Abstract
Undernutrition is responsible for up to 45% of deaths in children under five, with low- and middle-income countries disproportionately affected. Adipokines are known modulators of metabolism and have been linked to growth rates and neurocognition during infancy. We examined the relationship(s) between cord blood adiponectin and leptin and both longitudinal growth and cognition during the first year of life using generalized estimating equations. Infants were classified as underweight (weight-for-age z-score [WAZ]), stunted (height-for-age z-score [HAZ]) or wasted (weight-for-height z-score [WHZ]) using WHOAnthro software. Cord blood adiponectin and leptin levels were highly correlated (r = 0.35, P < 0.0001) and positively associated with birth WAZ (r = 0.34 and r = 0.45, P < 0.0001, respectively). Adipokines were independently, inversely associated with weight gain. Infants in the highest quintile of adipokine production had a lower risk of being stunted, while neither was associated with lower WAZ or WHZ in final adjusted models. Cognition was not found to be independently related to cord blood leptin or adiponectin. The negative association with adipokines and rate of weight gain during infancy may reflect heightened nutritional status at birth rather than a direct hormonal influence. The relationship between leptin or adiponectin and longitudinal length gains suggests that both adipokines may promote linear growth during infancy.
Similar content being viewed by others
Introduction
Stunting, or low height-for-age, is an indicator of chronic undernutrition, and poses a substantial risk to the long-term health and cognitive development of an estimated 155 million children globally, with 30% of all children under the age of five in the Philippines affected by the condition1. Adipose tissue is a potent endocrine organ, capable of influencing a variety of organ systems through the dynamic production of adipokines. Key among these are leptin and adiponectin, which exert opposing effects on energy metabolism; leptin being related to insulin resistance, and adiponectin an insulin sensitizer. Both are produced by the fetus, and both have been shown to be altered in cases of fetal growth restriction (FGR) in humans2. Many of these formerly in utero growth-restricted individuals go on to display altered response to nutrient availability after gestation2.
Cord blood levels of metabolic hormones have been associated with long-term growth and metabolic indicators such as pancreatic β-cell function3,4,5. Although specific mechanisms remain somewhat elusive, impacts on hypothalamic circuitry and/or epigenetic regulation have been suggested as means by which adipokines are modulated in a lasting manner in utero6. In addition, children who experience stunting during early childhood are at greater risk of impaired cognitive outcomes7,8,9,10. Fetal leptin in particular has been associated with poor neurodevelopmental outcomes, such as cognitive development in infants and hyperactivity/inattention in children11,12. Whether specific adipokines influence cognitive development independently, or act through undernutrition or stunting is currently unknown.
Herein, we utilized an existing cohort of infants in rural Philippines to examine the association of cord blood adiponectin and leptin with longitudinal growth during infancy and neurocognition captured by the Bayley’s Scales of Infant Development III (BSID III) at 1 year of age. These infants were recruited based on their mother’s participation in a randomized controlled trial examining the impact of praziquantel administration for the treatment of schistosomiasis during pregnancy on birth outcomes (NCT00486863).
Materials/subjects and methods
Ethical considerations and informed consent
This study was approved by the institutional review boards of Rhode Island Hospital and The Research Institute for Tropical Medicine (Philippines). All subjects provided informed consent prior to enrollment. All methods were performed in accordance with the relevant guidelines and regulations.
Study site and population
Detailed recruitment and characteristics of this study population have been described elsewhere13. Briefly, women were recruited from 72 barangays in rural northeastern Leyte, the Philippines. This region is endemic for schistosomiasis and soil-transmitted helminths, but not malaria. HIV prevalence in the area was under 1%. Women were eligible to participate in the original study if they were over 18 years of age, otherwise healthy as determined by medical history, physical examination and laboratory studies (renal and liver function tests, complete blood count), and carrying a singleton pregnancy free from congenital defects as determined by ultrasound between 12 and 16 weeks of gestation. All women were positive for schistosomiasis and were randomized to praziquantel treatment to resolve infection, or placebo at 14 ± 2 weeks gestation. Of note, treatment did not impact birth weight, risk for low birth weight (LBW), small for gestational age (SGA), or neurodevelopmental outcomes at 12 months of age13,14. Women were enrolled (n = 346) at 14 ± 2 weeks of gestation, and characteristics including socioeconomic status, age and current alcohol consumption were obtained. BMI was calculated based on weight and height measurements taken at enrollment, with women with a BMI of > 25 classified as overweight. All women in this cohort delivered vaginally and infants were followed until 12 months of age.
Sample collection
At maternal enrollment, we collected health related epidemiologic and demographic data, described in our original publication13. Geohelminth (A. lumbricoides, hookworm, T. trichuria) and schistosome infections were determined at enrollment and again approximately 8 weeks after treatment. Cord blood was collected directly from the umbilical cord after delivery of the placenta and cleaning of the outside of the umbilical cord with alcohol wipe(s). Blood samples were collected in a purple top vacutainer tubes and plasma obtained after centrifuging for 15 min at 5000×g.
Anthropometric measurements
Newborn weight and length were measured within 24 h of delivery, and height, weight and mid-upper arm circumference (MUAC) and head circumference collected at 1 month, 6 months and 1 year of age. Breast feeding status (exclusive, mixed, none) was collected via questionnaires administered to the mother at each visit. Low birth weight was classified as infants weighing < 2500×g at birth. Infants were classified as preterm if delivery was prior to 37 weeks gestation, as determined by ultrasound at enrollment. Small-for-gestational age (SGA) was calculated using the application developed by the INTERGROWTH-21th project15. Height-for-age z-scores (HAZ), weight-for-age z-score (WAZ), weight-for-height z-scores (WHZ) and MUAC z-scores were calculated using the 2006 World Health Organization Anthro Growth Standards (www.who.int/childgrowth/software/en/). Infants with individual z-scores below two standard deviations of the mean were considered stunted (HAZ), underweight (WAZ) or wasted (WHZ) during infancy.
Adipokine measurement
Total adiponectin levels in cord blood plasma were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN). Intra assay %CV for adiponectin was 5.54%, inter assay %CV was 4.03%. Leptin was analyzed by generation of a bead-based Luminex system (BioRad, Hercules, CA), with the lower limit of detection being 7.8 pg/mL. Intra assay %CV for leptin was 4.41%, inter assay %CV was 8.2%.
Cognition assessments
Cognition was assessed at 12 months using the Bayley Scales of Infant and Toddler Development test, third edition (BSID-III) by trained study pediatricians16. This test consists of three primary developmental categories: cognitive, language (consisting of receptive and expressive language subscales), and motor (consisting of fine and gross motor subscales). As reported previously, the social scales were not included in this examination14. Cognition, receptive language, expressive language, fine motor and gross motor skills were scaled according to age grouping by month and examined for relationship(s) with either cord blood adiponectin or leptin.
Statistical analysis
Statistical analysis was carried out using SAS and generalized estimated equations (GEE) using a Poisson distribution with log link, an unstructured correlation structure, and robust variance estimation17. All models included SES and breastfeeding status. A stepwise selection approach was used for each model selection. We considered predictors with P < 0.2 in univariable analysis for inclusion in multivariable analysis. We included predictors with P < 0.05 in the multivariable models in the presence of adiponectin or leptin variables. P < 0.05 was considered as significant.
Results
General characteristics of the study population are presented in Table 1. Adiponectin levels in cord blood were not different based on maternal factors including first trimester BMI, socioeconomic status (SES), age, or level of education. Females did show higher levels of adiponectin than male infants (P = 0.05). The highest levels of adiponectin were also statistically associated with breastfeeding practices (P = 0.024); we therefore included breastfeeding status in all subsequent statistical modeling. Cord blood leptin levels were not associated with maternal SES, age, level of education or alcohol consumption during pregnancy. Cord blood leptin levels were positively associated with maternal BMI. Similar to what was observed with adiponectin, cord blood leptin levels were higher in female neonates.
Association of cord blood adipokines with growth during infancy
Cord blood levels of leptin and adiponectin were highly correlated with one another, regardless of maternal parameters (Table 2). Both cord plasma adiponectin and leptin were independently correlated with weight for gestational age at delivery, as captured by SGA (Table 1), with both adipokines higher in heavier newborns. Additionally, there was a trend for a positive association between cord blood leptin and length at delivery (Table 1). Cord blood leptin levels were also positively correlated with weight-for-age (WAZ) and height-for-age (HAZ) at 1 year (Tables 1 and 2). In contrast, leptin and adiponectin in cord blood were both negatively correlated with the change in WAZ over the first year of life in this cohort (Table 2). Similarly, cord blood leptin was negatively correlated with the change in WHZ over the first year of infancy (Table 2).
In univariate analysis, cord blood adiponectin was positively associated with weight-for-age z-score (WAZ) early in infancy (delivery and 1 month; Fig. 1A), positively associated with height-for-age z-score (HAZ) at 1 and 6 months (Fig. 1B), but negatively associated with weight-for-height z-score (WHZ) at 1 month (Fig. 1C). All associations between cord blood adiponectin and infant growth parameters were lost by 12 months of age. Similarly, cord blood leptin levels were positively associated with HAZ at 1 and 6 months of age (Fig. 1E) and negatively with WHZ at 1 month of age (Fig. 1F). The positive association between cord blood leptin and WAZ however was more persistent than that observed with adiponectin, lasting through the first year of infancy (Fig. 1D).
Weight-for-age z-score (WAZ), height-for-age z-score (HAZ), and weight-for-height z-score (WHZ) according to quintiles of adiponectin (A–C) and leptin (D–F) in cord blood. Error bar indicates standard deviation. Filled circle: quintile 1—the lowest level, filled square: quintile 2, filled triangle: quintile 3, filled inverted triangle: quintile 4, filled diamond: quintile 5—the highest level. *P value < 0.05, **P value < 0.01, ***P value < 0.001.
Adipokines as predictors of growth during infancy
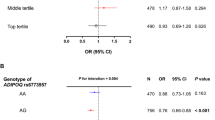
Univariate analysis of the relationship between cord blood adiponectin and leptin and longitudinal growth are presented in Supplemental Table 1. We have previously reported the association of maternal and infant characteristics with poor growth outcomes during infancy in this population18, and used these data to inform our multivariable analysis of the relationship between cord blood adiponectin and leptin and growth during infancy (Table 3). We found the highest level of adiponectin levels in cord blood to be negatively associated with risk of stunting during infancy (RR: 0.32 (0.16–0.64)). Similarly, elevated leptin levels in cord blood were negatively associated with risk of stunting in infancy (RR: 0.39 (0.18–0.83) and RR: 0.36 (0.17–0.76) for the two highest quintiles). These associations remained when adiponectin and leptin were analyzed as continuous variables (Table 4). Neither adipokine was associated with risk of developing underweight or wasting during infancy.
Associations with cognitive outcomes
In univariate analysis, the highest quintile of cord blood adiponectin was negatively associated with receptive language and fine motor scores, while not significantly associated with cognition, expressive language or gross motor skills scores. Cord blood leptin was not associated with any of the cognitive scales examined in univariate analysis (Table 1). We have previously reported on predictors of cognition in this population, such as maternal iron status, infant growth parameters, maternal education and cognition14. Multivariable regression models with specific predictors for a given cognitive outcome included failed to show an association between either adiponectin or leptin and most of the cognitive outcomes assessed. The exception was the negative association between cord blood adiponectin and fine motor skills was retained after inclusion of maternal cognition and education (β: − 0.058, P = 0.004), two factors we previously showed to be associated with fine motor skill development in this population (data not shown)14.
Discussion
Herein, we have examined the association of fetal adiponectin and leptin with growth during the first year of life. Since its inception19, the Developmental Origins of Health and Disease hypothesis has been substantiated by a wealth of studies, and the role of the metabolic axis as established during gestation on subsequent growth in infancy is substantial. Infancy, in turn, represents a critical period of growth and development, both because of the heightened trajectory by which both of these processes occur during this period, and also due to the known associations between growth in infancy and long-term impacts on the affected individual, including cognition, longitudinal growth potential, and establishment of risk for metabolic diseases late in life. As such, identification of biomarker(s) which may predict growth trajectory during infancy may serve to identify those infants most at risk of poor growth characteristics in time for an appropriate intervention to be made.
Infancy and early childhood is the most likely time period during which stunting will occur, largely due to this being the period of life with the greatest growth velocity, poor quality of weaning foods, multiple infectious disease insults that can affect appetite and gut health, and the need for energy dense foods20. The importance of growth during the period of infancy on adult attained height and adiposity has been demonstrated by others21. Growth trajectory during the first 2 years of life has been associated with adult size, independent of BMI at birth or 2 years, highlighting that the change in growth during this period as critical to determining adult size21. In addition, children born SGA who failed to display catch up growth have reduced adiponectin and insulin sensitivity in childhood, compared to height matched children born AGA22. Levels of adiponectin in cord blood are also positively associated with birth weight, being lower in small-for-gestational-age (SGA) infants in some, but not all studies23,24,25,26,27,28,29. Our study substantiates these findings, showing significantly more low birth weight infants falling into the lowest quintiles for cord blood adiponectin.
Studies examining the association with cord blood adiponectin and growth and nutritional status during infancy have reported mixed results, with overall consensus suggesting a slight positive association with cord adiponectin and neonatal adiposity30. Cord blood adiponectin has also been negatively associated with weight gain during early infancy31, and weight-for-age in the first year of life32, however others have reported a positive association or none at all5,29,33,34. Indeed, a recent report suggests cord blood adiponectin is predictive of central adiposity at 3 years of age35. While we observed a significant association with the highest levels of cord blood adiponectin and weight-for-age z-scores, these effects were limited to only the first month of life, and overall cord adiponectin was actually negatively associated with weight-for-age gain during infancy. This suggests that adiponectin’s relationship with weight for age is driven by its effects on size at birth. In addition, the relationship between cord blood adiponectin and growth trajectories may display sex discordance36,37. Our data also substantiate these findings, with elevated adiponectin in female neonates and disparate rates of growth between the sexes18.
Adiponectin has also been associated with long bone growth, and evidence in mouse models show it plays a role in promoting osteogenesis38. These data are in line with our observation of higher cord blood adiponectin being related to decreased risk of stunting (or to higher HAZ) during infancy, but not underweight or wasting. Interestingly, others have reported an association with neonatal adiponectin and wasting, but not stunting39. This study examined intrauterine stunting as a subtype of SGA, therefore our definition of stunting based on growth during infancy could help explain our divergent results, as well as potential divergent genetic and environmental responses based on the unique study populations.
Cord blood leptin is known to be positively associated with birthweight28,40,41. Cord blood leptin has also been reported to exhibit a positive association with growth trajectory during infancy into early childhood in infants with normal growth patterns. In contrast, leptin has been inversely associated with both catchup growth during early infancy and, among SGA infants, with catchup growth, highlighting the importance of leptin in mediating the metabolic axis35,42. This is particularly important in a resource limited environment, and highlights the importance of understanding primary determinants of growth during infancy. We observed a positive association between cord blood leptin and weight-for-age at delivery, as well weight-for-age at 1 year of age. While we did not dichotomize our data based on SGA status, cord blood leptin was negatively associated with the weight-for-age gain/month during infancy, suggesting that our study may be in accordance with reports demonstrating a negative association between cord leptin and rates of catchup growth during infancy.
Cognitive development during infancy is known to be highly influenced by inflammatory events during this period, while growth is predominantly associated with maternal body size and birth anthropometry43. Adiponectin is known to exert anti-inflammatory effects, and in an aged model, adiponectin has been shown to improve cognitive performance through suppression of the inflammatory cascade, in addition to its impacts on appetite regulation and energy expenditure44. Similarly, cord blood leptin has been associated with a reduced risk of hyperactivity problems in school-aged children11. However plasma leptin in infants has also been reported to be negatively related to cognitive outcomes using the BSID-III test12. While we observed a slight negative association between the highest levels of cord blood adiponectin and receptive language and fine motor scores at 12 months of age, only the relationship between cord adiponectin and fine motor skills was maintained after adjustment for key confounders (i.e. maternal cognition score and education). We did not observe any association between cord blood leptin and any of the cognitive outcomes measured. These data suggest that while there may be a slight negative association between cord blood adiponectin and some cognitive development parameters, larger impacts for either adipokine, as reported by others, may be mediated indirectly through other pathways, such as growth and nutritional status.
Our study is limited by measurement of adiponectin and leptin only at delivery, and future studies examining the relationship of adiponectin and leptin and growth throughout infancy will enhance our understanding of the dynamic role these hormones play in mediating the metabolic axis during infancy. In addition, our study represents a secondary analysis from a cohort of infants, all of whom were born to mothers with schistosomiasis early in gestation. Half of these mothers received praziquantel for resolution of infection early in gestation, and treatment did not impact birth weight of the neonates13, such that we do not expect maternal treatment impacted our findings. Similarly, treatment group was not associated with cord blood adipokine levels. Our study was also limited to women residing in rural villages within the Philippines, potentially limiting the applicability of our findings to other ethnicities/regions. In addition, we measured total adiponectin, rather than focusing exclusively on the high molecular weight (HMW) form of adiponectin which is known to be the most bioactive form of adiponectin.
In conclusion, we failed to identify an association with either leptin or adiponectin cord blood levels and change in adiposity during the first year of infancy. Tracking of these adipokines throughout infancy as they relate to growth may help identify more nuanced associations throughout this highly dynamic period, and is an area of continued interest for our group. We did however observe a positive association with both cord blood adiponectin and leptin and gain in length during the first year. This association remained after controlling for length at delivery, suggesting that these adipokines may be independently associated with long bone growth during infancy, rather than simply a reflection of birth length or continued growth by a large neonate.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because the dataset contains confidential patient data but are available from the corresponding author on reasonable request.
References
World Health Organization, G. H. O. Child Stunting. http://apps.who.int/gho/data/node.sdg.2-2-viz-1?lang=en. (Accessed 14 September 2017).
Briana, D. & Malamitsi-Puchner, A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur. J. Endocrinol. 160, 337–347 (2009).
Simpson, J. et al. Programming of adiposity in childhood and adolescence: Associations with birth weight and cord blood adipokines. J. Clin. Endocrinol. Metab. 102, 499–506 (2017).
Zhang, D. et al. Cord blood insulin, IGF-I, IGF-II, leptin, adiponectin and ghrelin, and their associations with insulin sensitivity, B-cell function and adiposity in infancy. Diabet. Med. 35, 1412–1419 (2018).
Yeung, E., Sundaram, R., Xie, Y. & Lawrence, D. Newborn adipokines and early childhood growth. Pediatr. Obes. 13, 505–513 (2018).
Reynolds, C., Gray, C., Li, M., Segovia, S. & Vickers, M. Early life nutrition and energy balance disorders in offspring in later life. Nutrients 7, 8090–8111 (2015).
Woldehanna, T., Behrman, J. & Araya, M. The effect of early childhood stunting on children’s cognitive achievements: Evidence from young lives Ethiopia. Ethiop. J. Health Dev. 31, 75–84 (2017).
Berkman, D., Lescano, A., Gilman, R., Lopez, S. & Black, M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognitiion in late childhood: A follow-up study. Lancet 359, 564–571 (2002).
Pongcharoen, T. et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch. Pediatr. Adolesc. Med. 166, 411–416 (2012).
Walker, S., Grantham-McGregor, S., Powell, C. & Chang, S. Effects of growth restriction in early childhood growth, IQ, and cognition at age 11 to 12 years and the benefits of nutritional supplementation and psychosocial stimulation. J. Pediatr. 137, 36–41 (2000).
Minatoya, M. et al. Association between fetal adipokines and child behavioral problems at preschool age: The Hokkaido Study on Environment and Children’s Health. Int. J. Environ. Res. Public Health 15, E120 (2018).
Camargos, A. et al. Association betwen obesity-related biomarkers and cognitive and motor development in infants. Behav. Brain Res. 325, 12–16 (2017).
Olveda, R. et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 16, 199–208 (2016).
Park, S. et al. Mechanistic pathways from early gestation through infancy and neurodevelopment. Pediatrics 138, e20161843 (2016).
Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional study of the INTERGROWTH-21st project. Lancet 384, 857–868 (2014).
Bayley, N. Bayley Scales of Infant and Toddler Development (StatPearls, 2006).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Blake, R. et al. LBW and SGA impact longitudinal growth and nutritional status of Filipino infants. PLoS ONE 11, e0159461 (2016).
Barker, D. & Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081 (1986).
Martorell, R., Khan, L. & Schroeder, D. Reversibility of stunting: Epidemiological findings in children from developing countries. Eur. J. Clin. Nutr. 48, S45-57 (1994).
Slining, M., Herring, A., Popkin, B., Mayer-Davis, E. & Adair, L. S. Infant BMI trajectories are associated with young adult body composition. J. Dev. Orig. Health Dis. 4, 56–68 (2013).
Huang, Y. et al. Low serum adiponectin levels are associated with reduced insulin sensitivity and lipid disturbances in short children born small for gestational age. Clin. Endocrinol. 83, 78–84 (2015).
Goto, E. Maternal and cord blood adiponectin concentrations in small for gestational age: A meta-analysis. Ann. Nutr. Metab. 72, 57–64 (2017).
Daryasari, S. et al. Adiponectin levels in maternal serum and umbilical cord blood at birth by mode of delivery: Relationship to anthropometric measurements and fetal sex. BMC Pregn. Childbirth 19, 344 (2019).
Dawczynski, K. et al. Adiponectin serum concentrations in newborn at delivery appear to be of fetal origin. J Pediatr. Endocrinol. Metab. 27, 1–6 (2013).
Aramesh, M., Dehdashtian, M., Malekian, A., ShahAli, S. & Shojaei, K. Relation between fetal anthropometric parameters and cord blood adiponectin and high-sensitivity C-reactive protein in gestational diabetes mellitus. Arch. Endocrinol. Metab. 61, 228–232 (2017).
Yoshida, T., Nagasaki, H., Asato, Y. & Ohta, T. Early weight changes after birth and serum high-molecular-weight adiponectin level in preterm infants. Pediatr. Int. 53, 9926–9929 (2011).
Zemet, R. et al. Disparity in fetal growth between twin and singleton gestation: The role of adipokines. J. Perinatol. 38, 35–40 (2017).
Inami, I. et al. Imapct of serum adiponectin concentration on birth size and early postnatal growth. Pediatr. Res. 61, 604–606 (2007).
Bagias, C., Sukumar, N., Weldeselassie, Y., Oyebode, O. & Saravanan, P. Cord blood adipocytokines and body composition in early childhood: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 18, 1897 (2021).
Joung, K., Christou, H., Park, K. & Mantzoros, C. Cord blood levels of osteopontin as a phenotype marker of gestational age and neonatal morbidities. Obesity 22, 1317–1324 (2014).
Zhang, Z. et al. Maternal and cord blood adiponectin levels in relation to post-natal body size in infants in the first year of life: A prospective study. BMC Pregna. Childbirth 16, 189 (2016).
Cesur, G., Ozguner, F., Ylimaz, N. & Dundar, B. The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. J. Physiol. Sci. 62, 185–190 (2012).
Meyer, D. et al. Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 y. Pediatr. Res. 81, 745–751 (2017).
Mantzoros, C. et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics 123, 682–689 (2009).
Ashley-Martin, J. et al. An examination of sex difference in associations between cord blood adipokines and childhood adiposity. Pediatr. Obes. 15, e12587 (2020).
Simón-Muela, I. et al. Gender determines the actions of adiponectin multimers on fetal growth and adiposity. Am. J. Obstet. Gynecol. 208, e481–e487 (2013).
Oshima, K. et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem. Biophys. Res. Commun. 333, 520–526 (2005).
He, H. et al. Cord blood IGF-1, proinsulin, leptin, HMW adiponectin, and ghrelin in short or skinny small-for-gestational-age infants. J. Clin. Endocrinol. Metab. 106, e3049–e3057 (2021).
Schubring, C. et al. Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: Relation to neonatal and placental weight. J. Clin. Endocrinol. Metab. 82, 1480–1483 (1997).
Matsuda, J. et al. Serum leptin concentration in cord blood: Relationship to birth weigth and gender. J. Clin. Endocrinol. Metab. 82, 1642–1644 (1997).
Karakosta, P. et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism 65, 874–882 (2016).
Donowitz, J. et al. Role of maternal health and infant inflammation in nutritional and neurodevelopmental outcomes of two-year-old Bangladeshi children. PLoS Negl. Trop. Dis. 12, e0006363 (2018).
Rizzo, M., Fasano, R. & Paolisso, G. Adiponectin and cognitive decline. Int. J. Mol. Sci. 21, 2010 (2020).
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (U01AI066050 to J.F.F. and K01AI13068 to E.A.M.).
Author information
Authors and Affiliations
Contributions
Conception & design, acquisition, analysis or interpretation of data (S.P., Z.V., A.Z., P.I.B., J.F.F., E.A.M.); Drafting of article (S.P., J.F.F., E.A.M.); Final approval (S.P., Z.V., A.Z., P.I.B., J.F.F., E.A.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, S., Vargas, Z., Zhao, A. et al. Cord blood adiponectin and leptin are associated with a lower risk of stunting during infancy. Sci Rep 12, 15122 (2022). https://doi.org/10.1038/s41598-022-19463-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-19463-3