Abstract
Rim restriction surrounding the resection cavity of glioma is often seen on immediate post-op diffusion-weighted imaging (DWI). The etiology and clinical impact of rim restriction are unknown. We evaluated the incidence, risk factors and clinical consequences of this finding. We evaluated patients that underwent surgery for low-grade glioma (LGG) and glioblastoma (GBM) without stroke on post-operative imaging. Analyses encompassed pre- and postoperative clinical, radiological, intraoperative monitoring, survival, functional and neurocognitive outcomes. Between 2013 and 2017, 63 LGG and 209 GBM patients (272 in total) underwent surgical resection and were included in our cohort. Post-op rim restriction was demonstrated in 68 patients, 32% (n = 20) of LGG and 23% (n = 48) of GBM patients. Risk factors for restriction included temporal tumors in GBM (p = 0.025) and insular tumors in LGG (p = 0.09), including longer surgery duration in LGG (p = 0.008). After a 1-year follow-up, LGG patients operated on their dominant with post-op restriction had a higher rate of speech deficits (46 vs 9%, p = 0.004). Rim restriction on postoperative imaging is associated with longer duration of glioma surgery and potentially linked to brain retraction. It apparently has no direct clinical consequences, but is linked to higher rates of speech deficits in LGG dominant-side surgeries.
Similar content being viewed by others
Introduction
Maximal safe resection is the desired goal in surgery of both high- and low-grade gliomas, and it has also been linked to better overall survival1,2,3. However, postoperative deficits are still a major concern due to their association with impaired quality of life and even decreased survival4,5.
Diffusion-weighted imaging (DWI) restrictive changes are often seen transiently at the rim surrounding the resection cavity after surgery for gliomas6. Post-operative diffusion restriction is thought to result from cellular injury and swelling which decreases the movement of protons7,8. As opposed to intra-operative strokes which are occasionally demonstrated by post-operative DWI and have been discussed in depth in the past, the etiology and clinical impact of peri-resection rim restriction remain unclear9,10,11,12. Cases with this imaging finding have been either excluded from studies dealing with perioperative ischemic complications or incorporated into groups of combined ischemic lesions. Furthermore, these studies have often been composed of a variety of types of tumors10,12,13,14,15.
The purpose of this study was to characterize the various aspects of rim restrictions as depicted on imaging studies following resection of low grade glioma (LGG) and glioblastoma (GBM), including their incidence, risk factors, short- and long-term clinical, functional, and cognitive implications, as well as their associations with intraoperative events.
Results
Clinical and demographic data
Between 2013 and 2017, 468 patients underwent either surgical resection or biopsy at our center, of these 120 for LGG and 348 for GBM. Fourty-four cases of biopsy procedures alone (5 of LGG and 39 of GBM), as well as 23 patients who had no complete pre-and postoperative MRI data (4 of LGG and 19 of GBM), and 80 patients who had no detailed admission, surgery, and discharge or follow-up reports (29 of LGG and 51 of GBM) were excluded from the study. Nineteen LGG and 30 GBM patients who sustained an intraoperative stroke were also excluded. Our final cohort consisted of 272 patients (63 LGG and 209 GBM) who underwent resection and had full datasets that included a long-term clinical evaluation and no evidence of intra-operative stroke.
The combined population mean age at surgery was 53 ± 16 years, with LGG patients being significantly younger than those with GBM (37 ± 12 and 58 ± 14, respectively, p = 0.001). Of the combined study population, 102 (38%) were females with similar rates within each tumor study group. There were 100 (37%) awake operations and 92 (34%) recurrent operations. There was a significantly higher rate of temporal tumors among GBM patients (33% vs 11%, p = 0.001), while insular tumors were more common among LGG patients (21% Vs 9%, p = 0.013). Median preoperative KPS was significantly higher among LGG patients than in GBM (90, 70–100 vs 80, 30–100, p = 0.001). While pre-operative tumor volume was similar (34 ± 32 ml in LGG and 33 ± 25 ml in GBM, p = 0.750), EOR was significantly higher in the GBM group (96 ± 8%) vs 87 ± 11% in the LGG group (p = 0.001).
Incidence and risk factors
Rim restriction was demonstrated on the postoperative DWI studies of 68 patients (25%), including 20 LGG and 48 GBM patients.
We did not find significant differences in the rate of post-op rim restriction between LGG and GBM patients (32% vs 23%, p = 0.184).
We found no significant association between postoperative rim restriction and the evidence of blood in the surgical cavity that was demonstrated on the postoperative CT of 30% of the restriction cases and 28% of the non-restricted ones in LGG (p = 0.864), as well as 51% of GBM patients with restriction vs 49% in those without restriction (p = 0.177).
A univariate analysis that compared patients with and without rim restriction with respect to demographic, clinical, and pathological parameters showed no significant differences between those with and without rim-restriction in the combined GBM + LGG cohort (Table 1). Among GBM patients, rim restriction was more common in tumors located in the temporal lobes (32% Vs 18% in non-temporal areas, p = 0.025) and non-significantly more common in LGG of insular location (54% vs. 24%, p = 0.09). The calculated risk (odds ratio, OR) for post-op rim restriction among temporal GBM cases was 1.74 (95% CI 1.09–4.10, p = 0.027).
IOM
Full IOM of MEPs, anesthesiology and awake monitoring reports were available in 152, 267 and 98 of the combined study population (n = 272), respectively. Neither MEPs changes nor awake intraoperative monitoring parameters showed any significant associations with rim restriction. Duration of anesthesia in the combined GBM and LGG cohort was significantly longer among patients with rim restriction, when compared to those without it (306 ± 87 min and 276 ± 81 min, respectively, P = 0.009). The difference was even more pronounced in the LGG group analysis (375 ± 17 min vs 316 ± 13 min; p = 0.008), Table 2.
Clinical, functional, and cognitive outcomes
Overall and progression-free survival
The mean overall survival time in the LGG group was 56 months (95% confidence interval 52–60), and there was no perioperative mortality. There was no difference in survival between those with and without post-op restriction (p = 0.117). Mean progression free survival (PFS) in the entire LGG group was 44 months (95% CI 37–50) with no significant differences between the subgroups (p = 0.449).
Mean overall survival in the entire GBM group was 22 months (95% confidence interval 19–25). Interestingly, patients with immediate post-op restriction had a non-significant trend towards increased survival (26 months, 95% CI 19–32) as compared to those without (20 months, CI 95% 17–24, p = 0.076). Mean PFS in the GBM group was 13 months (95% CI 10–15) with a non-significant trend towards longer period among patients with immediate post-op restriction (15, 95% CI 19–21), as compared to those without rim restriction (11 months, 95% CI 8–14, p = 0.065).
Motor deficits
New or worsening immediate post-op motor deficits among LGG occurred in 4/20 (20%) of those with post-op restriction, similar to the rate in the non-restriction group (10/43 ,23%, p = 0.772). The rates were 9/48 (19%) in patients with post-op rim restriction and 19/161 (12%) of those without restriction (p = 0.215) in the GBM sub-population. In either group, no significant changes were noted in the rate of motor deficits over time, Tables 3 and 4.
Speech deficits
Speech deficits were analyzed in a subgroup of patients that underwent surgeries for tumors located in the dominant hemispheres (33 LGG and 103 GBM patients). We found a significantly higher rate of LGG patients with speech deficits in the post-op follow-up period in the restriction group, as compared to the non-restriction group (p = 0.004). All rim-restriction cases with speech deficits (n = 7) involved eloquent areas: either the left frontal, temporal or insular lobes. The percentage of patients with speech deficits in the post-op period decreased from 63 to 46% in the restriction group (p = 0.873) and from 18 to 9% (p = 0.989) in the non-restriction group after 1 year of follow-up, Table 3. Among GBM patients, no significant differences were noted in the rate of speech deficits in the post-op follow-up period between the restriction and non-restriction groups (p = 0.226), Table 4.
Functional assessment scores (KPS, MRS and cognitive tests)
We did not detect any significant differences in the KPS and MRS scores in either the LGG or GBM populations during our follow-up period (p > 0.05, see Tables 3 and 4).
Cognitive function tests, as previously described11, were performed in 33 LGG patients before and 3 months after surgery. We compared the results of 6 patients with post-op rim restriction and 27 with no restriction and could not detect any significant differences between the 2 groups in any of the functions assessed. Pre- and post-operative global cognitive scores were 98 ± 11and 95 ± 9 in the restriction group, and 95 ± 9 and 95 ± 9 in the non-restriction group, respectively (p = 0.194).
Discussion
To the best of our knowledge, this is the first study to specifically describe the incidence as well as the short- and long-term clinical correlations of a phenomenon, defined as rim restriction on postoperative imaging following resection of low-grade gliomas and GBM, while excluding cases that got complicated by ischemic stroke. The relevance of this data lies in the question whether this quite prevalent abnormal post-operative imaging finding is linked to certain intra-operative parameters and can predict surgical outcomes.
Rim restriction was seen on the postoperative DWI studies in 32% and 23% of LGG and GBM patients, respectively. The incidence rates of postoperative rim restriction reportedly ranged from 16 to 39%, yet previous studies encompassed various types of tumors and also included patients that sustained intra-operative ischemic strokes, which as well present with restrictive changes on post-operative DWI.12,13,23
We found that the duration of surgery was significantly longer among patients that developed rim restriction on postoperative imaging as opposed to those without (Table 2) and these differences were particularly noted in the LGG group. Proposed mechanisms for the development of postoperative DWI restrictive changes included local intraoperative brain ischemia by direct vascular damage, coagulation, or vasospasm, as well as kinking of small arteries or mechanical tissue pressure by brain retraction24,25. Prolonged brain retraction is a potential cause for rim restriction in surgeries of longer duration and minimizing retraction might reduce its occurrence9,24,25,26.
We found that rim restriction is more common after surgeries for GBM involving the temporal lobe (p = 0.025) and tends to be higher in LGG surgeries involving the insula (p = 0.09). Previous reports demonstrated a higher risk for restrictive changes on DWI as well as for ischemic complications in insular region surgeries13. The insula is mainly supplied by frequent perforating arteries with no collateral flow, and surgeries in this location often require prolonged brain retraction13,24,25,26,27,28. As we often use retractors during trans-cortical resections of either temporal or insular gliomas, we raise the possibility that even mild surgical retraction in a diseased temporal lobe might be enough to induce the micro-ischemic changes which are often seen post-op. We did not detect any additional peri-operative parameters to be associated with the occurrence of rim restriction, including previously reported ones, such as recurrent operations and decreased MAP or IOM abnormalities (Tables 1 and 2).15,25,26,29Findings of rim restriction have been associated with evidence of remnant blood products in the surgical cavity on postoperative imaging, yet no significant association were seen in our study9,30.
We found a higher rate of speech deficits among LGG patients with rim restriction after they had undergone dominant-side surgeries in either the frontal, temporal or insular lobes, even after adjusting for the duration of surgery. (Table 3). It was previously reported that rim-pattern DWI abnormalities were non-significantly more common among patients with new postoperative neurological deficits (35%), as opposed to those without (25%), p = 0.17710. However, most studies viewed this imaging pattern as displaying either normal postoperative changes or as part of a larger group of ischemic complications, which did not enable subgroup analyses9,13,15,23. It is possible that rim restriction in dominant-side surgeries, particularly insular cases, is an indicator for subtle parenchymal injuries during surgery that manifest in speech deficits. Such injuries may be related to retraction forces applied on the tissue, as discussed above24,27,28, which seem to lead to a long-lasting damage, as there was no significant decrease in the rate of speech deficits over time. The GBM population showed a significant decrease in the rate of seizures following surgical resection, and these results are in line with previous reports regarding the beneficial effects of surgery in alleviating seizures among glioma patients, yet no differences were noted in relation to post-op restriction status.31,32.
Interestingly, the GBM population showed a unique yet non-significant trend towards increased overall and progression-free survival among patients with post-op rim restriction (p = 0.076 and p = 0.065, respectively). We could not clearly explain this trend and additional research is needed in order to validate and explore these findings.
Glioma patients often sustain various preoperative cognitive abnormalities that may further deteriorate after surgery, depending upon the eloquence of tumor location. These functions often improve when analyzed up to 1 year after surgery33,34. With mild cognitive abnormalities having been related to small strokes in proximity to the resection cavity35, we now searched for any association between rim restriction and cognitive changes following surgery, yet our cohort was too small to detect significant differences between the groups.
Interestingly, a recent study on perilesional rim restriction after surgical removal of convexity meningiomas found that restriction thicker than 1 cm was associated with post-op neurological deficits that lasted more than 3 months, including motor and speech deficit and seizures, with the majority improving over time. Main risk factors for post-op restriction were increasing age, intra-operative blood loss, tumor location over the motor strip and pre-op peri-tumor edema36.
The main limitation of this study is its historical cohort design, which forced us to estimate and define the degree of several study parameters, such as MRS and KPS, based on the study patients’ medical records. In addition, in the case of LGG, the study follow-up period was too short (5 years) to reveal differences in long-term survival between groups of patients. Furthermore, molecular IDH status was not available for the entire study population, as it included patients treated between the years 2013–2017. Finally, this study was not large enough to perform certain subgroup analyses, such as cognitive changes following dominant-side surgeries.
Conclusions
Rim restriction on postoperative imaging is associated with longer durations of glioma surgeries, particularly of LGG, and more often occurs in cases of temporal and insular tumors. These imaging findings generally showed no apparent direct clinical consequences in either LGG or GBM, but they might be linked to a higher rate of speech deficits in dominant-side LGG surgeries.
Study population and methods
Study population
Our historical cohort included patients who underwent resection of LGG and GBM between January 2013 to December 2017. Preoperative clinical and intraoperative monitoring data were retrieved and documented, as were short- and long-term clinical outcomes. We selected patients that underwent surgical resection of LGG (World Health Organization 1 or 2) or GBM (WHO 4), and who had a full radiological and clinical dataset. The 2016 WHO classification became available in the middle of our study follow-up period (2013–2017). Due to the high ambiguity of WHO grade 3 that occasionally overlaps with grade II, but often tends to be more aggressive and overlap with grade IV, we decided not to include patients with the diagnosis of anaplastic astrocytoma in order to keep both study groups as uniform as possible. Patients who only underwent biopsies and those without full radiological and clinical data were excluded. In addition, in order to specifically focus on peri-resection rim restriction on post-operative imaging, we excluded patients who sustained intraoperative ischemic stroke (30 GBM and 19 LGG patients). The most recent surgery was considered as the index one in cases of patients who underwent more than one operation during the study period. This study was approved by the Tel-Aviv Sourasky Medical Center institutional ethics committee, reference number: 0768-17-TLV.
Clinical and demographic data
We reviewed admission, surgical, and discharge reports for each case. Clinical data on follow-up in the ambulatory or hospital setting up to one year after surgery were also collected. The following clinical and demographic pre-and post-operative parameters were analyzed: age, sex, body mass index, hand dominance, other malignancies, brain radiotherapy, cerebrovascular, cardiac and metabolic comorbidities, and recurrent tumors, Karnofsky Performance Status (KPS) and Modified Rankin Score (MRS). Mean overall and progression free survival were also measured. Neurological manifestations were documented, including motor and speech deficits as well as report of seizures before and immediately (within hours) after surgery, and at 3, 6, and 12 months of follow-up, when available. Due to the inherent prognostic differences between each pathology groups, these outcomes were measured separately, up to 6 months in GBM patients and up to 12 months among LGG patients.
Radiological data
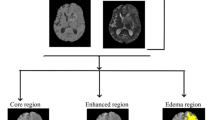
Data on tumor volume and location, tumor enhancement, and extent of resection (EOR) were derived from pre- and immediate postoperative MRI studies, which are routinely performed within 48 h after surgery.3,16 . The EOR for GBM and LGG was calculated using the following formula: (preoperative − postoperative tumor volume)/preoperative tumor volume × 100). In case of GBM, the volume of blood products rather than the volume of the residual tumor was confirmed by comparing T1-weighted gadolinium-enhanced and non-enhanced MRIs. FLAIR sequence was used for measuring the non-enhancing component of the tumors17. Rim-pattern restriction surrounding the resection cavity was detected by a neuroradiologist who was blinded to clinical outcomes, and it was based on DWI studies and apparent diffusion coefficient techniques (Fig. 1). Infarcts, which were excluded from this study, were distinguished by their typical wedge-shaped arterial territory and relatively rapid appearance on immediate post-op DWI. Areas of T1-weighted hyper-intensities, accompanied by areas of hyperdensity on post-operative computerized tomographs (CT) were considered as blood products11,14.
Neurocognitive analysis
LGG patients were evaluated by a battery of computerized cognitive tests before surgery and at the 3-month follow-up (Table 5). The NeuroTrax testing platform was used for the assessment of various cognitive functions, including visual and verbal memory, executive function, attention, naming, and visual spatial processing as was previously described11,18,19,20. Test scores were calculated by the software normalized for age and education level relative to a large normative database of healthy individuals and fit to an IQ-style scale, with higher scores reflecting better performance (mean:100, SD: 15).
Intraoperative neurophysiologic and anesthetics data
Changes in transcranial and direct cortical and subcortical motor evoked potentials (MEPs) of intraoperative monitoring (IOM) were evaluated as reported by our group3,16,21,22. Monitoring during awake craniotomies was performed by a trained neuropsychologist and it was based on continuous physical examination for motor functions, as well as language assessments for the detection of production and comprehension decline. Data on anesthetics included anesthesia duration and blood pressure measurements before and during surgery.
Statistical analysis
Characteristics of categorical data were compared using the Pearson’s χ2 test. Multivariate logistic regression analysis was used to evaluate risk factors for the occurrence of rim restriction. Overall and progression free survival (PFS) were analyzed based on the Kaplan–Meier method. Repetitively measured variables were analyzed using generalized linear models. Statistics were performed using SPSS 21.0 software (SPSS Inc, Chicago, IL).
Ethics approval
This study was approved by the Tel-Aviv Sourasky Medical Center institutional ethics committee, reference number: 0768-17-TLV. The study was performed in accordance with the relevant guidelines and regulations.
Consent to participate
The ethics committee of the Tel-Aviv Sourasky Medical Center waived the requirement of informed consent due to the retrospective nature of the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. In the future we may consider asking our patients for their permission to share clinical data for research puropses.
References
Jakola, A. S. et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas surgical resection vs waiting in low-grade gliomas. JAMA 308, 1881–1888. https://doi.org/10.1001/jama.2012.12807 (2012).
Brown, T. J. et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2, 1460–1469. https://doi.org/10.1001/jamaoncol.2016.1373 (2016).
Grossman, R. et al. Dynamics of FLAIR volume changes in glioblastoma and prediction of survival. Ann. Surg. Oncol. 24, 794–800. https://doi.org/10.1245/s10434-016-5635-z (2017).
McGirt, M. J. et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65, 463–469. https://doi.org/10.1227/01.neu.0000349763.42238.e9 (2009).
Hervey-Jumper, S. L. & Berger, M. S. Evidence for improving outcome through extent of resection. Neurosurg. Clin. N. Am. 30, 85–93. https://doi.org/10.1016/j.nec.2018.08.005 (2019).
Smith, J. S. et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: Distinguishing tumor recurrence from postresection injury. J. Neurosurg. 103, 428–438. https://doi.org/10.3171/jns.2005.103.3.0428 (2005).
Kessler, A. T. & Bhatt, A. A. Brain tumour post-treatment imaging and treatment-related complications. Insights Imaging 9, 1057–1075. https://doi.org/10.1007/s13244-018-0661-y (2018).
Le Bihan, D. & Iima, M. Diffusion magnetic resonance imaging: What water tells us about biological tissues. PLoS Biol. 13, e1002203. https://doi.org/10.1371/journal.pbio.1002203 (2015).
Ozturk, A., Oguz, K. K., Akalan, N., Geyik, P. O. & Cila, A. Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn. Intervent. Radiol. (Ankara, Turkey) 12, 115–120 (2006).
Jakola, A. S. et al. Surgically acquired deficits and diffusion weighted MRI changes after glioma resection—A matched case-control study with blinded neuroradiological assessment. PLoS ONE 9, e101805. https://doi.org/10.1371/journal.pone.0101805 (2014).
Berger, A. et al. Incidence and impact of stroke following surgery for low-grade gliomas. J. Neurosurg. https://doi.org/10.3171/2019.10.jns192301 (2019).
Strand, P. S. et al. Brain infarctions after glioma surgery: Prevalence, radiological characteristics and risk factors. Acta Neurochir. 163, 3097–3108. https://doi.org/10.1007/s00701-021-04914-z (2021).
Dutzmann, S. et al. Risk of ischemia in glioma surgery: comparison of first and repeat procedures. J. Neurooncol. 107, 599–607. https://doi.org/10.1007/s11060-011-0784-1 (2012).
Ulmer, S. et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67, 1668–1670. https://doi.org/10.1212/01.wnl.0000242894.21705.3c (2006).
Gempt, J. et al. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J. Neurosurg. 118, 801–808. https://doi.org/10.3171/2012.12.jns12125 (2013).
Zelitzki, R. et al. Comparison of motor outcome in patients undergoing awake vs general anesthesia surgery for brain tumors located within or adjacent to the motor pathways. Neurosurgery https://doi.org/10.1093/neuros/nyz007 (2019).
Grabowski, M. M. et al. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma: Clinical article. J. Neurosurg. JNS 121, 1115–1123. https://doi.org/10.3171/2014.7.jns132449 (2014).
Melton, J. T., Murray, J. R. & Lowdon, I. M. A simple clinical test of flexor pollicis longus rupture. J. Hand Surg. Br. 30, 624–625. https://doi.org/10.1016/j.jhsb.2005.06.005 (2005).
Schweiger, A. D. G., Dwolatzky, T., Jaffe, D. & Simon, E. S. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychol. 1, 407–413 (2003).
Dwolatzky, T. et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 3, 4. https://doi.org/10.1186/1471-2318-3-4 (2003).
Gonen, T. et al. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J. Neurosurg. 121, 1133–1138. https://doi.org/10.3171/2014.7.JNS132657 (2014).
Grossman, R. et al. Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann. Surg. Oncol. 20, 1722–1728. https://doi.org/10.1245/s10434-012-2748-x (2013).
Khan, R.B., Gutin, P.H., Rai, S.N., Zhang, L., Krol, G., & DeAngelis, L.M. Use of diffusion weighted magnetic resonance imaging in predicting early postoperative outcome of new neurological deficits after brain tumor resection. Neurosurgery 59, 60–66. https://doi.org/10.1227/01.neu.0000219218.43128.fc (2006) (discussion 60–66)
Rey-Dios, R. & Cohen-Gadol, A. A. Technical nuances for surgery of insular gliomas: Lessons learned. Neurosurg. Focus 34, E6. https://doi.org/10.3171/2012.12.focus12342 (2013).
Neuloh, G., Simon, M. & Schramm, J. Stroke prevention during surgery for deep-seated gliomas. Neurophysiol. Clin. Clin. Neurophysiol. 37, 383–389. https://doi.org/10.1016/j.neucli.2007.09.002 (2007).
Neuloh, G., Pechstein, U. & Schramm, J. Motor tract monitoring during insular glioma surgery. J. Neurosurg. 106, 582–592. https://doi.org/10.3171/jns.2007.106.4.582 (2007).
Kumabe, T., Higano, S., Takahashi, S. & Tominaga, T. Ischemic complications associated with resection of opercular glioma. J. Neurosurg. 106, 263–269. https://doi.org/10.3171/jns.2007.106.2.263 (2007).
Przybylowski, C. J., Baranoski, J. F., So, V. M., Wilson, J. & Sanai, N. Surgical morbidity of transsylvian versus transcortical approaches to insular gliomas. J. Neurosurg. 132, 1731–1738. https://doi.org/10.3171/2018.12.jns183075 (2019).
Bette, S. et al. Safe brain tumor resection does not depend on surgery alone—Role of hemodynamics. Sci. Rep. 7, 5585. https://doi.org/10.1038/s41598-017-05767-2 (2017).
Schaefer, P. W., Grant, P. E. & Gonzalez, R. G. Diffusion-weighted MR imaging of the brain. Radiology 217, 331–345. https://doi.org/10.1148/radiology.217.2.r00nv24331 (2000).
Wang, D. D. et al. Seizure outcome after surgical resection of insular glioma. Neurosurgery 83, 709–718. https://doi.org/10.1093/neuros/nyx486 (2018).
Borger, V. et al. Seizure outcome in temporal glioblastoma surgery: lobectomy as a supratotal resection regime outclasses conventional gross-total resection. J. Neurooncol. 152, 339–346. https://doi.org/10.1007/s11060-021-03705-x (2021).
Satoer, D. et al. Long-term evaluation of cognition after glioma surgery in eloquent areas. J. Neurooncol. 116, 153–160. https://doi.org/10.1007/s11060-013-1275-3 (2014).
Satoer, D. et al. Cognitive functioning early after surgery of gliomas in eloquent areas. J. Neurosurg. 117, 831–838. https://doi.org/10.3171/2012.7.jns12263 (2012).
Loit, M. P. et al. Hotspots of small strokes in glioma surgery: An overlooked risk?. Acta Neurochir. 161, 91–98. https://doi.org/10.1007/s00701-018-3717-3 (2019).
Magill, S. T. et al. Postoperative diffusion-weighted imaging and neurological outcome after convexity meningioma resection. J. Neurosurg. https://doi.org/10.3171/2020.8.jns193537 (2021).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design: R.R.G., A.B., G.T. Material preparation and data collection: A.B., G.T., M.S., P.V., A.M., A.K., N.K.L., D.A. Data analysis was performed by A.B. and G.T. The first draft of the manuscript was written by A.B. and G.T. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berger, A., Tzarfati, G.G., Serafimova, M. et al. Clinical and prognostic implications of rim restriction following glioma surgery. Sci Rep 12, 12874 (2022). https://doi.org/10.1038/s41598-022-16717-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-16717-y
This article is cited by
-
Impact of maximal and supramaximal resections on postoperative diffusion-weighted imaging changes and clinical outcomes in IDH-wildtype glioblastoma
Journal of Neuro-Oncology (2025)
-
Mechanism and significance of diffusion restriction followed by calcification in high-grade glioma treated with bevacizumab
Scientific Reports (2024)
-
Survival implications of postoperative restricted diffusion in high-grade glioma and limitations of intraoperative MRI detection
Journal of Neuro-Oncology (2024)
-
Risk factors and prognostic implications of surgery-related strokes following resection of high-grade glioma
Scientific Reports (2022)