Abstract
The survival rate of breast cancer patients is closely related to the pathological stage of cancer. The earlier the pathological stage, the higher the survival rate. Breast ultrasound is a commonly used breast cancer screening or diagnosis method, with simple operation, no ionizing radiation, and real-time imaging. However, ultrasound also has the disadvantages of high noise, strong artifacts, low contrast between tissue structures, which affect the effective screening of breast cancer. Therefore, we propose a deep learning based breast ultrasound detection system to assist doctors in the diagnosis of breast cancer. The system implements the automatic localization of breast cancer lesions and the diagnosis of benign and malignant lesions. The method consists of two steps: 1. Contrast enhancement of breast ultrasound images using segmentation-based enhancement methods. 2. An anchor-free network was used to detect and classify breast lesions. Our proposed method achieves a mean average precision (mAP) of 0.902 on the datasets used in our experiment. In detecting benign and malignant tumors, precision is 0.917 and 0.888, and recall is 0.980 and 0.963, respectively. Our proposed method outperforms other image enhancement methods and an anchor-based detection method. We propose a breast ultrasound image detection system for breast cancer detection. The system can locate and diagnose benign and malignant breast lesions. The test results on single dataset and mixed dataset show that the proposed method has good performance.
Similar content being viewed by others
Introduction
Breast cancer is one of the most prevalent type of cancer in women. According to the global cancer epidemic statistics released by the International Agency for Research on Cancer of the World Health Organization, there are approximately 2.89 million new female breast cancer cases worldwide each year, accounting for 24.2% of the total female cancer cases, ranking first1. Meanwhile, breast cancer incidence in developed countries is high, while the relative mortality in the less developed countries is the highest2. Clinical reports show that early detection and breast cancer treatment can significantly improve the survival rate3.
Mammography, digital breast tomosynthesis (DBT) and ultrasound imaging are three common imaging methods in clinical examination of breast cancer. However, mammography has the disadvantages of low specificity, high cost, and radioactivity4. Radioactivity causes health risks for patients, high cost increases the financial burden of patients, and low specificity (65–85%) leads to unnecessary biopsy operation4. DBT also has the disadvantages of high cost and radioactivity. In contrast, ultrasound imaging has the advantages of real-time imaging, no ionizing radiation, and low cost, and is commonly used in breast cancer screening or diagnosis. However, the diagnosis of ultrasound image is highly dependent on the skill level of the technician. Doctors with different training and different clinical experiences may make different diagnosis results5. Moreover, ultrasound images have high noise, significant artifacts, and low contrast between tissue structures. Therefore, it is desirable to develop a computer-aided breast cancer diagnosis system that can assist doctors in diagnosis.
Many researchers have studied the ultrasound diagnosis of breast cancer. Previous researches mainly applied traditional digital image processing techniques and machine learning technique to implement breast cancer detection6,7. For example, Drucker et al.8 first used radial gradient index filtering to detect the initial points of a region, examined the candidate areas from the background by maximizing the regional average radial gradient index of detection point growth, and classified the lesions using Bayesian neural networks. Finally, it achieved sensitivity of 87% at 0.76 false positive detection. As the most popular machine learning method, deep learning (DL) has gained a good reputation in computer vision and pattern recognition. In the medical field, many researchers have successfully applied DL to breast cancer detection9,10,11,12,13. Cao et al.14 comprehensively compared five object detection networks based on deep learning (Fast R-CNN15, Faster R-CNN16, you only look once (YOLO)17, YOLO V318, and single shot multibox detector (SSD)19), and demonstrated that SSD achieved the best performance in terms of precision and recall. In a study on breast lesion detection, Yap et al.20 used Faster R-CNN as their deep learning network. To reduce the impact of small sample datasets on the experiment, they applied transfer learning. At the same time, they proposed a three-channel fusion method, the original image, the sharpened image, and the contrast enhanced image (three single-channel images), are merged into a new three-channel image. However, the limitations of the prior work include: (1) they did not explore the impact of image preprocessing on experimental results; (2) their datasets14,20 are not publicly available and other researchers can not conduct comparative experiments; and (3) they all used anchor-based object detection networks and they did not examine the impact of anchor size settings on the experimental results. Therefore, we address the above issues by proposing an anchor-free object detection method for breast cancer detection. In addition, a segmentation-based enhancement (SBE) method is proposed for the detection performance improvement. The system flow chart is shown in Fig. 1. We focus on improving the contrast of ultrasound images and improving the detection precision of breast lesions. The key contributions include:
-
1.
We designed a segmentation-based ultrasound image contrast enhancement method.
-
2.
We explore the use of an anchor-free object detection network to detect breast cancer, avoiding the complex calculations of the anchor-based detection network.
-
3.
We propose a method of making object detection label by using lesion shape label.
The remainder of this paper is as follows, “Results” section presents the experimental results. “Discussion” and “Conclusions” section discuss and conclude our research, respectively. “Methods” section describes our experimental methods and procedures in detail.
Results
We evaluated the performance of our breast lesion detection system using various datasets. We also compared with many different enhancement methods and detection networks. The performance metrics and experimental results are described bellow.
Overview of datasets and breast lesion detection system
Datasets
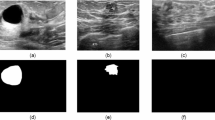
In this study, we used three public datasets, namely breast ultrasound (BUS)21, breast ultrasound image dataset (BUSI)22, and breast ultrasound image segmentation dataset (BUSIS)23. BUS was collected from the UDIAT Diagnostic Centre of the Parc Tauli Corporation, Sabadell (Spain). BUS contains 163 breast ultrasound images, of which 109 are benign and 54 are malignant. BUSI was collected from Baheya Hospital for Early Detection and Treatment of Women’s Cancer, Cairo, Egypt. The breast ultrasound images were collected from 600 female patients between 25 and 75 years old. BUSI contains 437 benign images, 210 malignant images, and 133 normal breast images, for a total of 730 breast ultrasound images. BUSIS was collected from the Second Affiliated Hospital of Harbin Medical University, the Affiliated Hospital of Qingdao University, and the Second Hospital of Hebei Medical University. BUSIS contains 562 images among women between 26 and 78 years old. These datasets contain multiple images for the same patient. The specific information of the datasets are shown in Table 1. In terms of image labels, BUS and BUSI include lesion shape labels and lesion benign and malignant classification labels (as shown in Fig. 2a,b), while BUSIS only contains lesion shape labels. In this study, we used BUSIS for image preprocessing and BUS and BUSI for breast lesion detecion.
Labels
The task of breast lesion detection is to identify and locate the exact localization of the lesion. Identification is to classify benign and malignant lesions and location is to give localization information of the lesion area. In BUS and BUSI datasets, the category labels of the lesions have been given, but there is no coordinate information of the lesions. We propose a method to obtain the lesion coordinates according to the lesion shape labels. As shown in Fig. 2b, we traverse all non-zero pixels in Fig. 2b, and find the largest and smallest horizontal and vertical coordinates \(x_{\min }\), \(x_{\max }\), \(y_{\min }\), \(y_{\max }\) among these non-zero pixels. We can obtain the upper left point \(p_{ul} = (x_{\min }, y_{\min })\) and the lower right point \(p_{lr} = (x_{\max },y_{\max })\) of the lesion area. The lesion area’s width w equal \(x_{\max } - x_{\min }\) and height h equal \(y_{\max } - y_{\min }\). We are then able to determine a bounding box of the lesion (Fig. 2). Finally, we use the five information set of \(p_{ul}\), \(p_{lr}\), w, h and lesion category as the label for breast lesion detection. However, in BUSIS dataset, because the lesion category is not given, it can not be used as the breast lesion detection data. Therefore, we use BUSIS in the image preprocessing step and we will introduce the use of BUSIS dataset in detail in the next section.
Overview of breast lesion detection system
Our system consists of two parts, the image preprocessing part and the breast lesion detection part. First, in the image preprocessing part, we use a new image enhancement method named segmentation-based enhancement (SBE). A deep learning method is used to segment the breast lesion region, and the segmented image is multiplied with the original image to obtain an enhanced image. Second, we input the enhanced image to an anchor-free object detection network (i.e., fully convolutional one-stage object detection network (FCOS)24) to detect the breast lesion.
Performance metrics
We used Precision, Recall, and mean average precision (mAP) as the performance metrics in our experiments. The calculation of Precision, Recall, and mAP depends on the following parameters.
-
IoU, in medical image analysis, IoU is also known as Jaccard Similarity Index or Jaccard Index. The IoU is defined by:
$$\begin{aligned} \text {IoU}=\dfrac{{\text {Area of Overlap}}}{{\text {Area of Union}}}. \end{aligned}$$(1)Among them, Area of Overlap refers to the area where the predicted bounding boxes (BBox) overlaps the label BBox, and Area of Union refers to the union of the predicted BBox and the label BBox. Based on IoU as the criteria, for each class, we can calculate the following parameters:
-
Confidence Probability of each class prediction.
-
True positives (TP) The prediction BBox with \(\text {IoU}>0.5\) and meeting the category confidence threshold.
-
False positives (FP) The prediction BBox with \(\text {IoU}<0.5\) and meeting the category confidence threshold.
-
False negatives (FP) \({\text {IoU}}=0\).
According to the above parameters, we have
By setting different category confidence thresholds, we can obtain the Precision–Recall (PR) curve. Average precision (AP) is the area under the PR curve, and mAP is the average of all categories of AP. We have
where N is the total number of categories of class.
Results
Comparison of the experimental results with different image enhancement methods
We used different enhancement methods (our proposed method SBE, recurrent residual convolutional neural network based on U-Net (R2U-Net)25, Attention U-Net26, and traditional method contrast limited adaptive histogram equalization (CLAHE)27) and tested them based on both single dataset and composite dataset (BUS+BUSI). The experimental results are shown in Tables 2 and 3 and the PR curves are shown in Fig. 5. The results show that we have achieved 8 best mAP in 9 sets of comparative experiments. In malignant lesion detection preformance (M-Recall), we achieved all best results. Notice that the boundary of malignant tumors is usually irregular and the contrast between malignant tumors and normal tissue is low, so that the malignant tumors are not easy to detect. However, with our proposed SBE, the contrast is greatly enhanced, making malignant tumors easier to be detected. The experimental result images are shown in Fig. 3. We also found that during SBE, some breast lesions were not segmented (Fig. 4b), and some incorrect segmentations occurred (Fig. 4f,j). However, our method can still correctly detect the lesion areas, as shown in Fig. 4, which demonstrates good detection performance. Finally, for easy viewing, we surround the predicted benign tumors with a green box and the predicted malignant tumors with a red box.
Comparison of the experimental results with different detection networks
To further verify the performance of our proposed method (i.e., combining FCOS with SBE), we compared it with a breast cancer ultrasound detection method proposed by Mo et al.28 in 2020. This method used YOLO V3 as the detection network and maked two changes to the original YOLO V3. First, Ref.28 adopted the K-Means++ algorithm and K-Mediods algorithm to optimize the original K-Means algorithm to set the anchor size. Second, the residual structure in the original YOLO V3 was changed, and a new residual network based on ResNet and DenseNet29 was constructed. We implement the method proposed by Ref.28 using our dataset for experimentation. We have obtained three different anchor size through K-Means++ and K-Mediods, and named the network that changed the anchor size as YOLO V3-anchor. Three sets of anchors The sizes are (34, 45), (40, 45), (40, 54), (60, 80), (66, 109), (88, 99), (90, 99), (94, 217), (164, 220) for BUS+BUSI; (25,50), (35, 69), (76, 62), (89, 128), (95, 100), (107, 192), (164, 220), (187, 341), (196, 208) for BUSI; (26, 27), (29, 59), (31, 78), (40, 54), (48, 57), (60, 80), (62, 134), (162, 134), (201, 361) for BUS. We reproduced a new residual structure according to the method proposed by Ref.28 and named it as YOLO V3-res. The experimental results are shown in Table 4. Notice that the performance of our method is not the best in all cases. However, as shown in Table 4, our method achieves the best results on both Precision and Recall of the detection of malignant lesions. More importantly, our method achieves the best results on the mAP performance measure.
Discussion
The above results show that our breast lesion detection system can detect the lesion region and classify the benign and malignant regions. When building this system, we mainly research two aspects. The first is the preprocessing of breast ultrasound images. We compared the effects of images under different enhancement methods on the detection results, including no enhancement, CLAHE, and SBE. After comparison, we found that the image processed by SBE can better improve the detection performance. Moreover, it can be proved that good local enhancement is helpful to the detection system. At the same time, we designed a new segmentation network. This network combines the characteristics of R2U-Net and Attention U-Net, and integrates the recurrent mechanism and attention mechanism into the network. The results show that the images enhanced by our network have achieved the best detection results on a variety of datasets. Second, we research the application of anchor-free detection network in breast lesion detection. We use YOLO V3 as a comparison network to prove the effectiveness of the anchor-free detection network in breast detection. In a variety of datasets, anchor-free detection network can achieve the highest mAP.
Conclusions
This paper proposes an automatic breast cancer ultrasound image detection method based on deep learning, using anchor-free network FCOS as a breast cancer detection network, which can determine the location of breast cancer lesions and identify benign versus malignant. Our method can assist doctors in diagnosing breast lesions during ultrasound breast cancer screening, automatically locating lesions and classifying them (i.e., benign or malignant). We also propose a segmentation-based ultrasound image enhancement method to improve the breast cancer detection method’s performance. We use three public datasets, which are obtained from 8 different ultrasound acquisition devices, to compare our proposed method with anchor-basde method. Our proposed method can reach an mAP of 0.902, which demonstrates that our proposed method has good generalization ability and high clinical application value.
Methods
This section covers image preprocessing methods of breast ultrasound images, an anchor-free detection network, and implementation process of our experiment.
In this study, we used data from three publicly available datasets, and our study is carried out in accordance with relevant guidelines and regulations.
Image preprocessing
Due to the low contrast of ultrasound images and a large amount of speckle noise, appropriate preprocessing methods are essencial for subsequent image analysis. In this study, the preprocessing of ultrasound images consists of three steps. The first is to use traditional methods to enhance the contrast of the image and then denoise. Finally, we use our SBE method to further enhance the image’s contrast.
Traditional methods
We use CLAHE to enhance the image. The algorithm of CLAHE is as follows.
Step I First, divide the original picture into \(\text {N}\times \text {N} \) subregions, and calculate the cumulative distribution function \(\text {CDF}_{i}\), histogram \(\text {Hist}_{i}\), and mapping function \(\text {n}_{i}\) of the histogram in each subregion. We have,
Take the derivative of \(\text {n}_{i}\) to get the slope K of the subregion. Set a threshold T, cut off the part of \(\text {Hist}_{i}\) where K is greater than T, and evenly distribute it to the original image histogram to obtain a new histogram. Simultaneously, to avoid the blocking effect caused by the block operation, the bilinear interpolation method needs to be used to reconstruct each pixel’s gray value.
Step II The original image’s noise is enhanced for the ultrasound image calculated by CLAHE and the image needs to be denoised. Anisotropic diffusion30 is a denoising method based on partial differential equations, which can preserve image details while denoising.
Let \(I_{p}^{t}\) denote the discrete sampling of the current image, p the coordinate of the sampled pixel, \(I_{q}^{t}\) the neighborhood discrete sampling of \(I_{p}^{t}\), \(\partial _{p}\) denotes the neighborhood space of p, \(|\partial p|\) denotes the size of the neighborhood space, and \(\lambda \) control the diffusion strength. The iterative expression of anisotropic diffusion is
Let k be the gradient threshold, then \(c(I_{p}^{t}-I_{q}^{t})\) is
Anisotropic diffusion needs to set the number of iterations n, gradient threshold k, and diffusion strength \(\lambda \) to adjust the denoising effect.
Segmentation-based enhancement method
After CLAHE and anisotropic diffusion, we obtain the contrast-enhanced image, as shown in Fig. 6. However, we found that the contrast of ultrasound images was still low. Therefore, we develop a segmentation-based enhancement method to further enhance the contrast of ultrasound images.
We integrated R2U-Net and Attention U-Net and designed R2AttU-Net. The downsampling part of R2AttU-Net is from R2U-Net, and the upsampling part is from Attention U-Net. R2AttU-Net network structure is shown in Fig. 7. We use BUSIS as training data of R2AttU-net and BUS and BUSI as test datas. We input the original ultrasound image (as shown in Fig. 8a) into R2AttU-net. After processing by R2AttU-net, the image in Fig. 8b is generated. Set the white part in Fig. 8b to 1 and the black part to 0.6, and multiply the image in Fig. 8b with the image in Fig. 8a to obtain a contrast-enhanced image shown in Fig. 8c. From Fig. 8, it can be seen that the contrast of the ultrasound image is substantialy enhanced.
Implementation
Lesion detection
Through the steps described above, we have obtained the enhanced image. In this section, we will introduce the last step of the whole breast lesion detection process.
Detection network
We adopted an anchor-free detection network, FCOS, as the detection network for breast lesions. FCOS outputs five sizes of heads to facilitate object detection of different sizes. Three loss functions (classification loss, center-ness loss and regression loss) are used to calculate the loss of the object category, center point, and bounding-box size, respectively. Compared with anchor-based object detection networks (such as Faster R-CNN, YOLO V3), anchor-free networks do not need to set anchor boxes in advance, so that can significantly reduce the number of parameters and reduce the large number of calculations due to anchor boxes (For example, the intersection over union (IoU) calculation and matching of anchor boxes and ground-truth boxes in training). These advantages over anchor-based object detection networks lead to faster detection and simpler training process in FCOS.
The overall experimental steps of this study is shown in Fig. 9. In Fig. 10, we show our experimental steps in the form of a network structure. BUSI dataset includes 697 images containing lesions, but we found some duplicate images. We deleted the duplicate images and selected 610 breast ultrasound images from BUSI. Finally, we obtained a total of 773 images from the BUS dataset and the BUSI dataset. All breast ultrasound images were randomly selected for training data, validation data, and testing data according to the ratio of 8:1:1 and resized to \(224 \times 224\).
We used FCOS based on the mmdetection object detection toolbox31. Using ResNet5032 as a backbone of FCOS, a total of 300 epochs are trained. The FCOS output detection box coordinates are mapped to the original breast ultrasound image and the final output result is obtained. We feedback/map the detection boxes to the original image, rather than the enhanced image, to avoid the segmentation results from interfering with the doctor’s diagnosis. The hyperparameters of the R2AttU-Net used in the image preprocessing stage and the FCOS used in the breast lesion detection stage are shown in Table 5.
Data availability
The datasets analysed during the current study are available in https://scholar.cu.edu.eg/?q=afahmy/pages/dataset, https://ieeexplore.ieee.org/abstract/document/goo.gl/SJmoti, and http://cvprip.cs.usu.edu/busbench.
Abbreviations
- AP:
-
Average precision
- BUS:
-
Breast ultrasound
- BUSI:
-
Breast ultrasound image dataset
- BUSIS:
-
Breast ultrasound image segmentation dataset
- CLAHE:
-
Contrast limited adaptive histogram equalization
- DL:
-
Deep learning
- FCOS:
-
Fully convolutional one-stage object detection
- mAP:
-
Mean average precision
- PR:
-
Precision–Recall
- R2U-Net:
-
Recurrent residual convolutional neural network based on U-Net
- SBE:
-
Segmentation-based enhancement
- SSD:
-
Single shot multibox detector
- YOLO:
-
You only look once
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Ghoncheh, M., Pournamdar, Z. & Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 17, 43–46 (2016).
Shin, H. J., Kim, H. H. & Cha, J. H. Current status of automated breast ultrasonography. Ultrasonography 34, 165 (2015).
Cheng, H.-D., Shan, J., Ju, W., Guo, Y. & Zhang, L. Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recogn. 43, 299–317 (2010).
Qian, X. et al. Prospective assessment of breast cancer risk from multimodal multiview ultrasound images via clinically applicable deep learning. Nat. Biomed. Eng. 5, 522–532 (2021).
Alvarenga, A. V., Pereira, W. C., Infantosi, A. F. C. & Azevedo, C. M. Complexity curve and grey level co-occurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med. Phys. 34, 379–387 (2007).
Murali, S. & Dinesh, M. Classification of Mass in Breast Ultrasound Images Using Image Processing Techniques (2012).
Drukker, K. et al. Computerized lesion detection on breast ultrasound. Med. Phys. 29, 1438–1446 (2002).
Wang, Y. et al. Deeply-supervised networks with threshold loss for cancer detection in automated breast ultrasound. IEEE Trans. Med. Imaging 39, 866–876 (2019).
Kumar, V. et al. Automated and real-time segmentation of suspicious breast masses using convolutional neural network. PLoS ONE 13, e0195816 (2018).
Behboodi, B., Amiri, M., Brooks, R. & Rivaz, H. Breast lesion segmentation in ultrasound images with limited annotated data. In 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), 1834–1837 (IEEE, 2020).
Moon, W. K. et al. Computer-aided tumor detection in automated breast ultrasound using a 3-D convolutional neural network. Comput. Methods Prog. Biomed. 190, 105360 (2020).
Li, Y., Wu, W., Chen, H., Cheng, L. & Wang, S. 3D tumor detection in automated breast ultrasound using deep convolutional neural network. Med. Phys. 47, 5669–5680 (2020).
Cao, Z., Duan, L., Yang, G., Yue, T. & Chen, Q. An experimental study on breast lesion detection and classification from ultrasound images using deep learning architectures. BMC Med. Imaging 19, 51 (2019).
Girshick, R. Fast R-CNN. In Proc. IEEE International Conference on Computer Vision, 1440–1448 (2015).
Ren, S., He, K., Girshick, R. & Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. IEEE Trans. Pattern Anal. Mach. Intell. 39, 1137–1149 (2016).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. In Proc. IEEE Conference on Computer Vision and Pattern Recognition, 779–788 (2016).
Redmon, J. & Farhadi, A. Yolov3: An incremental improvement. Preprint at http://arxiv.org/abs/1804.02767 (2018).
Liu, W. et al. SSD: Single shot multibox detector. In European Conference on Computer Vision, 21–37 (Springer, 2016).
Yap, M. H. et al. Breast ultrasound region of interest detection and lesion localisation. Artif. Intell. Med. 107, 101880 (2020).
Yap, M. H. et al. Automated breast ultrasound lesions detection using convolutional neural networks. IEEE J. Biomed. Health Inform. 22, 1218–1226 (2017).
Al-Dhabyani, W., Gomaa, M., Khaled, H. & Fahmy, A. Dataset of breast ultrasound images. Data Brief 28, 104863 (2020).
Xian, M. et al. A Benchmark for Breast Ultrasound Image Segmentation (BUSIS) (Infinite Study, 2018).
Tian, Z., Shen, C., Chen, H. & He, T. FCOS: Fully convolutional one-stage object detection. In Proc. IEEE International Conference on Computer Vision, 9627–9636 (2019).
Alom, M. Z., Hasan, M., Yakopcic, C., Taha, T. M. & Asari, V. K. Recurrent residual convolutional neural network based on U-Net (R2U-Net) for medical image segmentation. Preprint at http://arXiv.org/1802.06955 (2018).
Oktay, O. et al. Attention U-Net: Learning where to look for the pancreas. Preprint at http://arxiv.org/abs/1804.03999 (2018).
Zuiderveld, K. Contrast limited adaptive histogram equalization. Graph. Gems 1, 474–485. https://doi.org/10.1016/B978-0-12-336156-1.50061-6 (1994).
Mo, W., Zhu, Y. & Wang, C. A method for localization and classification of breast ultrasound tumors. In International Conference on Swarm Intelligence, 564–574 (Springer, 2020).
Huang, G., Liu, Z., Van Der Maaten, L. & Weinberger, K. Q. Densely connected convolutional networks. In Proc. IEEE Conference on Computer Vision and Pattern Recognition, 4700–4708 (2017).
Perona, P. & Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 12, 629–639 (1990).
Chen, K. et al. MMDetection: Open mmlab detection toolbox and benchmark. Preprint at http://arxiv.org/abs/1906.07155 (2019).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In Proc. IEEE Conference on Computer Vision and Pattern Recognition, 770–778 (2016).
Author information
Authors and Affiliations
Contributions
Y.W.: Problem definition and formulation, method design, experiments, result analysis, paper writing. Y.Y.: Problem definition and formulation, paper writing and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Yao, Y. Breast lesion detection using an anchor-free network from ultrasound images with segmentation-based enhancement. Sci Rep 12, 14720 (2022). https://doi.org/10.1038/s41598-022-18747-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-18747-y
This article is cited by
-
Explainable AI based hybrid DRM-Net transfer learning model for breast cancer detection and classification using ultrasound images
Scientific Reports (2025)
-
Innovative breast cancer detection using a segmentation-guided ensemble classification framework
Biomedical Engineering Letters (2025)
-
A Multi-attention Triple Decoder Deep Convolution Network for Breast Cancer Segmentation Using Ultrasound Images
Cognitive Computation (2024)
-
Tumor detection based on deep mutual learning in automated breast ultrasound
Multimedia Tools and Applications (2024)