Abstract
There is a clear relationship between rheumatoid arthritis (RA) and major osteoporotic fracture (MOF), although there is limited evidence on the effect of continuity of care (COC) on MOF in these patients. We investigated the association between COC and risk of MOF, including fractures of the lumbar spine and pelvis, forearm, and hip, among newly diagnosed RA patients aged ≥ 60 years. A total of 8715 incident RA patients from 2004 to 2010 were included from the Korean National Health Insurance Service-Senior cohort database. Participants were categorized into a good and bad COC group according to the COC index. The cumulative incidence of MOF was higher in RA patients with bad than in those with good COC (p < 0.001). The incidence rates of MOF were 4439 and 3275 cases per 100,000 person-years in patients with bad and good COC, respectively. RA patients with bad COC had an increased incidence of overall MOF (adjusted hazard ratio, 1.32; 95% confidence interval, 1.14–1.53), with the highest increase in risk being that of forearm fracture. An increased MOF risk in patients with bad COC was predominantly observed in females. This study suggested that interventions that can improve COC in patients with RA should be considered.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a progressive inflammatory disease characterized by chronic symmetrical polyarticular and extra-articular involvement1. Patients with RA may develop skeletal complications, including generalized bone loss, osteopenia, and osteoporotic fracture2,3. Among the comorbidities, major osteoporotic fracture (MOF) not only reduces the quality of life in the elderly population but also increases hospitalization and immediate- and long-term mortality risk; it is thus clinically important to find preventable factors associated with MOF in RA patients4,5. RA per se is a risk factor for the development of MOF, and risk of MOF increases as the disease progresses and chronic systemic inflammation increases6,7. Moreover, medications for RA are also associated with fracture risk8. The appropriate treatment for RA patients can improve the quality of life and increase life expectancy in the elderly with RA by preventing future MOF.
Continuity of care (COC), an essential concept for high-quality patient care, is the process by which patients and providers maintain an ongoing partnership to effectively meet the patients' healthcare needs9. Better COC is known to improve patient outcomes and satisfaction10,11. However, patients with chronic diseases involving a relatively long treatment period do not receive COC and often demonstrate “doctor-shopping” behavior12. In RA specifically, the goal of treatment is remission rather than cure, and relapse is common when medications are arbitrarily stopped or tapered after remission13. Subsequently, the patient may mistakenly believe that the recurrence resulted from poor quality of care at the initial hospital and may seek care from other hospitals. There is especially no regulation due to the attributes of the medical system in the Republic of Korea in which there is no primary care physician acting as a gatekeeper; rather, a specialist oversees primary care at the clinic14. This can lead to many fragmented visits.
Although there are several published studies reporting that better COC can reduce comorbidities in chronic diseases, such as diabetes and hypertension, only a few studies report the effect of COC on comorbidities in RA patients14,15,16,17. Therefore, this study aimed to investigate the association between the continuity of ambulatory care and incidence of MOF defined as fracture of the lumbar spine and pelvis, forearm, or hip in elderly patients with RA using a nationally representative sample from the National Health Insurance Service (NHIS)-Senior cohort 2002–2013.
Methods
Data and sample
This study used data from the 2002–2013 NHIS–Senior cohort provided by the NHIS of the Republic of Korea. The NHIS is the sole medical insurer managed by the government in the Republic of Korea that provides a system of universal healthcare coverage to the citizens of the Republic of Korea. All citizens, except those eligible for medical aid, are obligated to enroll in the NHIS. The National Health Information Database was developed by the NHIS and contains personal information and demographic details of all those enrolled for the purpose of collecting insurance premium and subscription data for reimbursement18.
The NHIS–Senior cohort refers to a representative sample cohort created by randomly selecting 558,147 adults aged ≥ 60 years, comprising 10% of the total eligible population in the Republic of Korea in 2002. Participants were followed-up for a period of 12 years until 2013, unless they were disqualified due to death or emigration19. The database includes information on reimbursement for each medical service and includes basic patient information, diagnostic codes, expenses incurred, and death-related information.
Study sample
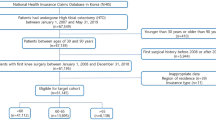
Our study sample included only patients who met the following criteria: (1) newly diagnosed RA patients in 2004–2010 and (2) at least four visits to an ambulatory clinic for RA within 2 years after initial diagnosis. Incident RA patients were defined as those who were diagnosed with diseases under the M05 code of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) during outpatient treatment. To enumerate only new onset RA patients, those diagnosed with RA between January 1, 2002 and December 31, 2003 were excluded20. Finally, to secure a time interval of at least 2 years to measure COC, patients diagnosed with RA for the first time after 2011 were excluded.
Identification of MOF
The primary outcome was defined as an incidence of MOF that met the following criteria: (1) patients diagnosed with fracture of the lumbar spine and pelvis, forearm, or hip (ICD-10 codes, S32, S52, and S72), (2) patients with a history of two or more outpatient visits or one or more hospitalizations owing to fracture after diagnosis, and (3) cases where the fracture occurred 2 years after the diagnosis of RA to consider the period for calculating the COC index.
Measuring COC
The characteristics of the medical delivery system in the Republic of Korea, which is not limited in terms of selecting the patient's preferred primary care provider, were considered in the measurement of COC. Therefore, the COC index, in this case, refers to the consistency of care17. There are various methods for measuring COC, of which the one most widely used in research is classification based on whether or not a primary health-care provider is designated21. The COC index, proposed by Bice et al., is the most representative index and is measured by combining the two aspects of visit concentration and visit distribution. The formula for measuring the COC index is as follows:
where nj was defined as the number of visits to the provider j, M is the number of medical service providers, and N is the total number of visits22. The COC index ranges from 0 to 1, where 0 means outpatient visits are distributed to different providers, and 1 means they are focused on one provider.
We selected a 2-year exposure period to ensure longitudinal continuity, as in the previous study23, and reflected the COC index of participants with four or more visits23,24. Therefore, the COC index within the first 2 years of RA diagnosis is determined according to all outpatient visits (Fig. 1). The reason for specifying the minimum number of visits when calculating the COC index is that the COC index is relatively easy to reach the maximum value of 1 or the minimum value of 0 with a small number of visits24. In this study, we used a cut-off point of 0.75 for the COC index, which has been extensively validated in previous studies25,26. Patients were thus classified as having good or bad COC depending on whether more or less than 75% of the outpatient visits were from the same physician during the 2 years following diagnosis of RA27. Since there are several methods of measuring COC, a sensitivity analysis was also performed by calculating the usual provider of care (UPC), another representative method. As in the previous study, if the UPC was 0.75 or higher, the patient was defined as having good COC28.
Covariates
This study considered age (60–64, 65–69, 70–74, 75–79, 80 + years), sex (male and female), income-level quintile, residential area (urban or rural), registered disability (yes or no), Charlson Comorbidity index (CCI; 0–1, 2, 3, or ≥ 4), history of osteoporosis (yes or no), RA severity (mild or severe), systemic glucocorticoid exposure (yes or no), and hospital-level variables as covariates. Hospital-level variables included hospital classification (general hospital, hospital, or clinic), hospital location (metropolitan, urban, or rural), number of beds (< 30, < 300, < 1000, or ≥ 1000), and ownership (public, corporate, or private). The CCI was calculated by the weighting and scoring of comorbidity conditions using Quan’s method, with additional points given to comorbidities that affect the health outcomes of patients29,30. Patients were classified as having severe RA according to the prescription claim for tumor necrosis factor inhibitors (TNFis), biologics, (infliximab, adalimumab, golimumab, certolizumab pegol, or etanercept), non-TNFi biologics, (abatacept, rituximab, tocilizumab, or anakinra), or Janus kinase inhibitors (JAKis) (tofacitinib)31,32,33. Patients who had used oral glucocorticoids for ≥ 6 months were categorized into the systemic glucocorticoid exposure group34. The hospital-level variables were based on the health-care institution most frequently visited by the patient for outpatient treatment17.
Statistical analyses
We used the Chi-square test for categorical variables to compare the distribution of baseline characteristics. Aalen-Johansen estimators were used to determine the cumulative incidence of MOF and 95% confidence interval (CI), and Gray’s k-sample test was conducted to compare the cumulative incidence35,36. A generalized estimating equation using a Poisson distribution was conducted to calculate the incidence rate (IR) of MOF and 95% CI. The IR was expressed as the number of MOF per 100,000 person-years. The effect size was expressed as a hazard ratio (HR) using the Cox proportional hazards model. We set time zero (index date) as 1 day after 2 years of RA diagnosis for each patient. We defined the survival time used in the survival analyses as the number of months from time zero to the date of MOF development, date of death, or December 31, 2013, whichever came first. The log transformation for the negative log of the estimated survivor function and Schoenfeld residuals were used to assess proportional hazard assumption. A Fine and Gray competing risk model with death as a competing risk was conducted along with cause-specific hazard model. Furthermore, since age may act as an important confounder in this study, an additional analysis was performed using age as a continuous variable and attained age (age as time scale)37. For the sensitivity analysis, the association between COC and the risk of MOF was investigated using the COC index segregated into four categories by 0.25 units and UPC. Stratified analyses according to age group, sex, and history of osteoporosis were also performed. Additionally, dependent subgroup analyzes were performed to examine whether COC had different effects depending on the MOF subtype. All calculated p-values were two–sided; p-values < 0.05 were considered significant. We performed all analyses using SAS software version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.0.3 (Vienna, Austria; Rproject.org/).
Ethics approval and consent to participate
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine (IRB no. 4-2021-0984). The need of informed consent was waived by the IRB of Severance Hospital at Yonsei University College of Medicine, as data of the NHIS–Senior cohort do not contain any personally identifiable information.
Results
From January 1, 2002 to December 31, 2013, a total of 134,732 RA patients met the inclusion criteria. Among them, patients with RA were excluded during the washout period of 2002–2003 (n = 40,620). As a next step, we excluded patients who had no outpatient visits for RA within 2 years after the first RA diagnosis (n = 60,197) and patients who had fewer than four outpatient visits within 2 years (n = 21,397). Among the initially selected patients with incident RA, those diagnosed with RA after 2011(n = 2030), with MOF before the RA diagnosis (n = 289) or index date (n = 1173), incident RA patients who died before the index date (n = 276), and those with missing covariates (n = 35) were also excluded. Finally, 8715 new onset RA patients from 2004 to 2010 were included in this study (Fig. 2).
The baseline characteristics of the study population according to the COC index are described in Table 1. Of the total 8715 RA cohort, 1088 (12.5%) patients had a bad COC index. Patients with bad COC index tended to have a history of osteoporosis and be exposed to systemic glucocorticoid. The mean follow-up period was 4.4 years, and 38,228 person-years were observed. During the follow-up period, 1310 (15.0% of the RA cohort) patients with RA developed MOF.
We observed a significant difference in the cumulative incidence of developing MOF during the entire follow-up period between RA patients with good and bad COC indices (p < 0.001 for the Gray’s test, Supplementary Fig. 1). The 8-year cumulative incidence risks of MOF were 27.91% and 21.00% in RA patients with bad and good COC indices, respectively (Table 2).
During the entire follow-up period, 222 new MOF cases were identified from 1088 RA patients with a bad COC index (Table 3). After adjusting for all covariates, RA patients with a bad COC index were 1.32 times more likely to develop MOF than those with a good COC index (adjusted HR, 1.32; 95% CI, 1.14–1.53; Table 3). When the age was adjusted as a continuous variable and the cox model with attained age was conducted, the results were similar to the main results (Table 3). The results using the Fine and Gray subdistribution hazard models (adjusted HR, 1.32; 95% CI, 1.13–1.54) and the cause-specific model were similar. Unadjusted and confounder-adjusted estimates are presented in Supplementary Table 1.
In the sensitivity analyses, when the COC index was divided into four groups, the risk of MOF was highest when the COC index was the worst (adjusted HR, 1.82; 95% CI, 1.02–3.24; Fig. 3). Similar results were obtained when measurements were made using the UPC rather than the COC index (adjusted HR, 1.23; 95% CI, 1.02–1.49; Fig. 3).
Sensitivity analyses using several measurements of continuity of care. (A) Analysis of the association between continuity of care (COC) and risk of major osteoporotic fracture (MOF) by classifying the COC index into four categories. (B) Analysis of association between COC and risk of MOF by different cut-offs in COC index and usual provider of care (UPC). UPC = Nu/N; where N is the total number of outpatient visits and Nu is the number of visits to routine health care providers. COC continuity of care, MOF major osteoporotic fracture, UPC usual provider of care.
Table 4 shows the association of the COC index with the risk of MOF subtype and risk of hospitalization for MOF treatment in RA patients. After adjusting for all covariates, the risk of fracture of the lumbar spine and pelvis increased by 1.33 times (adjusted HR, 1.33; 95% CI, 1.09–1.62) and the risk of fracture of the forearm by 1.49 times (adjusted HR, 1.49; 95% CI, 1.11–1.99) in RA patients with a bad COC index compared with those with a good COC index. RA patients with a bad COC index were 1.29 times more likely to be hospitalized for MOF treatment (adjusted HR, 1.29; 95% CI, 1.07–1.56).
Table 5 presents the association between the COC index and risk of MOF according to sex, age, and history of osteoporosis. The subsequent risk of fracture in RA patients significantly increased in female patients with a bad COC index (adjusted HR, 1.33; 95% CI, 1.14–1.62), and in patients in their 60 s (adjusted HR, 1.53; 95% CI, 1.23–1.90). Furthermore, a bad COC index increased the risk of fracture regardless of a history of osteoporosis.
Discussion
In this study, we examined the association between COC and the risk of MOF in patients with RA. RA patients with a bad COC had a 32% greater risk of MOF after adjusting for all covariates. The cumulative incidence of MOF was 6.91% greater for patients with a bad COC than for those with a good COC index.
The association between RA and MOF is higher with chronic systemic inflammation, decreased physical activity, and vitamin D deficiency7. A recent study reported that drugs used for RA, including disease-modifying antirheumatic drugs, in addition to glucocorticoids, which are classically known as risk factors for fracture, are associated with fracture risk8. Our study suggests that better COC may reduce the risk of MOF in elderly RA patients. However, the underlying mechanism was not elucidated. One possible hypothesis is that COC may benefit patients by enhancing their knowledge about the disease, motivating them to adhere to their physician's advice38. The increase in compliance according to COC may have a positive effect on the disease course, such as reducing chronic inflammation.
Results of the stratified analyses showed an increased risk of MOF with a bad COC index in female RA patients. Both genetic and hormonal factors influence sex differences in the association between a bad COC index and the risk of MOF. Female RA patients have higher disease activity scores and more severe dysfunction, which may have influenced this association39,40. Furthermore, a bad COC index was associated with subsequent risk of MOF in RA patients in the younger sector of the study population. It is well known that the prevalence of associated systemic symptoms, disease progression, and functional outcomes may vary depending on the age of onset of RA41. It is assumed that distinct characteristics of laboratory findings or phenotypes in late-onset RA, which are different from those of younger-onset RA42, affect RA and the subsequent risk of MOF, although additional studies should be considered.
A bad COC index among RA patients did not increase the risk of hip fracture, although the risk of lumbar spine and pelvis, and forearm fractures increased significantly, concurring with previous studies that risk factors for fractures vary depending on the skeletal site43. Particularly, the differences between the risk factors for the distal radius and those for hip fracture may be the cause of this44,45.
The paradigm of recognition and treatment of RA has changed over the past two decades, and remission and damage prevention have become the major treatment goals46. The prioritization of evaluation of suspected inflammatory arthritis within a few weeks of onset, frequent re-evaluation to achieve objective determination of remission, and aggressive adjustment of treatment are widely accepted as the standard of care and feature as major treatment guidelines31,32.
To support these principles and to achieve these standards in practice, COC is important in patients with RA. The government in the Republic of Korea introduced the Chronic Disease Care System (CDCS) for patients with hypertension and diabetes in 2012 to improve the quality of treatment and to contain costs. Participation of patients in the CDCS program assures a reduction of outpatient out-of-pocket costs from the usual 20 to 30% of the total cost; there is also the provision for health support services, such as education, if hypertension or diabetes patients choose their preferred primary clinic and continue to receive treatment at the same institution47. COC can be improved in diabetic patients with the implementation of the CDCS pilot project47. Our results suggest that chronic disease management initiatives to improve COC may not be limited to patients with hypertension and diabetes but could be extended to RA patients as well.
Our study has some limitations. The first is the accuracy in diagnosing MOF. However, in defining patients with MOF, we tried to overcome the limitation of claim data by including only those patients who visited the outpatient clinic twice or more or were hospitalized at least once. Second, unhealthy behaviors such as smoking and alcohol consumption may also affect the risk of MOF; however, these could not be confirmed in the absence of relevant information in the original dataset. Third, the prescriptions of biologics, including TNFis or non-TNFi biologics, were used to adjust the severity of RA. However, the severity could not be fully reflected, as the laboratory tests of each RA patient were not confirmed due to the nature of the claim data. Lastly, causality cannot be inferred owing to the observational nature of the study, even though we adjusted for possible confounders. There might also be reverse causality in RA patients with physical limitation due to MOF that lead to less visits of clinic despite excluding patients diagnosed with MOF prior to the onset of RA. Future well-designed prospective studies are warranted to confirm the causality between COC and risk of MOF in RA patients.
Despite these limitations, this study has several notable strengths. First, to the best of our knowledge, this is the first study to investigate the association between COC and the risk of MOF. Subsequently, due to the characteristics of Korea’s NHIS, the NHIS–Senior cohort, which is based on claim data, does not target patients only in a specific hospital, institution, or area, but rather represents the entire population over 60 years of age in the Republic of Korea. Thus, this study was conducted on a representative population of RA patients aged ≥ 60 years in the Republic of Korea. Furthermore, as the NHIS–Senior cohort used in this study is a large sample from a 12-year period with a relatively small number of follow-up losses, the association between COC and the risk of MOF could be observed for a sufficient duration.
Conclusions
In conclusion, senior RA patients with a bad COC index had an increased risk of developing MOF compared with those with a good COC index. Among MOF subtypes, a bad COC index was associated with increased subsequent risk of fracture of the lumbar spine, pelvis, and forearm. The association between COC and the subsequent risk of developing MOF in RA patients was prominent in female patients. Due to the nature of observational studies, causality cannot be inferred, but it is necessary to educate RA patients that COC is a good way to improve disease progression in clinical practice. Moreover, policymakers should consider to adopt policies to improve COC in these patients, as well as in patients with chronic diseases such as hypertension and diabetes.
Data availability
No data are available as the National Health Information Database is accessible only by researchers authorized by the National Health Insurance Service.
Abbreviations
- CCI:
-
Charlson comorbidity index
- CDCS:
-
Chronic disease care system
- CI:
-
Confidence interval
- COC:
-
Continuity of care
- HR:
-
Hazard ratio
- ICD-10:
-
10th revision of the International Statistical Classification of Diseases and Related Health Problems
- IR:
-
Incidence rate
- JAKi:
-
Janus kinase inhibitor
- MOF:
-
Major osteoporotic fracture
- NHIS:
-
National Health Insurance Service
- RA:
-
Rheumatoid arthritis
- TNFi:
-
Tumor necrosis factor inhibitor
- UPC:
-
Usual provider of care
References
Kwoh, C. K. et al. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 46, 328–346 (2002).
van Staa, T. P., Geusens, P., Bijlsma, J. W., Leufkens, H. G. & Cooper, C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 54, 3104–3112 (2006).
Jin, S. et al. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int.: A J. Establ. Res. Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 29, 1263–1275 (2018).
Bliuc, D. et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301, 513–521 (2009).
Pongchaiyakul, C. et al. Asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: A long-term prospective study. J. Bone Min. Res.: Offi. J. Am. Soc. Bone Min. Res. 20, 1349–1355 (2005).
El Maghraoui, A. et al. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology (Oxford) 49, 1303–1310 (2010).
Briot, K., Geusens, P., Em Bultink, I., Lems, W. F. & Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int.: A J. Establ. Res. Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 28, 3301–3314 (2017).
Ozen, G., Pedro, S., Wolfe, F. & Michaud, K. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 78, 1041–1047 (2019).
Gulliford, M., Naithani, S. & Morgan, M. What is ‘continuity of care’?. J. Health Serv. Res. Policy 11, 248–250 (2006).
Rogers, J. & Curtis, P. The concept and measurement of continuity in primary care. Am. J. Public Health 70, 122–127 (1980).
van Walraven, C., Oake, N., Jennings, A. & Forster, A. J. The association between continuity of care and outcomes: A systematic and critical review. J. Eval. Clin. Pract. 16, 947–956 (2010).
Sato, T., Takeichi, M., Shirahama, M., Fukui, T. & Gude, J. K. Doctor-shopping patients and users of alternative medicine among Japanese primary care patients. Gen. Hosp. Psychiatry 17, 115–125 (1995).
Haschka, J. et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: Interim results from the prospective randomised controlled RETRO study. Ann. Rheum. Dis. 75, 45–51 (2016).
Cho, K. H. et al. Impact of continuity of care on preventable hospitalization of patients with type 2 diabetes: A nationwide Korean cohort study, 2002–10. Int. J. qual. Health Care: J. Int. Soc. Qual. Health Care 28, 478–485 (2016).
Nam, Y. S., Cho, K. H., Kang, H. C., Lee, K. S. & Park, E. C. Greater continuity of care reduces hospital admissions in patients with hypertension: An analysis of nationwide health insurance data in Korea, 2011–2013. Health Policy (Amsterdam, Netherlands) 120, 604–611 (2016).
Choi, D. et al. Impact of continuity of care on cardiovascular disease risk among newly-diagnosed hypertension patients. Sci. Rep. 10, 19991 (2020).
Jang, Y. J., Choy, Y. S., Nam, C. M., Moon, K. T. & Park, E. C. The effect of continuity of care on the incidence of end-stage renal disease in patients with newly detected type 2 diabetic nephropathy: A retrospective cohort study. BMC Nephrol. 19, 127 (2018).
Moon, J. Y. et al. Association between open-angle glaucoma and the risks of Alzheimer’s and Parkinson’s diseases in South Korea: A 10-year nationwide cohort study. Sci. Rep. 8, 11161 (2018).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC) South Korea. Int. J. Epidemiol. 46, e15 (2017).
Jang, S. Y., Kang, K. W., Jo, M. & Park, M. Risk of new-onset acute coronary syndrome and atrial fibrillation in patients with rheumatoid arthritis compared with a risk-set and propensity score-matched cohort- a nationwide cohort study. Circ. J. 85, 194–200 (2021).
Saultz, J. W. Defining and measuring interpersonal continuity of care. Ann. Fam. Med. 1, 134–143 (2003).
Bice, T. W. & Boxerman, S. B. A quantitative measure of continuity of care. Med. Care 15, 347–349 (1977).
Wolinsky, F. D. et al. Continuity of care with a primary care physician and mortality in older adults. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 65, 421–428 (2010).
Nyweide, D. J. et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern. Med. 173, 1879–1885 (2013).
Menec, V. H., Sirski, M., Attawar, D. & Katz, A. Does continuity of care with a family physician reduce hospitalizations among older adults?. J. Health Serv. Res. Policy 11, 196–201 (2006).
Worrall, G. & Knight, J. Continuity of care is good for elderly people with diabetes: Retrospective cohort study of mortality and hospitalization. Can. Fam. Phys. Med. Fam. Can. 57, e16-20 (2011).
Cho, K. H. et al. The association between continuity of care and all-cause mortality in patients with newly diagnosed obstructive pulmonary disease: A population-based retrospective cohort study, 2005–2012. PLoS ONE 10, e0141465 (2015).
Cho, K. H. et al. Effects of continuity of care on hospital admission in patients with type 2 diabetes: Analysis of nationwide insurance data. BMC Health Serv. Res. 15, 107 (2015).
Lee, Y. R., Oh, S. S., Jang, S. I. & Park, E. C. Statin adherence and risk of all-cause, cancer, and cardiovascular mortality among dyslipidemia patients: A time-dependent analysis. Nutr. Metab Cardiovasc. Dis. 30, 2207–2214 (2020).
Quan, H. et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173, 676–682 (2011).
Singh, J. A. et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. (Hoboken, N.J.) 68, 1–26 (2016).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699 (2020).
Chandran, U., Reps, J., Stang, P. E. & Ryan, P. B. Inferring disease severity in rheumatoid arthritis using predictive modeling in administrative claims databases. PLoS ONE 14, e0226255 (2019).
Kim, D. et al. Glucocorticoids are associated with an increased risk for vertebral fracture in patients with rheumatoid arthritis. J. Rheumatol. 45, 612–620 (2018).
Andersen, P. K., Abildstrom, S. Z. & Rosthøj, S. Competing risks as a multi-state model. Stat. Methods Med. Res. 11, 203–215 (2002).
Thölking, G. et al. Increased renal function decline in fast metabolizers using extended-release tacrolimus after kidney transplantation. Sci. Rep. 11, 15606 (2021).
Korn, E. L., Graubard, B. I. & Midthune, D. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale. Am. J. Epidemiol. 145, 72–80 (1997).
Gray, D. P. et al. Towards a theory of continuity of care. J. R. Soc. Med. 96, 160–166 (2003).
Iikuni, N. et al. The influence of sex on patients with rheumatoid arthritis in a large observational cohort. J. Rheumatol. 36, 508–511 (2009).
Sokka, T. et al. Women, men, and rheumatoid arthritis: Analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res. Ther. 11, R7 (2009).
Yazici, Y. & Paget, S. A. Elderly-onset rheumatoid arthritis. Rheum. Dis. Clin. North Am. 26, 517–526 (2000).
Turkcapar, N. et al. Late onset rheumatoid arthritis: Clinical and laboratory comparisons with younger onset patients. Arch. Gerontol. Geriatr. 42, 225–231 (2006).
Papaioannou, A. et al. Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: The Canadian multicentre osteoporosis study (CaMos). Osteoporos. Int.: A J. Establ. Res. Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 16, 568–578 (2005).
Furuya, T. et al. Risk factors associated with the occurrence of hip fracture in Japanese patients with rheumatoid arthritis: A prospective observational cohort study. Osteoporos. Int.: A J. Establ. Res. Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 24, 1257–1265 (2013).
Ochi, K. et al. Risk factors associated with the occurrence of distal radius fractures in Japanese patients with rheumatoid arthritis: A prospective observational cohort study. Clin. Rheumatol. 33, 477–483 (2014).
Chatzidionysiou, K. & Sfikakis, P. P. Low rates of remission with methotrexate monotherapy in rheumatoid arthritis: Review of randomised controlled trials could point towards a paradigm shift. RMD Open 5, e000993 (2019).
Kim, W., Choy, Y. S., Lee, S. A. & Park, E. C. Implementation of the chronic disease care system and its association with health care costs and continuity of care in Korean adults with type 2 diabetes mellitus. BMC Health Serv. Res. 18, 991 (2018).
Acknowledgements
We would like to thank the NHIS, which provided the representative sample cohort data.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Author information
Authors and Affiliations
Contributions
S.H.K. and E.C.P. were responsible for the conception and design of the study. S.H.K., S.H.J. and H.K. performed the data acquisition and research execution. S.H.K. and S.Y.J. made contributions to analysis and interpretation of the data. SHK was drafted the manuscript. E.C.P. and S.Y.J. were performed writing review and editing, and all authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.H., Kim, H., Jeong, S.H. et al. Impact of continuity of care on risk for major osteoporotic fracture in patients with new onset rheumatoid arthritis. Sci Rep 12, 10189 (2022). https://doi.org/10.1038/s41598-022-14368-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-14368-7
This article is cited by
-
First fracture in rheumatoid arthritis: analysis by fracture site, gender, age, and comorbidities
Osteoporosis International (2025)