Sulphanilic Acid) Copolymer with Highly Improved Electrical Conductivity and Ion Exchange Properties. Chem. Methodol. 4(6), 707–719 (2020).
Hency, T., Ram, V., Dave, P.N. Plant Mediated Synthesis of Iron Nanoparticles and Their Applications: A Review. Prog. chem. biochem. res. 2(3), 84–91 (2019).
Abdullahi, A. et al. Isotherms, Kinetics, Equilibrium, and Thermodynamic Studies on the Uptake of Hexavalent Chromium Ions from Aqueous Solution Using Synthetic Hydroxyapatite. Adv. J. Chem. B. 2(4), 214–225 (2020).
Dhiman, P. et al. Rapid visible and solar photocatalytic Cr (VI) reduction and electrochemical sensing of dopamine using solution combustion synthesized ZnO–Fe2O3 nano heterojunctions: Mechanism elucidation. Ceram. Int. 46(8), 12255–12268 (2020).
Shakoor, M. B. et al. The evaluation of arsenic contamination potential, speciation and hydrogeochemical behaviour in aquifers of Punjab, Pakistan. Chemosphere 199, 737–746 (2018).
Borthakur, P. et al. Experimental and molecular dynamics simulation study of specific ion effect on the graphene oxide surface and investigation of the influence on reactive extraction of model dye molecule at water–organic interface. J. Phys. Chem. C 120(26), 14088–14100 (2016).
Raza, M., Hussain, F., Lee, J. Y., Shakoor, M. B. & Kwon, K. D. Groundwater status in Pakistan: A review of contamination, health risks, and potential needs. Crit. Rev. Environ. Sci. Technol. 47(18), 1713–1762 (2017).
Suljevic, D., Sulejmanovic, J., Focak, M., Halilovic, E., Pupalovic, D., Hasic, A., Alijagic, A., Assessing hexavalent chromium tissue-specific accumulation patterns and induced physiological responses to probe chromium toxicity in Coturnix japonica quail. Chemosphere, 129005 (2020).
Wise, J.T.F., Shi, X., Zhang, Z., Toxicology of Chromium (VI) Encyclopedia of Environmental Health(Second Edition), pp. 1–8 (2019).
Moradi, F. et al. A pilot study of the effects of chromium picolinate supplementation on serum fetuin-A, metabolic and inflammatory factors in patients with nonalcoholic fatty liver disease: A double-blind, placebo-controlled trial. J. Trace Elements Med. Biol. 63, 126659 (2021).
Rafiaee, S., Samani, M. R. & Toghraie, D. Removal of hexavalent chromium from aqueous media using pomegranate peels modified by polymeric coatings: Effects of various composite synthesis parameters. Synthetic Metals J. 265, 116416 (2020).
Kumar, A. et al. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review. Chem. Eng. J. 382, 122937 (2020).
Tasrin, S., Fazil, S. M., Senthilmurugan, S. & Selvaraju, N. Facile preparation of nanocellulose embedded polypyrrole for dye removal: Unary and binary process optimization and seed toxicity. Int. J. Environ. Sci. Technol. 18(2), 365–378 (2021).
Patil, D. S., Chavan, S. M. & Oubagaranadin, J. U. K. A review of technologies for manganese removal from solutionss. J. Environ. Chem. Eng. 4, 468–487 (2016).
Youssef, A. M., El-NaggarMalhat, M. E. & El-Sharkawi, F. M. Efficient removal of pesticides and heavy metals from solutions and the antimicrobial activity of f-MWCNTs/PVA nanocomposite film. J. Clean. Prod. 206, 315–325 (2019).
Seliem, M. K. & Komarneni, S. Equilibrium and kinetic studies for adsorption of iron from aqueous solution by synthetic Na-A zeolites: Statistical modeling and optimization. Micropor. Mesopor. Mat. 228, 266–274 (2016).
Abdullahi, A. et al. Isotherms, Kinetics, Equilibrium, and Thermodynamic Studies on the Uptake of Hexavalent Chromium Ions from Aqueous Solution Using Synthetic Hydroxyapatite. Adv. J. Chem. B. 2(4), 214–225 (2020).
Guo, Y. et al. A novel process for comprehensive resource utilization of hazardous chromium sludge: Progressive recovery of Si, V, Fe and Cr. J. Hazardous Mater. 405, 124669 (2021).
Abdullah, N., Yosof, N., Lau, W. J., Jaafar, J. & Ismail, A. F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 76, 17–38 (2019).
Wei, W. et al. Selective adsorption of organic dyes by porous hydrophilic silica aerogels from aqueous system. Water Sci. Technol. 78, 402–414 (2018).
Malik, D. S., Jain, C. K. & Yadav, A. K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 7, 2113–2136 (2017).
Shao, P. et al. Lattice-defect-enhanced adsorption of arsenic on zirconia nanospheres: A combined experimental and theoretical study. ACS Appl. Mater. Interfaces. 11, 29736–29745 (2019).
Cai, C., Wang, R., Liu, S., Yan, X., Zhang, L., Wang, M., Tong, Q., Jiao, T., Synthesis of self-assembled phytic acid-MXene nanocomposites via a facile hydrothermal approach with elevated dye adsorption capacities. Colloid. Surface. A., 589 (2020). Article 124468.
Shahnaz, T., Padmanaban, V. C. & Narayanasamy, S. Surface modification of nanocellulose using polypyrrole for the adsorptive removal of Congo red dye and chromium in binary mixture. Int. J. Biol. Macromol. 15(151), 322–332 (2020).
Shahnaz, T., Priyan, V. V., Pandian, S. & Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of Ciprofloxacin and Diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 268, 115494 (2021).
Awwad, A., Amer, M., Al-aqarbeh, M., TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: Adsorption and thermodynamics of Pb (II) and Cd (II) ions in aqueous solution. Chem Int (2020).
Alaqarbeh, M., Shammout, M. & Awwad, A. Nano platelets kaolinite for the adsorption of toxic metal ions in the environment. Chem. Int. 6, 49–55 (2020).
Alasadi, A., Khaili, F. & Awwad, A. Adsorption of Cu (II), Ni (II) and Zn (II) ions by nano kaolinite: thermodynamics and kinetics studies. Chem. Int. 5, 258–326 (2019).
Soliman, N. K. & Moustafa, A. F. Industrial solid waste for heavy metals adsorption features and challenges: A review. J. Market. Res. 9(5), 10235–10253 (2020).
Tran, A. T. K. et al. From waste disposal to valuable material: Sulfonating polystyrene waste for heavy metal removal. J. Environ. Chem. Eng. 8(5), 104302 (2020).
Sandoval, V. J. L. et al. Valorization of agri-food industry wastes to prepare adsorbents for heavy metal removal from water. J. Environ. Chem. Eng. 8(5), 104067 (2020).
Kera, N. H., Bhaumik, M., Pillay, K., Ray, S. S. & Maity, A. m-Phenylenediamine-modified polypyrrole as an efficient adsorbent for removal of highly toxic hexavalent chromium in water. Mater. Today Commun. 1(15), 153–164 (2018).
Moorthy, M., Kumar, V. B., Porat, Z. & Gedanken, A. Novel polymerization of aniline and pyrrole by carbon dots. N. J. Chem. 42, 535–540 (2018).
Taghizadeh, A. et al. Conductive polymers in water treatment: A review. J. Molecular Liq. 312, 113447 (2020).
Wang, X. et al. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 115, 100704 (2021).
Dinari, M., Neamati, S., Surface modified layered double hydroxide/polyaniline nanocomposites: synthesis, characterization and Pb2+ removal. Colloid Surface Physicochem. Eng. Asp., p 124438 (2020).
Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chemistry 74, 1–54 (2020).
Deng, X. et al. Polyaniline-TiO2 composite photocatalysts for light-driven hexavalent chromium ions reduction. Sci. Bull. 65(2), 105–112 (2020).
Deng, J., Li, X., Wei, X., Liu, Y., Liang, J., Song, B., Shao, Y., Huang, W., Hybrid silicate-hydrochar composite for highly efficient removal of heavy metal and antibiotics: Coadsorption and mechanism. Chem. Eng. J., p 11 (2020). Article 124097.
Wang, Y., Tran, H. D., Liao, L., Duan, X. & Kaner, R. B. Nanoscale morphology, dimensional control, and electrical properties of oligoanilines. J. Am. Chem. Soc. 132(30), 10365–10373 (2010).
Syed, A. A. & Dinesan, M. K. Polyaniline—A novel polymeric material. Talanta 38(8), 815–837 (2009).
Kumar, R. et al. Adsorption modeling and mechanistic insight of hazardous chromium on para toluene sulfonic acid immobilized-polyaniline@CNTs nanocomposites. J. Saudi Chem. Soc. 23(2), 188–197 (2019).
Riahisamani, M. & Toghraie, D. Removal of hexavalent chromium from water using polyaniline/wood sawdust/polyethylen glycol composite: An experimental study. J. Environ. Health Sci. Eng. 17, 53–62 (2019).
Hsini, A. et al. Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: Equilibrium, reusability and process optimization. J. Mol. Liquids 316, 113832 (2020).
Shyaa, A. A., Hasan, O. A. & Abbas, A. M. Synthesis and characterization of polyaniline/zeolite nanocomposite for the removal of chromium(VI) from aqueous solution. J. Saudi Chem. Soc. 19(1), 101–107 (2015).
Hosseini, H. & Mousavi, S. M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 278, 123817 (2021).
Mahmud, H. N. M. E., Huq, A. O. & Binti Yahya, R. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. Rsc Adv. 6(18), 14778–14791 (2016).
Alidokht, L., Khataee, A. R., Reyhanitabar, A. & Oustan, S. Reductive removal of Cr (VI) by starch-stabilized Fe 0 nanoparticles in aqueous solution. Desalination 270(1), 105–110 (2011).
Priyan, V. V., Shahnaz, T., Suganya, E., Sivaprakasam, S. & Narayanasamy, S. Ecotoxicological assessment of micropollutant Diclofenac biosorption on magnetic sawdust: Phyto, Microbial and Fish toxicity studies. J. Hazardous Mater. 403, 123532 (2021).
Rambabu, K., Bharath, G., Banat, F., Show, P.L., Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. P 187 (2020). Article 109694.
Emam, A. A., Ismail, L. F. M. & AbdelKhalek, M. A. AzzaRezhan, Adsorption study of some heavy metal ions on modified kaolinite clay. Int J. Advan. Eng. Technol. Manag. Appl. Sci. 3(7), 152–163 (2016).
Lu, J., Xu, K., Yang, J., Hao, Y. & Cheng, F. Nano iron oxide impregnated in chitosan bead as a highly efficient sorbent for Cr(VI) removal from water. Carbohydr. Polym. 173, 28–36 (2017).
Cao, Y. et al. Poly(vinylidene fluoride) derived fluorine-doped magnetic carbon nanoadsorbents for enhanced chromium removal. Carbon 115, 503–514 (2017).
Senberber, F. T., Yildirim, M., Mermer, N. K. & Derun, E. M. Adsorption of Cr (III) from aqueous solution using borax sludge. Acta Chim. Slov. 64, 654–660 (2017).
Shobier, A. H., Sadaawy, M. M. E. & Said, G. F. E. Removal of hexavalent chromium by ecofriendly raw marine green alga Ulva fasciata: Kinetic, thermodynamic and isotherm studies. Egypt. J. Aquatic Res. 46(4), 325–331 (2020).
Soliman, N. et al. Removal of chromium and cadmium ions from aqueous solution using residue of Rumex dentatus L. Plant waste. Desalin Water Treat 149, 181–193 (2019).
Soliman, N., Mohamed, H.S., Ahmed, S.A., Sayed, F.H., Elghandour, A.H., Ahmed,S.A., Cd2+ and Cu2+ removal by the waste of the marine brown macroalga Hydroclathrus clathratus. Environ. Technol. Innov. (2019). Article 100365.
Khamis Soliman, N., Moustafa, A. F., Aboud, A. A. & Halim, K. S. A. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J. Mater. Res. Technol. 8(2), 1798–1808 (2019).
Mohamed, H. S. et al. Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low-cost adsorbent. Heliyon 5, e01287 (2019).
Shahnaz, T., Patra, C., Sharma, V. & Selvaraju, N. A comparative study of raw, acid-modified and EDTA-complexed Acacia auriculiformis biomass for the removal of hexavalent chromium. Chem. Ecol. 36(4), 360–381 (2020).
Ghorbani-Khosrowshahi, S. & Behnajady, M. A. Chromium (VI) adsorption from aqueous solution by prepared biochar from Onopordom Heteracanthom. Int. J. Environ. Sci. Technol. 13(7), 1803–1814 (2016).
Ghnimi, S. M. & Srasra, N. F. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 179, 105151 (2019).
Hussain, S., Amin, N. U. & Ali Khan, S. Quartzite an efficient adsorbent for the removal of anionic and cationic dyes from aqueous solutions.. Arab. J. Chem. 13, 4731–4740 (2020).
Liu, Z. et al. Geopolymerization enhanced hydrothermal synthesis of analcime from steel slag and CFBC fly ash and heavy metal adsorption on analcime. Environ. Technol. 41, 1753–1765 (2020).
Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Franklin Inst. 183, 102–105 (1917).
Olad, A. & Farshi, A. F. A study on the adsorption of chromium (VI) from aqueous solutions on the alginate-montmorillonite/polyaniline nanocomposite. Desalin. Water Treat. 52(13–15), 2548–2559 (2014).
Gałuszka, A., Migaszewski, Z. & Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices, TrAC, Trends. Anal. Chem. 50, 78–84 (2013).
Ren, G. et al. Chromium(vi) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci. Total Environ. 607–608, 900–910 (2017).
Mortazavian, S., An, H., Chun, D. & Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 353, 781–795 (2018).
Zhang, K., Li, H., Xu, X. & Yu, H. Synthesis of reduced graphene oxide/NiO nanocomposites for the removal of Cr(vi) from aqueous water by adsorption. Microporous Mesoporous Mater. 255, 7–14 (2018).
Preethi, J., Prabhu, S. M. & Meenakshi, S. Effective adsorption of hexavalent chromium using biopolymer assisted oxyhydroxide materials from aqueous solution. React. Funct. Polym. 117, 16–24 (2017).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosseini, S.A., Samani, M.R. & Toghraie, D. Investigating the hexavalent chromium removal from aqueous solution applying bee carcasses and corpses modified with Polyaniline. Sci Rep 11, 19117 (2021). https://doi.org/10.1038/s41598-021-97518-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-97518-7
This article is cited by
-
Assessment of biochar filter application in improving chromium stress tolerance and plant physiology in Chinese cabbage (Brassica rapa) under a flow-through water setup
BMC Biotechnology (2025)
-
Highly porous biochars from different biomasses as potential adsorbents for chromium removal: optimization by response surface methodology
International Journal of Environmental Science and Technology (2024)
-
Application of “Three-in-One” Strategy Based on MOF-199 in Determination of Phenylenediamine Isomers and Cr(VI)
Journal of Analysis and Testing (2024)
-
A comprehensive review on human health effects of chromium: insights on induced toxicity
Environmental Science and Pollution Research (2022)