Abstract
Improving yield and secondary metabolites production of medicinal plants through nutrition management recently has been considered. The present study was done to determine the effects of different ammonium (NH4+) to nitrate (NO3−) ratios (100:0, 75:25, 50:50, 25:75, 0:100) on morphophysiological, nutrient contents (N, P, K, Ca, and Mg), phenolic compounds (Total phenolics (TPC) and flavonoid (TFC) contents and individual phenolics including chlorogenic acid, rosmarinic acid, gallic acid, cinnamic acid, caffeic acid, rutin, p-Coumaric acid, apigenin, and quercetin by HPLC–DAD), essential oil composition (by GC and GC–MS), and antioxidant capacity (by DPPH and FRAP assays) of Moldavian balm (Dracocephalum moldavica L.) in deep water culture (DWC) system. The highest biomass and morphological traits values of D. moldavica observed in 0:100 ratio of NH4+:NO3−. Also, the highest TPC and TFC was earned in plants that supplied with 0:100 ratio of NH4+:NO3−. Using the 25:75 ratio of NH4+:NO3− caused the highest nutrient contents (N, Ca and Mg) in the leaves. p-Coumaric acid was detected as the major abundant phenolic compound in extracts and the application of 75:25 ratio of NH4+:NO3 resulted in the highest amounts of p-Coumaric acid, gallic acid, rosmarinic acid, caffeic acid, quercetin, and rutin. The highest antioxidant capacity by both FRAP and DPPH assays was obtained in 75:25 ratio of NH4+:NO3−. Also, the highest geranial and geranyl acetate, geraniol, and neral were obtained in 75:25, 25:75, and 50:50 ratios of NH4+:NO3−, respectively. Plants supplied with the 0:100 ratio of NH4+:NO3−, had the highest total carotenoids, while the highest chlorophyll a and b content gained with 75:25 ratio of NH4+:NO3−. These results suggest that the management of N source in nutrient recipe could contribute to enhance of morphophysiological traits, antioxidant activity and phytochemical compounds in Moldavian balm.
Similar content being viewed by others
Introduction
Moldavian balm or Moldavian dragonhead (Dracocephalum moldavica L.) is an aromatic and medicinal herb that belongs to the Lamiaceae family. It is native to central Asia and is naturalized in North Africa eastern and central Europe, Northeastern United States, and China1. Leaves and hydro-distilled aqueous extract of Moldavian balm are used in Iranian traditional medicine especially in the West and East Azerbaijan provinces that have aphrodisiac, sedative and diaphoretic, digestive and stomachic effects. Essential oil and extracts from Moldavian balm are used widely in the food, cosmetic, flavoring and pharmaceutical industries2. Moldavian balm as a herbal medicine has been used for treating headaches, colds, general weakness, nerve pains, stomach and kidney spasms, and in composition of mouthwash and anti-tumor drugs3. Many chemical compounds have been identified in D. moldavica essential oil. The major of these compounds are geranial, neral, geranyl acetate and geraniol, which are oxygenated monoterpenes and are most abundant at the flowering stage4,5,6. In addition to valuable essential oil, D. moldavica is a rich source for antioxidants, flavonoids, phenolic acids, tannins and diterpenes7.
Hydroponic culture is an emerging technique that seems to be nature-friendly and can be an effective production system in countries with poor soils, land shortage and in challenge with low water resources. Therefore, hydroponics provide an excellent potential to use in that situations8,9. In addition to nutrient management in hydroponic systems, it is also possible to monitor and control the pH of the nutritional solution10,11. Deep water culture (DWC) as one of the hydroponic techniques. Since the roots are constantly supplied with oxygen-rich nutrient solution in DWC, the plants grow very quickly and vigorously. Crop production under DWC, give some advantages such as good management of pest and diseases, higher yield and notable production of secondary metabolites compared to soil culture8,9,12. Due to the various changes that nitrogen undergoes in the soil media, hydroponic systems are suitable to evaluate the effect of nitrogen forms in morphophysiological, growth and phytochemical traits, as well as on proteome analysis13. Moreover, hydroponic techniques are more suitable for assessment of root and shoot morphology and already have been used to evaluation of the effect of different nitrogen forms (NH4+ and NO3−) and their uptake efficiency in plant species14. In deep water culture systems, well-rooted plants are placed in a net pot on a floating plate in the liquid reservoir, like a raft, and the method is so-called as “floating culture”. To stabilize the plant, the net pot may be filled with substrate, e.g. clay balls or agricultural grade perlite. The nutrient solution reservoir, commonly have a depth at least 30 cm to allow well root growth and development. The nutrient solution regularly checked for pH and electrical conductivity to prevent nutrient imbalance. In DWC system, the plants are grown with their bare roots dipping directly into the nutrient solution which is very well oxygenated. This is done by means of an air pump and aeration stones, which introduce very fine air bubbles into the water. Considering to some plants of the same family (Lamiaceae), such as basil (Ocimum basilicum L.), which is grown simply in floating culture systems, good growing of Moldavian balm plant in that type of hydroponics is expected15,16,17.
Nitrogen is an essential element in plant nutrition and plays a critical role in the biosynthesis of proteins, nucleic acids and coenzymes. It is also a major component of the chlorophyll molecule and therefore plays an vital role in photosynthesis18. In addition, nitrogen is needed for growth, development, and productivity of higher plants. The two major forms of nitrogen normally used by higher plants are nitrate (NO3−) and ammonium (NH4+)19. Available forms of N has a considerable effect on growth, yield, photosynthesis, and plant quality20,21. In general, most plants prefer the nitrate form as a source of nitrogen, while plant roots can also absorb the ammonium form in nitrate deficiency conditions. When ammonium is provided as a main source of nitrogen with high concentrations for plants, their leaves number was decreased, growth decreased, roots stunted, and in severe condition this leads to yellowing of leaf tissue and ammonium toxicity22. For most species, a mixture of nitrate and ammonium is recommended as an excellent fertilizer rather than just nitrate or ammonium. The optimal ratio of nitrate to ammonium for plant growth and development depends on the genotype, environmental conditions, growth stage and the total concentration of N provided23,24. Increased rates of nitrogen and microelements have positive effects on leaf yield and essential oil production and the application of nitrogen and microelements can be an important role in increasing essential oil content of Moldavian balm25.Treating dragonhead plants with nitrogen increased plant height and branching. Fertilization with nitrogen and application of the mineral mixture caused the highest fresh herb yield and more essential oil production. Geranyl acetate, geranial and geraniol were identified as the three major compounds in the essential oil26. Results of a research revealed that the application of recommended rates of a NPK fertilizer in Moldavian balm growing, resulted in the highest plant height, highest number of branches per plant, highest fresh and dry weights as well as highest volatile oil and plant pigments production27.
Several studies have been done to evaluate the response of different species to ammonium and nitrate as a source of nitrogen. Depending on the nitrogen form supplied, species type, and environmental condition, plants show different morphophysiological and biochemical responses28,29,30,31,32,33. Different ratios of ammonium to nitrate in nutrient recipes has a great impact on quantitative and qualitative aspects of plants such as photosynthesis, growth and yield34. Increasing the ratio of ammonium to nitrate in the nutrient solution decreased most growth indices in basil (Ocimum basilicum)17. Effect of different ratios of ammonium to nitrate studied by Wang et al.35 Results showed that by reducing the ratio of ammonium to nitrate, the fresh and dry weight of roots and stem increased and the highest amount of the mentioned traits was gained at the 25:75 ratio of ammonium to nitrate.
Improving crop yield and enhancing secondary metabolites production of medicinal plants through nutrient minerals management recently has been considered. On the other hand, new researches have shown that the Moldavian balm, can be seen as a new unpolished diamond amongst medicinal herbs due to the variety of its possible applications in food and pharmaceutical industries. Due to the different behavior of plant species in the uptake of NO3− and NH4+, finding the appropriate ratios of nitrate to ammonium is very important. In addition, the effect of various aspects of hydroponic culture (including the ratio of nitrate to ammonium in nutrient solution) on the production of secondary metabolites of plants is a topic that is less discussed. Due to the many uses of essential oils and other products of Moldavian balm, this plant becomes a matter of great importance in world. In the current study, in order to improve the phytochemical properties of Moldavian balm plants grown on DWC system in greenhouse, the effect of different ammonium to nitrate ratios on morphophysiological and phytochemical properties was evaluated.
Materials and methods
Plant materials
In order to improve the phytochemical properties of Moldavian balm (Dracocephalum moldavica L.) medicinal plant under greenhouse conditions and in deep water culture system, an experiment was conducted with a completely randomized design (CRD) with five treatments and three replications (five observations were used for each replication). In this experiment, the effect of different ratios of ammonium (NH4+) to nitrate (NO3−) (100:0, 75:25, 50:50, 25:75, 0:100) on the morphophysiological and phytochemical characteristics of Moldavian balm was investigated. It should be noted that plants that supplied with 100:0 ratio of NH4+:NO3− was destroyed in mid-growth stages, due to sever ammonium toxicity. This experiment was done in the greenhouse unit and laboratories of the Urmia University in 2021. The seeds of Moldavian balm prepared from a legal local seed supplier and first sowed in 288 cells plug trays. The culture medium used for cultivation seeds was peat moss and perlite (70:30). Three weeks later, when the seedlings reached 3-4 leaves stage, they were transferred on DWC system for more growth and proceeding the main experiment. The used nutrient solutions recipe for each ratio as a treatment are shown in Table 1. In this table, the amount of all fertilizers expressed in grams. The dash signs below specific NH4+:NO3− columns and in front of some fertilizers in Table 1, means that kind of fertilizer not used for preparing the related ratio of NH4+:NO3−. Research steps and D. moldavica plants with different treatments are exhibited in Fig. 1.
Morphological traits
Morphological traits were measured during full flowering stage. Plant height (cm), number of lateral branches, fresh and dry weight of aerial parts and root (g), were recorded using meter measuring tape and sensitive scale.
Nutrient content (N, P, K, Ca and Mg)
The amount of N in Moldavian balm leaves was measured following Tedesco et al.36 The other elements were measured via atomic spectroscopy absorption (K, Ca, and Mg) and colorimetry (P) methods37. The nutrient content of Moldavian balm leaves was expressed as% in dry matter.
Photosynthetic pigments measurement
Chlorophyll a and b (Cla and Clb) and total carotenoid content (TCC) was determined following the method of Lichtenthaler (1987) with some modification. To obtain the acetone extract, 0.5 g of dried aerial parts powder was added to 10 ml of acetone/water (80%, v/v) and ultrasound assisted extraction process was conducted (Elmasonic, Germany) at 30 °C for 35 min. The acetone extract was centrifuged at 6000 rpm for 15 min. The absorbance of the extracts was read at 645, 662 and 470 nm against the blank (80% acetone) using spectrophotometer. The amount of total chlorophyll and carotenoid content was calculated based on the following formulas:
Preparation of methanolic extract
To obtain the methanolic extract, 1 g of dried aerial parts powder was added to 10 ml of methanol/water (80%, v/v) and ultrasound assisted extraction process was conducted (Elmasonic, Germany) at 30 °C for 35 min. It was filtered using Whatman No.1 filter paper. The resulting extracts were kept at 4 °C prior to analysis38.
Total phenolic content (TPC)
The Folin–Ciocalteu method was used with some modification (Slinkard and Singleton39). Briefly, 5 µl of extract solution was mixed with 1 ml of diluted (1:10) Folin–Ciocalteu and then, 480 µl of 7.5% sodium carbonate was added to mixture. After the solution was left for 30 min at lab temperature, the absorbance at 760 nm was measured by using a UV–vis spectrophotometer (UNICO, China). The results of total phenolic content were calculated using a standard curve provided using gallic acid (GAE) and were expressed as mg of gallic acid equivalents per gram of dry matter (mg GAE/g DW).
Total flavonoid content (TFC)
The amount of TFC in methanolic extract of Moldavian balm was measured following Shin et al.40 The methanolic extract (5 μl) was added to to 200 μl of 5% NaNO2 and then followed after 5 min by 300 μl of 10% AlCl3. After further 5 min, the solution was treated with 0.2 ml of 1 mM NaOH. Finally, the solution was diluted to 1 ml with distilled water and the absorbance was recorded at 380 nm. The results of TFC were estimated using a standard curve prepared using quercetin and were expressed as mg of quercetin equivalents per gram of dry matter (mg QUE/g DW).
Phenolic compound analysis by HPLC
The methods described by Gholizadeh-Moghadam et al.41 were used to quantify the phenolic compounds in alcoholic extracts using by an Agilent Technologies 1100 series HPLC (Agilent Technologies, Wilmington, DE, USA) equipped with a Dionex UltiMate 3000 system consists of a 20 μl manual sample loop, ultrasound degasser (Hwashin, Korea), quaternary pump (LPG-3400RS), column oven, and photodiode array detector with detection wavelengths of 272, 250, 310, and 360 nm (DAD-3000RS). The flow rate of 1.5 ml/min was used to separate 20 μl filtered extract (through a 0.45 μm syringe filter, Sartorius, Germany) by ZORBAX Eclipse XDB column (4.6 mm × 250 mm, 5 μm pore size, Dr. Mainsch, Germany) which was thermostatically controlled at 28 °C. Acetonitrile (solvent A) and acetic acid solution pH 3.0 (1.0% V/V in water) (solvent B) were used as mobile phases with the gradient elution program of 10%A/90%B (0–5 min), 15%A/85%B (1.5 min), 20%A/80%B (1.5 min), 25%A/75%B (1.5 min), 25-65%A/75-35%B (increase in solvent A concentration on 5% from 25-65% and decrease in solvent B concentration on 5% from 75-35 during 10-20 min), remaining on 65%A/35%B (20–25 min) and 10%A/90%B (25–35 min). Peak identification of the phenolic compounds was achieved using the retention time and photodiode array spectra comparison of commercially standard with real samples.
Antioxidant activity
DPPH assay
The antioxidant activity of methanolic extract of Moldavian balm was determined using a DPPH ((2,2-diphenyl-1-picrylhydrazyl)) assay. 2000 μl of DPPH solution (0.006 gr DPPH in 150 ml methanol 80%) was added to a certain amount of extract. After 30 min of incubation at laboratory temperature, the absorbance of the solution was recorded (λ=517 nm)42. The inhibition% of the DPPH radical was obtained by using the following equation:
Abs control: Absorbance of DPPH solution mixtures without extract.
Abs sample: Absorbance of DPPH mixtures containing extract.
Ferric reducing antioxidant power (FRAP) assay
The antioxidant activity of methanolic extract of Moldavian balm was measured by FRAP (ferric reducing antioxidant power) assay. For this, 900 μl of fresh FRAP reagent (mixing 2.5 ml of 10 mM of TPTZ solution in 40 mM HCl, 25 ml of 0.3 M acetate buffer (pH 3.6), and 20 mM FeCl3) was diluted with a certain amount of methanolic extract (1:10 v/v). Then, the resulting solution was heated up to 36 °C in a water bath. Finally, the absorbance of the mixture was measured at 593 nm. FeSO4.7H2O were used for the calibration curve, and shown as mM Fe++/g DW43.
Essential oil analysis
Essential oil (EO) extraction.
The aerial parts of Moldavian balm were harvested during the flowering stage and dried in well ventilated shade for EO extraction. 40 g dried sample were powdered using a laboratory mill and then were subjected to hydro distillation using a Clevenger instrument. The resulting essential oil of D. moldavica was collected in tightly closed dark vials, and kept in darkness at 4 °C until analysis.
GC and GC-MS analysis
D. moldavica EO were analyzed by Agilent 6890 N gas chromatography (GC) system coupled with a mass spectrophotometer GC-MS-5973 with a HP-5MS non-polar capillary column (30 m × 0.25 mm × 0.250 μm) for peak separation. The oven temperature was set with heating rate of 60 to 250 °C at 5 °C/min and remaining for isothermal mode for 10 min. The injection port temperature using split mode helium gas (1:100 with a flow rate of 1 ml/min) and FID temperature were programmed at 250 °C and 280 °C, respectively. Ionization energy was 70 eV. The 1 μl sample of D. moldavica EO were diluted with 1:20 (v:v) n-hexane and injected through a split injector. The range of 2-800 amu was used for mass range of compounds. Retention indices (RI) were calculated by using n-alkanes (C6–C24) with the same injection conditions, and in order to identify the essential oil compounds compared with previously published data. The mass spectra of compounds were collected using X Calibur (2.07). The levels of compounds (%) were obtained according to the area normalization technique, without consideration of response factors (Davies51).
Ethical approval
Authors confirm that the use of plants in the present study complies with international, national and/or institutional guidelines.
Results and discussion
Effects of NH4+:NO3− ratios on morphological traits
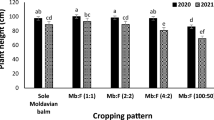
The highest biomass and morphological traits of D. moldavica in response to the different ratios of NH4+:NO3−, were gained at 0:100 ratio and the lowest values observed in the 75:25 ratio of NH4+:NO3−, with intermediate values obtained from 50:50 and 25:75 ratios of NH4+:NO3− (Fig. 2). Plants that supplied with 100:0 ratio of NH4+:NO3−, was destroyed in mid-growth stages, due to ammonium toxicity. The lower dry matter content of plants in response to higher NH4+ as N source may be associated with decrease in plant height, lateral branches, fresh weight of aerial organs and roots. Improvement of growth characteristics at higher nitrate ratios has been shown in many studies. High ammonium levels in many species causes toxicity and consequently poor growth30. A significant increment in plant biomass was found with the increment in nitrate to ammonium ratios in Citrullus Lanatus. Decreasing in biomass accumulation of tomato seedlings also shown when ammonium predominated. On the other hand, NH4+ leads to a rudimentary root system development30. Therefore, the growth was negatively influenced by a high levels of NH4+ in the solution29. Nitrogen fertilizer is one of most important factors affecting in Ocimum basilicum production. In O. basilicum, the supply of ammonium (NH4+), even in the presence of nitrate (NO3−), significantly impaired plant growth (Biesiada & Kuś, 2010; Kiferle et al.17). There are various reasons and mechanisms for ammonium toxicity in different species including disturbance in acid–base balance, reduced protein glycosylation, acidification of the external environment, and the energy lost exporting excess ammonium44. In addition, root growth in plant species is sensitive to excess NH4+45 and inhibition of root development may related to ethylene signaling46 or auxin transport30,47. Photosynthesis is perhaps the most important chemical process in plant growth. Species supplied with ammonium as main N source are suffered by decreased rates of net photosynthesis30,48. The negative effect of NH4+ on net photosynthesis rate has been attributed to excessive concentration of NH4+ in leaf tissue, which can cause separation of the electron transport reactions from phosphorylation in chloroplasts49.
The biomass and morphological traits of D. moldavica in response to the different NH4+:NO3− ratios: (a) plant height, (b) fresh weight of plant, (c) fresh weight of root, (d) dry weight of plant, (e) dry weight of root (f) number of lateral branches. *The plants of 100 NH4+:0 NO3− treatment was destroyed in mid-growth stages, due to ammonium toxicity.
Nutrient content (N, P, K, Ca and Mg)
Though, the beneficial components of medicinal plants that possess therapeutic values are mostly classified to tannins, alkaloids, steroids, polyphenolic acids and etc., the macro and micro nutrients are generally essential for metabolites production in the medicinal plants50. The macronutrients are consumed in larger quantities and are present in plant tissues51,52. The different NH4+:NO3− ratios in the hydroponic nutrient solution significantly (p < 0.01) changed the N, P, K, Ca and Mg content in the leaf tissue. The highest and lowest leaf N, Ca and Mg contents of D. moldavica were observed in 25:75 and 75:25 ratio of NH4+:NO3−, respectively. Low NH4+:NO3− ratios in the hydroponic nutrient solution generally resulted in greater leaf N, Ca and Mg content (Table 2). Also, Leaf P and K contents of D. moldavica were the highest with 75:25 ratio of NH4+:NO3−, whereas the lowest values obtained with 0:100 and 50:50 ratios of NH4+:NO3− (Table 2).
Prior researches has reported that plant species fed with ammonium as the main N source, contain higher concentrations of anions such as P and lower concentrations of cations such as Ca and Mg than those species fed with nitrate as the main N source28,53. The likely reason for the high concentration of K in plants grown in high ammonium is that plants use K accumulation as an alleviative mechanism to NH4+ toxicity54. Plants fed with ammonium, generally contain less low-molecular weight anions (nitrate) and thus have less negative charges to balance (Zhang et al.55). Phosphorus have a critical role in in providing anion and charge balancing the ammonium-fed plant. Therefore, high levels of phosphorus in ammonium-fed nightshade plants may be participate in positive and negative charges balancing in the plant. Generally, high levels of some nutrients such as N and P were observed in plants fed with mixture of ammonium and nitrate as N source30. Compared to the sole NO3− as N source (0:100 ratio of NH4+NO3−), the supply of NH4+ affect the balance of N, P, and K in plant organs (Zhang et al.32). A decrease in Mg and K contents was observed in Eustoma grandiflorum plants fed with the sole NH4+ as N source56.
Photosynthetic pigments
Chlorophyll plays a vital role in the photosynthetic processes in plants. It is not only responsible for capturing solar energy to carry out photosynthesis, but also plays a role in photoprotective processes and shows antioxidant activity, all of which contribute to effective biomass and oxygen production57,58,59.
The amounts of chlorophyll a, chlorophyll b, and total carotenoid content of aerial parts in the different treatments are exhibited in Fig. 3a–c. Moldavian balm plants fertilized with the 0:100 ratio of NH4+:NO3−, had the highest total carotenoid content, while the highest and lowest chlorophyll a and b contents were observed in 75:25 and 50:50 ratios of NH4+:NO3−, respectively. There was no significant difference between other treatments. Nitrogen is an essential nutrient in plant cells found in many (macro) molecules such as proteins, chlorophyll, pigments, nucleic acids and secondary metabolites60,61. Different nitrogen forms have a various effect on the amount of photosynthetic rate and pigments of higher plants (Xiao et al.62). In the present study, higher NO3− and NH4+ levels both increased photosynthetic pigments (chlorophyll a, b and carotenoid) content. These results are in conflict with some findings mentioned in other scientific papers and this may be due to unrevealed matters in uptake of both forms of N. Several previous studies reported that using NO3− as main N source, increases the availability of nitrogen and magnesium. These elements are a part of photosynthetic pigments and increases their content. Similar results were found by Liu et al.29 and Qadir et al.63.
The phytochemical content of D. moldavica in response to the different NH4+:NO3− ratios: (a) chlorophyll a (Cla) (b) chlorophyll b (Clb) (c) total carotenoid content (TCC) d) total phenolic content (TPC) e) total flavonoid content (TFC). *The plants of 100 NH4+:0 NO3̄ treatment was destroyed in mid-growth stages, due to ammonium toxicity.
Effect of the NH4+:NO3− ratios on TPC and TFC
Phenolic compounds as well as flavonoids are important bioactive agents that have long been interested due to their benefits for human health64,65,66,67. The results showed that TPC and TFC in D. moldavica were affected by various ratios of NH4+:NO3− (p < 0.01). The highest and lowest TPC and TFC in D. moldavica were obtained in 0:100 and 75:25 ratios of NH4+:NO3−, respectively, which are shown in Fig. 3d,e. Moldavian balm plants treated by nutrient solution with high concentrations of NO3−, produced greater amounts of these compounds, significantly. The result of present study is in agreement with some previous works and supports their results32,33. Decreasing of phenolics content in plant tissues (leaves and roots) due to the supply of NH4+ as sole N source reported in several plant species such as pea68, corn69, and Echinacea angustifolia70. Ammonium may by altering intracellular acidity affect the biosynthesis of metabolites including phenylpropanoid pathway71. There is less information on how the N source affects the biosynthesis and accumulation of various secondary metabolites including phenolics and most mechanisms involved are still unclear72.
Phenolic compound analysis by HPLC-DAD
Table 3 shows the retention time of the phenolic compounds studied, calibration curve, the correlation coefficient and the limits of detection (LOD) and quantification (LOQ). The chromatograms of phenolic acids and flavonoids standards are exhibited in Fig. 4. Phenolics as antioxidant are important due to their therapeutic potential related with many diseases such as anti-inflammatory, Alzheimer's and anti-cancer73. The contents of individual phenolics (gallic acid, rosmarinic acid, chlorogenic acid, caffeic acid, cinnamic acid, p-Coumaric acid, apigenin, quercetin, and rutin) by HPLC-DAD in the different treatments of NH4+:NO3− are exhibited in Table 4. The results revealed that phenolic composition in methanolic extracts of D. moldavica were considerably influenced by the various ratios of NH4+:NO3− (p < 0.01). p-Coumaric acid was identified as the most abundant compound in extracts. The highest amounts of p-Coumaric acid, gallic acid, rosmarinic acid, caffeic acid, quercetin, and rutin were observed in 75:25 ratio of NH4+:NO3−, followed by the ratio of 0:100. Moreover, the highest amounts of chlorogenic acid, cinnamic acid and apigenin were detected in the 0:100 ratio of NH4+:NO3−. When plants were exposed to higher levels of NH4+ showed greater amounts of rosmarinic acid and p-Coumaric acid. The plant responses to ammonium are profoundly influenced by the plant nutritional status, with particular regard to the availability of nitrate74. High levels of ammonium in the nutrient solution appear to cause stress in plants, and on the other hand stimulate the production of metabolites such as phenolics in Moldavian balm. It has been suggested that PAL activity is increased at high level of NH4+ and the production of metabolites is induced through the phenylpropanoid pathway75. However, there is not further information available concerning the impact of different N forms on accumulation of phenolic compounds. Phenolics are interesting specially due to their antioxidant, anti-inflammatory and anti-diabetic properties65,66 . They are considered to be a vital human dietary component and exhibit a tremendous antioxidant activity as well as other health benefits67. This research also shows that the providing of ammonium to Moldavian balm plants in DWC system could have lower impact than nitrate on the quantity enhancement of these constituents, indicating that this nutritional strategy could be suitable to improve the technological and nutraceutical value of Moldavian balm.
Antioxidant activity by DPPH and FRAP assays
Natural antioxidants are widely distributed in foods and medicinal plants. The effective extraction and proper assessment of antioxidants from food and medicinal plants are crucial to explore the potential antioxidant sources and promote their application in functional foods, pharmaceuticals and food additives76,77,78.
The results showed that antioxidant activity by FRAP and DPPH assays in D. moldavica was influenced by different ratios of NH4+:NO3− (p < 0.01). The highest and lowest antioxidant activity in both DPPH and FRAP methods was obtained in 75:25 and 0:100 ratios of NH4+:NO3−, respectively, which are exhibited in Fig. 5. Moldavian palm plants treated with high concentration of ammonium in the nutrient solution, showed significantly a greater amounts of antioxidant activity. The evaluated phenolic compounds (including gallic acid, caffeic acid, p-coumaric acid, rosmarinic acid and quercetin) had strong correlation with FRAP assay than the DPPH method. These compounds most widely used as a donor of electrons and prevents oxidative damages arising from free radicals79,80. In addition, results suggested that coumaric acid and quercetin are potent antioxidants with proven therapeutic efficacy81,82. Our experiment is also in consistent with other researches (Jakovljević et al.84, Okello et al.83, Prinsi et al.31). On a large scale, high phenolics content are directly related to higher resistance of plants to pests and diseases, and thus a precise regulation of N supplementation could present agroecosystem related advantages by reducing pesticide usage in agriculture. Generally, these natural antioxidants, especially polyphenols and carotenoids, exhibit a wide range of biological effects, such as anti-inflammatory, antibacterial, antiviral, anti-aging, and anti-cancer77,78. Considering their beneficial effects for health, the efficient extraction methods of natural antioxidants, appropriate assessment of antioxidant activity as well as their main resources from food and medicinal plants are drawing great attention in food science and nutrition.
GC and GC-MS analysis of essential oil (EO)
Essential oils are widely used in pharmaceutical, sanitary, cosmetics, agriculture and food industries for their bactericidal, virucidal, fungicidal, anti-parasitical and insecticidal properties85,86,87. The detailed volatile constituents of D. moldavica EO were analyzed by GC and GC/MS in different treatments, and fifty-seven compounds were detected (Table 5). The major compounds in D. moldavica EO were identified as neral, geranial, geraniol and geranyl acetate. Our results confirms previous studies conducted in Iran, reported that neral, geranial, geraniol and geranyl acetate are the predominant components of Moldavian balm EO88,89. The amount of EO components were widely varied by different NH4+:NO3− ratios (Table 5). By applying different ratios of NH4+:NO3−, the geranial and geranyl acetate contents in D. moldavica EO decreased from 32.76 to 19.89% and from 31.08 to 12.24% respectively, which are presented in Table 5. With different ratios of 75:25, 50:50, 25:75 and 0:100, 21, 14, 18 and 45 compounds were identified, respectively. The highest geranial and geranyl acetate content were obtained in 75:25 ratio of NH4+:NO3−, whereas the lowest geranial and geranyl acetate content were obtained in 0:100 ratio of NH4+:NO3−. Also, the highest (26.34%) and lowest (4.9%) amounts of geraniol was identified in 25:75 and 75:25 ratios of NH4+:NO3−, respectively. The highest (42.40%) content of neral was identified in 50:50 ratio of NH4+:NO3−, and the application of 0:100 ratio of NH4+:NO3−, resulted in lowest (14.83%) content of neral. In addition, the maximum (87.48%) and minimum (63.85%) levels of total dominant compounds (neral+geranial+geraniol+geranyl acetate) were obtained in 25:75 and 0:100 ratios of NH4+:NO3−. The best treatment for major compounds yield was 25:75 ratio of NH4+:NO3−. The variations in the volatile constituents of D. moldavica EO at different NH4+:NO3− ratios may be due to supply various quantities of nitrate (NO3−) to the D. moldavica. Nitrogen plays a key role in the production of EO in medicinal herbs90. N also as an important factor stimulates specific biosynthetic pathways of EOs in medicinal herbs91. Nitrogen increases the efficiency of photosynthesis by increasing leaf area, chlorophyll content, and enzymes activity thereby improving the production of EO in medicinal plants92. Furthermore, N is as important element in higher plants involved to biosynthesis of many organic structures including amino acids, enzymes, and etc. which are essential for EO production pathway93.
Conclusion
Crop production in hydroponic systems take many advantages compared to open fields. Higher yield, good quality and shorter growing periods are some benefits of hydroponically plant growing. Since the roots are constantly supplied with oxygen-rich nutrient solution in DWC, the plants grow very quickly and vigorously. Along with the aboveground parts, plant roots obtained from this system are not spoiled by substrate particles and can be easily removed from the nutrient solution without any damage or lose. This is important especially when such plants with valuable roots should be grown. Nitrogen is the most important macro nutrient in plant nutrition that have many roles in plant. In addition to nitrogen concentration in nutrient solution, the available forms of nitrogen and their ratio have significant effects on growth, photosynthesis, yield and plant quality. The two major forms of nitrogen normally used by higher plants are nitrate and ammonium. There is not further information available concerning the impact of different N forms on Moldavian balm. The present study was done to determine the effect of different ammonium to nitrate ratios on morphophysiological, nutrient contents, phenolic compounds, essential oil components, and antioxidant capacity of D. moldavica in DWC system. The results revealed that studied traits of D. moldavica were considerably influenced by the different ratios of NH4+:NO3−. The NH4+ as sole N source showed toxic effects being visible on growth and morphological traits. The supply of NO3− as N source increased the accumulation of some phytochemicals (TPC, TFC, and TCC) in plant tissues. For most traits, a mixture of nitrate and ammonium is recommended as an excellent fertilizer rather than just nitrate or ammonium. These results suggest that the management of N source could contribute to improve morphophysiological traits, antioxidant activity, and phytochemical compounds in Moldavian balm.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Naderifar, M., Sonboli, A. & Gholipour, A. Pollen morphology of Iranian Dracocephalum L. (Lamiaceae) and its taxonomic significance. Bangladesh J. Plant Taxon. 22, 99–110 (2015).
Dmitruk, M. & Weryszko-chmielewska, E. Non-glandular and glandular trichomes on Dracocephalum moldavicum L. Shoots. 63, 11–22 (2010).
Hussein, M. S., Khalil, M. Y., Naguib, N. Y. & Aly, S. M. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 108, 322–331 (2006).
Maham, M., Akbari, H. & Delazar, A. Chemical composition and antinociceptive effect of the essential oil of Dracocephalum moldavica L. Pharm. Sci. 18, 187–192 (2013).
Vafadar-Yengeje, L., Amini, R. & Nasab, A. D. M. Chemical compositions and yield of essential oil of Moldavian balm (Dracocephalum moldavica L.) in intercropping with faba bean (Vicia faba L.) under different fertilizers application. J. Clean. Prod. 239, 118033 (2019).
Fallah, S., Rostaei, M., Lorigooini, Z. & Surki, A. A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead- soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crops Prod. https://doi.org/10.1016/j.indcrop.2018.02.003 (2018).
Kakasy, A. Z. et al. Comparative study of traditional essential oil and supercritical fluid extracts of Moldavian dragonhead (Dracocephalum moldavica L.). Flavour Fragr. J. https://doi.org/10.1002/ffj.1569 (2006).
Marques, G., Aleixo, D., & Pitarma, R. Enhanced Hydroponic Agriculture Environmental Monitoring: An Internet of Things Approach. 11538 LNCS, 658–669 (Springer ,2019).
Pradhan, B. & Deo, B. Soilless farming - The next generation green revolution. Curr. Sci. 116, 728–732 (2019).
Bar-Yosef, B., Mattson, N. S. & Lieth, H. J. Effects of NH4:NO3:urea ratio on cut roses yield, leaf nutrients content and proton efflux by roots in closed hydroponic system. Sci. Hortic. (Amsterdam) 122, 610–619 (2009).
Corrêa, R. M. et al. A comparison of potato seed tuber yields in beds, pots and hydroponic systems. Sci. Hortic. (Amsterdam) 116, 17–20 (2008).
Abeysinghe, D. C., Wijerathne, S. M. N. K. & Dharmadasa, R. M. Secondary metabolites contents and antioxidant capacities of Acmella Oleraceae Grown under different growing systems. World J. Agric. Res. 2, 163–167 (2014).
Hajari, E., Snyman, S. J. & Watt, M. P. The effect of form and level of inorganic N on nitrogen use efficiency of sugarcane grown in pots. J. Plant Nutr. https://doi.org/10.1080/01904167.2016.123764840,248-257 (2016).
Robinson, N. et al. Nitrate paradigm does not hold up for sugarcane. PLoS ONE 6, e19045 (2011).
Kiferle, C. et al. Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Open Life Sci. 6, 946–957 (2011).
Kiferle, C., Maggini, R. & Pardossi, A. Influence of root hypoxia and NaCl salinity on sweet basil (Ocimum basilicum L.) grown hydroponically for the production of rosmarinic acid. Agrochimica 56, 257–267 (2012).
Kiferle, C., Maggini, R. & Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop Sci. 7, 321–327 (2013).
Barker, A. V & Pilbeam, D. J. Handbook of Plant Nutrition https://doi.org/10.1201/B18458 (2015).
Tschoep, H. et al. Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ. 32, 300–318 (2009).
Lošák, T., Hlušek, J. & Krá, S. The effect of nitrogen and sulphur fertilization on yield and quality. 697–703 (2008).
Ali, S. et al. Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol. Plant. 57, 758–763 (2013).
Tabatabaei, S. J., Yusefi, M. & Hajiloo, J. Effects of shading and NO3:NH4 ratio on the yield, quality and N metabolism in strawberry. Sci. Hortic. (Amsterdam) 116, 264–272 (2008).
Zhonghua, T., Yanju, L., Xiaorui, G. & Yuangang, Z. The combined effects of salinity and nitrogen forms on Catharanthus roseus: The role of internal ammonium and free amino acids during salt stress. J. Plant Nutr. Soil Sci. 174, 135–144 (2011).
Guo, X.-R., Zu, Y.-G. & Tang, Z.-H. Physiological responses of Catharanthus roseus to different nitrogen forms. Acta Physiol. Plant. 34, 589–598 (2012).
Nejatzadeh-Barandozi, F., Shahvaladi, E. H. & Gholami-Borujeni, F. Nitrogen fertilization and microelements influences growth index and yield in (Dracocephalum moldavica L). Bulg. J. Agric. Sci. 21, 266–269 (2015).
Said-Al Ahl, H. A. H., Hussein, M. S. & Abd El-Kader, A. A. Effect of nitrogen fertilizer and/or some foliar application on growth, herb yield, essential oil and chemical composition of dragonhead. J. Med. Food Plants 2(1), 12–28 (2010).
Mostafa, H.S., Dawoud, G.T. & Ashraf, S. M. Studies on the impact of NPK fertilization, compost and ascorbic acid on chemical and biological composition of dragonhead (Dracocephalum moldavica) plants. 378–393 (2019).
Helali, S. M. et al. Influence of nitrate-ammonium ratio on growth and nutrition of Arabidopsis thaliana. Plant Soil 336, 65–74 (2010).
Liu, G., Du, Q. & Li, J. Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. (Amsterdam) 214, 41–50 (2017).
Na, L., Li, Z., Xiangxiang, M. & Ara, N. Effect of nitrate/ammonium ratios on growth, root morphology and nutrient elements uptake of watermelon (Citrullus Lanatus) Seedlings. J. Plant Nutr. 37(11), 1859–1872. https://doi.org/10.1080/01904167.2014.911321 (2014).
Prinsi, B., Negrini, N., Morgutti, S. & Espen, L. Nitrogen starvation and nitrate or ammonium availability differently affect phenolic composition in green and purple basil. Agronomy 10(4), 498 (2020).
Zhang, J. et al. Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (Capsicum annuum L.). Agronomy 9(11), 683 (2019).
Zhu, Z. B. et al. Effects of ammonium to nitrate ratio on growth, nitrogen metabolism, photosynthetic efficiency and bioactive phytochemical production of Prunella vulgaris. Pharm. Biol. 52, 1518–1525 (2014).
Tabatabaei, S., Fatemi, L. & Fallahi, E. Effect of ammonium: Nitrate ratio on yield, calcium concentration, and photosynthesis rate in strawberry. J. Plant Nutr. 29, 1273–1285 (2006).
Wang, J., Zhou, Y., Dong, C., Shen, Q. & Putheti, R. Effects of NH4+-N/ NO3–N ratios on growth, nitrate uptake and organic acid levels of spinach (Spinacia oleracea L.). Afr. J. Biotechnol. 8, 3597–3602 (2009).
Tedesco, M.J., Volkweiss, S.J., Bohnen, H. Análise de solo, plantas e outros materiais. Universidade Federal do Rio Grande do Sul, Porto Alegre https://scholar.google.com/scholar?hl=fa&as_sdt=0%2C5&q=Tedesco%2C+M.J.%2C+Volkweiss%2C+S.J.%2C+Bohnen%2C+H.%2C+1985&btnG= (1985).
Bernardo, E. et al. Análise química para avaliação da fertilidade de solos tropicais (2001).
Alirezalu, A. et al. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of iran. Int. J. Food Prop. 21, 452–470 (2018).
Slinkard, K. & Singleton, V. L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 28(1), 49–55 (1977).
Shin, Y., Liu, R. H., Nock, J. F., Holliday, D. & Watkins, C. B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 45, 349–357 (2007).
Gholizadeh-Moghadam, N., Hosseini, B. & Alirezalu, A. Classification of barberry genotypes by multivariate analysis of biochemical constituents and HPLC profiles. Phytochem. Anal. 30, 385–394 (2019).
Nakajima, J. I., Tanaka, I., Seo, S., Yamazaki, M. & Saito, K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004, 241–247 (2004).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of ‘“antioxidant power”’: The FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Liu, Y. & Von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 68, 2581–2592 (2017).
Li, Q., Li, B., Kronzucker, H. J. & Shi, W. M. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ. 33(9), 1529–1542 (2010).
You, W. & Barker, A. V. Ethylene evolution and ammonium accumulation by tomato plants after root-applied glufosinate-ammonium treatment in the presence of ethylene inhibitors. Commun. Soil Sci. Plant Anal. 35, 1957–1965 (2004).
Cao, Y., Glass, A. & Crawford, N. M. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiol. 102(3), 983–989 (1993).
Claussen, W. & Lenz, F. Effect of ammonium and nitrate on net photosynthesis, flower formation, growth and yield of eggplants (Solanum melongena L.). Plant Soil 171, 267–274 (1995).
Guo, H. X., Liu, W. Q. & Shi, Y. C. Effects of different nitrogen forms on photosynthetic rate and the chlorophyll fluorescence induction kinetics of flue-cured tobacco. Photosynth. 44, 140–142 (2006).
Lasisi, A. A., Ejelonu, B. C., Nwosu, F. O., Olayiwola, M. A. & Yusuff, A. A. Heavy metals and macronutrients content in selected herbal plants of South-Western Nigeria. Hamdard Med. 49, 71–76 (2006).
Aggett, P. J. & Davies, N. T. Some nutritional aspects of trace metals. J. Inherit. Metab. Dis. 6, 22–30 (1983).
Praveeena, S., Pasula, S. & Sameera, K. Trace elements in diabetes mellitus. J. Clin. Diagn. Res. JCDR 7, 1863 (2013).
Roosta, H. R. & Schjoerring, J. K. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 30, 1933–1951 (2007).
Roosta, H. R. & Schjoerring, J. K. Effects of nitrate and potassium on ammonium toxicity in cucumber plants. J. Plant Nutr. 31, 1270–1283 (2008).
Zhang, Y., Lin, X., Zhang, Y. & Zheng, S. J. Effects of nitrogen levels and nitrate/ammonium ratios on oxalate concentrations of different forms in edible parts of spinach. J. Plant Nutr. 28(11), 2011–2025. https://doi.org/10.1080/01904160500311086 (2011).
Hernández-Pérez, A. et al. Effects of ammonium and calcium on lisianthus growth. Hortic. Environ. Biotechnol. 57, 123–131 (2016).
Hynninen, P. H. & Leppäkases, T. S. The functions of chlorophylls in photosynthesis. EOLSS Oxford UK 5, 1–9 (2002).
Pareek, S. et al. Chlorophylls: Chemistry and biological functions. Fruit Veg. Phytochem. Chem. Hum. Heal. Second Ed. 1, 269–284 (2017).
Kuczynska, P., Jemiola-Rzeminska, M. & Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 13, 5847–5881 (2015).
Tuncay, Ö., Eşiyok, D., Yaǧmur, B. & Okur, B. Yield and quality of garden cress affected by different nitrogen sources and growing period. Afr. J. Agric. Res. 6, 608–617 (2011).
Andrews, M., Raven, J. A. & Lea, P. J. Do plants need nitrate? the mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 163, 174–199 (2013).
Xiao, K., Zhang, S. H., Zou, D. H. & Zhang, R. X. The effects of different nitrogen nutrition forms on photosynthetic characteristics in wheat leaves. Acta Agron. Sin. 26, 53–58 (2000).
Qadir, O., Siervo, M., Seal, C. J. & Brandt, K. Manipulation of contents of nitrate, phenolic acids, chlorophylls, and carotenoids in lettuce (Lactuca sativa L.) via contrasting responses to nitrogen fertilizer when grown in a controlled environment. J. Agric. Food Chem. 65, 10003–10010 (2017).
Tungmunnithum, D., Thongboonyou, A., Pholboon, A. & Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 5, 93 (2018).
Hemmati, M. et al. Anti-atherogenic potential of jujube, saffron and barberry: anti-diabetic and antioxidant actions. EXCLI J. 14, 908 (2015).
Siriamornpun, S., Weerapreeyakul, N. & Barusrux, S. Bioactive compounds and health implications are better for green jujube fruit than for ripe fruit. J. Funct. Foods 12, 246–255 (2015).
Kumar, N. & Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 24, e00370 (2019).
Domínguez-Valdivia, M. D. et al. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and -sensitive plants. Physiol. Plant. 132, 359–369 (2008).
Vuletić, M., Šukalović, V. H. T., Marković, K. & Maksimović, J. D. Antioxidative system in maize roots as affected by osmotic stress and different nitrogen sources. Biol. Plant. 54, 530–534 (2010).
Wu, C. H., Dewir, Y. H., Hahn, E. J. & Paek, K. Y. Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J. Plant Biol. 49, 193–199 (2006).
Dixon, R. A. & Paiva, N. L. Stress-induced phenylpropanoid metabolism. Plant Cell 7, 1085–1097 (1995).
Naik, P. M., Manohar, S. H. & Murthy, H. N. Effects of macro elements and nitrogen source on biomass accumulation and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol. Plant. 33, 1553–1557 (2011).
Shahidi, F. & Yeo, J. D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 19, 1–16 (2018).
Britto, D. T. & Kronzucker, H. J. Review NH 4 + toxicity in higher plants: A critical review. J. Plant Physiol 159, 567–584 (2002).
Olsen, K. M. et al. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant. Cell Environ. 32, 286–299 (2009).
Xu, D. P. et al. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 18, 20–31 (2017).
Peng, C. et al. Biology of ageing and role of dietary antioxidants. Biomed Res. Int. 2014, 831841 (2014).
Zheng, J. et al. Spices for prevention and treatment of cancers. Nutrients 8, 495 (2016).
Wang, X. et al. Correlation between the adsorption ability and reduction degree of graphene oxide and tuning of adsorption of phenolic compounds. Carbon N. Y. 69, 101–112 (2014).
Martínez, L., Jongberg, S., Ros, G., Skibsted, L. H. & Nieto, G. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation. Food Res. Int. 129, 108789 (2020).
Shen, Y. et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 111, 579–587 (2019).
Xie, X. et al. Depression caused by long-term stress regulates premature aging and is possibly associated with disruption of circadian rhythms in mice. Physiol. Behav. 199, 100–110 (2019).
Okello, O. P., Gweyi, J. P. O., Nawiri, M. P. & Musila, W. Effects of water stress on phenolic content and antioxidant activity of African nightshades. Biofarmasi J. Nat. Prod. Biochem. 15, 79–95 (2017).
Jakovljević, D., Topuzović, M. & Stanković, M. Nutrient limitation as a tool for the induction of secondary metabolites with antioxidant activity in basil cultivars. Ind. Crops Prod. 138, 111462 (2019).
Krishna, A., Tiwari, R. & Kumar, S. Aromatherapy-an alternative health care through essential oils. J. Med. Aromat. Plant Sci. 22, 798–804 (2000).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475 (2008).
Bayala, B. et al. Anticancer activity of essential oils and their chemical components - a review. Am. J. Cancer Res. 4, 591 (2014).
Ehsani, A. et al. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum 8, 223 (2017).
Amini, R., Ebrahimi, A. & Nasab, A. D. M. Moldavian balm (Dracocephalum moldavica L) essential oil content and composition as affected by sustainable weed management treatments. Ind. Crops Prod. 150, 112416 (2020).
Oniszczuk, T. et al. Active polyphenolic compounds, nutrient contents and antioxidant capacity of extruded fish feed containing purple coneflower (Echinacea purpurea (L.) Moench.). Saudi J. Biol. Sci. 26, 24–30 (2019).
Baričevič, D. & Zupančič, A. The impact of drought stress and/or nitrogen fertilization in some medicinal plants. J. Herbs Spices Med. Plants 9, 53–64 (2002).
Hosseinpour, M., Ebadi, A., Habibi, H., Nabizadeh, E. & Jahanbakhsh, S. Enhancing enzymatic and nonenzymatic response of Echinacea purpurea by exogenous 24-epibrassinolide under drought stress. Ind. Crops Prod. 146, 112045 (2020).
Banica, F. et al. Determination of the total polyphenols content and antioxidant activity of echinacea purpurea extracts using newly manufactured glassy carbon electrodes modified with carbon nanotubes. Processes 8, 833 (2020).
Acknowledgements
This research was supported by the Urmia University, Urmia, Iran.
Author information
Authors and Affiliations
Contributions
A.A. contributed to the study conception and design. Data collection and analysis were performed by A.N. and P.N. The first draft of the manuscript was written by K.A. A.A. prepared research material and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naseri, A., Alirezalu, A., Noruzi, P. et al. The effect of different ammonium to nitrate ratios on antioxidant activity, morpho-physiological and phytochemical traits of Moldavian balm (Dracocephalum moldavica). Sci Rep 12, 16841 (2022). https://doi.org/10.1038/s41598-022-21338-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-21338-6
This article is cited by
-
A Novel UV Spectrophotometric Method Measuring Ammonium Ions for Protein Determination in Animal Food Source
Food Analytical Methods (2026)
-
Synergistic effect of ammonium and potassium on carrot growth, physio-biochemical mechanisms, and water use efficiency under varying irrigation regimes
Scientific Reports (2025)
-
Nitrogen source and solution strength modulate cannabinoid and antioxidant profiles in medicinal cannabis grown in a deep-water culture system
Scientific Reports (2025)
-
Phytochemical and morpho-physiological response of Melissa officinalis L. to different NH4+ to NO3̄ ratios under hydroponic cultivation
BMC Plant Biology (2024)
-
The effectiveness of hot-air, infrared and hybrid drying techniques for lemongrass: appearance acceptability, essential oil yield, and volatile compound preservation
Scientific Reports (2023)







Omar A. Reyes Vuelvas
Hello,
Can someone please help me clarify and understand how the amounts of fertilizer complies with the ammonium to nitrate ratios?
Thanks in advance to anyone who can help me!