Abstract
In patients with acute ST-elevation myocardial infarction (STEMI), it is essential to restore myocardial perfusion as soon as possible. However, a considerable proportion of patients have no-reflow. No-reflow increases the risk of major adverse cardiac events and even death. The role of blood eosinophil count in predicting no-reflow in STEMI patients has not been determined, particularly after primary percutaneous coronary intervention (pPCI). The present study aimed to evaluate the predictive value of eosinophil counts for no-reflow in patients with STEMI who underwent pPCI. A total of 674 STEMI patients who underwent pPCI were enrolled. The subjects were divided into two groups according to eosinophil counts for primary analysis and with or without T2DM for secondary analysis. Logistic regression analysis was used to determine whether eosinophil count was an independent predictor of no-reflow in the entire cohort, and subgroup and receiver operating characteristic (ROC) curves were explored to evaluate its predictive value. DeLong’s test was used to compare the area under curves of the three ROC curves. The low eosinophil count was an independent predictor for no-reflow in whole cohort (adjusted OR: 2.012, 95% CI 1.242–3.259, p = 0.004) and in patients with T2DM (adjusted OR: 4.312, 95% CI 1.878–9.900, p = 0.001). In patients without T2DM, hemoglobin, but not low eosinophil count, was an independent predictor of no-reflow. The results of the ROC curve analysis revealed that a low eosinophil count had moderate predictive efficiency for predicting no-reflow in patients with T2DM, and the power was superior to all populations and patients without T2DM. Our data suggest that decreased eosinophil count was an independent risk factor for no-reflow in patients with STEMI who underwent pPCI, especially in T2DM patients, which provides guidance for clinicians to identify patients at a higher risk of developing no-reflow and lowering their risk.
Similar content being viewed by others
Introduction
Myocardial perfusion should be restored as soon as possible in patients with acute ST-elevation myocardial infarction (STEMI)1. Primary percutaneous coronary intervention (pPCI) to achieve a resumption of optimal blood flow is the preferred method of reperfusion, which significantly prevents further necrosis of the myocardium and improves the quality of life of patients with acute myocardial infarction (AMI)2,3. However, a considerable proportion of patients who undergo pPCI have no-reflow, which can lead to adverse left ventricular remodeling and poor healing of the infarct, increasing the risk of major adverse cardiac events (MACE) and even death4,5. The impact of no-reflow on clinical outcomes has been well documented, and the incidence of no-reflow ranges from 2 to 60%6,7. Although no-reflow has been intensively studied, its detailed molecular mechanisms remain unclear8,9.
Accumulating evidence showed that inflammation was pivotal in developing no-reflow10,11. Many studies have evaluated the involvement of classic inflammatory responses in no-reflow, such as neutrophils, monocytes/macrophages, and T cells12,13,14. A previous study showed that C-reactive protein (CRP), albumin, white blood cell count (WBC) count, neutrophil-to-lymphocyte ratio (NLR), and CRP/albumin ratio (CAR) can be used to predict no-reflow in patients with STEMI who treated with pPCI. Moreover, the authors also found that the CAR has a stronger prediction ability than that of CRP, WBC, and NLR15. However, in addition to classic inflammation, both experimental and clinical studies suggest that allergic inflammation is also involved in the pathogenesis of coronary artery disease (CAD) and MACE following stent implantation16,17,18. Moreover, allergic inflammation, largely mediated by eosinophils, has recently been found to contribute to atherosclerotic plaque formation and thrombosis18. Previous studies have shown that eosinophil levels emerged as a strong predictor of mortality in patients with CAD undergoing PCI19, In fact, previous studies have unearthed that eosinophils levels emerged as a strong predictor of mortality in patients with CAD undergoing PCI20, and in patients with acute heart failure (HF)21. The role of blood eosinophil counts in predicting no-reflow in patients with STEMI, particularly following pPCI, has not been determined.
In light of the above, we aimed to assess the possible relationship between blood eosinophils count and no-flow in patients with STEMI who are undergoing pPCI. In addition, we studied their relationship in a subgroup of patients with and without T2DM.
Methods
Study design and patient selection
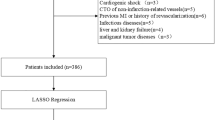
This was a single-center retrospective observational cohort study. Patients with STEMI who underwent pPCI at the Liaocheng People’s Hospital between June 2016 and November 2019 were enrolled in this study. ESC guidelines for managing STEMI were defined as diagnostic criteria22. The inclusion criteria were STEMI patients who underwent pPCI with stent implantation or percutaneous transluminal coronary angioplasty. Patients hospitalized for STEMI but with severe infections, severe renal and liver diseases, immune system diseases, aortic dissection, or cancer were excluded. Ultimately, 674 patients were included in the analysis. The study flowchart is shown in Fig. 1. All protocols were approved by the ethics committee of Liaocheng People’s Hospital. As this was a retrospective study, informed consent forms were exempted from the ethics committee of Liaocheng People’s Hospital.
Study variables and laboratory testing
Clinical characteristics and demographic profiles were obtained from the hospital’s computerized information system. All venous blood samples were collected from patients before the pPCI procedure at admission. All laboratory tests were performed at our hospital emergency laboratory. Whole-blood eosinophils counts (reference range: 0.02–0.52 × 109/L) were determined on a CELL DYN 4000 Abbott analyzer (Abbott Diagnostics, Santa Clara, California, USA), which was calibrated daily; and 0.02–0.52 × 109/L was defined as normal.
Primary PCI and no-reflow
Upon admission, all patients received a standard loading dose of medications (300 mg aspirin and 180 mg ticagrelor or 300 mg clopidogrel) upon the diagnosis of STEMI. A total of 2500 IU of heparin was administered, and a weight-dependent dose (up to 100 IU/kg) was added for PCI. All surgical procedures and decisions were made by experienced cardiologists. No-reflow phenomenon was defined according to coronary thrombolysis in myocardial infarction (TIMI) flow, and TIMI flow grade of 0–II after vessel reopening without coronary stenosis, dissection, and spasm was defined as “no-reflow.”
Statistical analysis
Statistical analysis was performed using SPSS 23.0 (IBM Corp.) and MedCalc Statistical Software, version 16.8.4 (Ostend, Belgium.). Normally distributed continuous data were expressed as mean ± standard deviation and were compared using the Student’s t-test. Non-normally distributed data were expressed as median (interquartile range) and compared using the Mann–Whitney U test. Categorical variables are expressed as frequencies (percentages) and compared using the chi-square or Fisher’s exact test. Logistic regression analysis was used to determine whether eosinophil count was an independent predictor of no-reflow in the total study population and in patients with and without T2DM. Variables with an unadjusted p value < 0.05 in the univariate analysis were subsequently evaluated using a multivariate logistic regression model. Receiver operating characteristic (ROC) curves were used to determine the best cut-off values of eosinophil count for predicting no-reflow in the study population and patients with and without T2DM, respectively. DeLong’s test was used to compare area under curves (AUCs) of the three ROC curves. A p value < 0.05 was the criteria for statistical significance in this analysis.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Liaocheng People’s Hospital. All procedures were in accordance with principles of Helsinki Declaration. Since it is a retrospective study, informed consent forms were exempted by the Ethics Committee of the Liaocheng People’s Hospital.
Results
Patient characteristics
Between June 2016 and November 2019, 674 patients who underwent pPCI were included in this study. Of the 674 patients, 455 patients (67.5%) had normal eosinophil counts (≥ 0.02 × 109/L) and 219 patients (32.5%) had decreased eosinophil counts (< 0.02 × 109/L). The baseline characteristics of the two groups are shown in Table 1. Patients who had decreased eosinophil counts were nonsmokers (p < 0.001), had higher levels of white blood cells (p < 0.001) and D-dimer (p = 0.026), and a higher incidence of no-reflow (p = 0.001).
In this study, the patients were divided into two groups for further analysis depending on the presence or absence of T2DM: 167 (24.8%) patients with T2DM and 507 (75.2%) patients without T2DM; the results are shown in Table 2. Patients with T2DM were male (p = 0.004), non-smokers (p < 0.001), had a higher triglyceride level (p = 0.012), and a higher incidence of no-reflow (p < 0.001).
The results of multivariate logistic regression analysis
For predicting no-reflow in the overall population, the multivariate logistic regression model, including variables such as age, sex, smoking status, T2DM, and low eosinophil count, showed that low eosinophil count was an independent predictor for no-reflow in the overall population (adjusted OR: 2.012, 95% CI 1.242–3.259, p = 0.004). Variables such as smoking status, T2DM, and hemoglobin level were independent factors for no-reflow (Table 3).
In patients with T2DM, the variables in the multivariate logistic regression model were smoking status, history of hypertension, and low eosinophil count. The results revealed that low eosinophil count was an independent predictor of no-reflow in the T2DM population (adjusted OR: 4.312, 95% CI 1.878–9.900, p = 0.001) (Table 3).
In patients without T2DM, the variables in the multivariate logistic regression model were age, smoking status, sex, and hemoglobin level. The results revealed that hemoglobin, but not low eosinophil count, was an independent predictor of no-reflow in patients without T2DM (adjusted OR: 0.972, 95% CI 0.955–0.990, p = 0.002) (Table 3).
The results of ROC curves
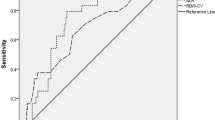
ROC curves were generated to evaluate the potential predictive power of low eosinophil count for no-reflow (Fig. 2). The results of the ROC curve analysis revealed that a low eosinophil count had moderate predictive efficiency for predicting no-reflow in patients with T2DM, and the power was superior to all populations and patients without T2DM. The performances of low eosinophil count in predicting no-reflow are shown in Table 4. DeLong’s test was used to compare the area under curves (AUCs) of the three ROC curves. It showed that there was no significant difference between the three ROC curves (Table 5).
Discussion
In this retrospective study, eosinophil count was an independent predictor of no-reflow in patients with STEMI who underwent pPCI. In the subgroup of patients with T2DM, eosinophil level was an independent risk factor for no-reflow, but not in patients without T2DM.
An increasing number of studies have demonstrated the value of plasma eosinophil counts in patients with CAD. However, the role of eosinophils in CAD remains unclear. Gao et al.20 revealed that the percentage of eosinophils was lower in patients with CAD, especially in those with AMI. Furthermore, low eosinophil count was strongly associated with severe CAD and acute coronary arterial thrombotic events. Jiang et al.23 showed that patients with AMI presenting with decreased eosinophil counts had serious myocardial damage. This study indicated that eosinophils play an important role in thrombosis in patients with CAD. Data from the CALIBER study showed a strong correlation between low eosinophil count, HF, and death24. In a prospective series of 620 patients with STEMI, lower minimum eosinophil counts were associated with more extensive edema, microvascular obstruction, infarct size, and a higher rate of cardiac events (death, reinfarction, or heart failure) during follow-up25. In a recent animal study, an increase in heart and blood eosinophils post-MI represented a compensatory mechanism to protect the heart from ischemic injury26. Furthermore, genetic and pharmacological eosinophil depletion leads to increased adverse remodeling in experimental AMI27. These results indicate that a higher eosinophil count is protective in patients with CAD. However, other studies have suggested a destructive effect of eosinophils on CAD. Increased eosinophils were an independent predictor of death in 8943 consecutive patients with triple-vessel CAD after a median of 7.5 years of follow-up28. A high preprocedural eosinophil count was associated with improved outcomes within the first 6 months; however, after this period, there was an increased risk of mortality29. Therefore, the value of eosinophils in CAD remains controversial.
However, the association between eosinophil levels and no-reflow was not explored in the studies above. Briefly, the present study results followed the findings of previous studies, and our study is the first to reveal an association in the pPCI population and further elucidate the T2DM-related difference in plasma eosinophil count predicting no-reflow. Further analysis with a larger population sample might help clarify the role of plasma eosinophil count in patients with CAD, especially in predicting no-reflow. A previous study showed that eosinophils contribute to atherosclerotic plaque formation and thrombosis through their interplay with platelets. Additionally, they found high numbers of eosinophils in coronary artery thrombi, and female patients with stent thrombosis had the highest eosinophil counts19. Therefore, we speculated that the eosinophil count was decreased in the peripheral blood. Mechanisms that explain the value of eosinophil counts in predicting the incidence of no-reflow in patients with STEMI are warranted. In our study, DeLong’s test show that there was no significant difference between the three ROC curves. This may be due to the small sample size. Multi center research with large sample size is needed in future research.
This study has several limitations. This was a single-center study, and plasma eosinophil count was not dynamically monitored. Moreover, our study did not collect data such as the total ischemic time, culprit lesion characteristics, and procedural details. Finally, further studies are required to better understand the pathophysiological role of eosinophils and to explore the potential therapeutic implications.
Conclusions
In this retrospective cohort study, a decreased plasma eosinophil count was an independent risk factor for no-reflow in patients with STEMI who underwent pPCI, especially in T2DM patients. This analysis highlighted the importance of eosinophil count and guided clinicians in identifying patients at a high risk of developing no-reflow and lowering their risk.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- STEMI:
-
ST-segment elevation myocardial infarction
- pPCI:
-
Primary percutaneous coronary intervention
- T2DM:
-
Type 2 diabetes mellitus
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curves
- OR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- AMI:
-
Acute myocardial infarction
- MACE:
-
Major adverse cardiac events
- CRP:
-
C-reactive protein
- WBC:
-
White blood cell count
- NLR:
-
Neutrophil-to-lymphocyte ratio
- CAR:
-
CRP/albumin ratio
- CAD:
-
Coronary artery disease
- TIMI:
-
Thrombolysis in myocardial infarction
- TG:
-
Total glyceride
References
Niccoli, G. et al. Optimized treatment of ST-elevation myocardial infarction. Circ. Res. 125(2), 245–258 (2019).
Jneid, H. et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 70(16), 2048–2090 (2017).
Mehta, S. R. et al. Complete revascularization with multivessel PCI for myocardial infarction. N. Engl. J. Med. 381(15), 1411–1421 (2019).
Niccoli, G., Kharbanda, R. K., Crea, F. & Banning, A. P. No-reflow: again prevention is better than treatment. Eur. Heart J. 31(20), 2449–2455 (2010).
Rezkalla, S. H., Stankowski, R. V., Hanna, J. & Kloner, R. A. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc. Interv. 10(3), 215–223 (2017).
Ashraf, T. et al. Clinical and procedural predictors and short-term survival of the patients with no reflow phenomenon after primary percutaneous coronary intervention. Int. J. Cardiol. 294, 27–31 (2019).
Bouleti, C., Mewton, N. & Germain, S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 108(12), 661–674 (2015).
Niccoli, G., Burzotta, F., Galiuto, L. & Crea, F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 54(4), 281–292 (2009).
Jaffe, R., Dick, A. & Strauss, B. H. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: A systematic approach. JACC Cardiovasc. Interv. 3(7), 695–704 (2010).
Eeckhout, E. & Kern, M. J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 22(9), 729–739 (2001).
Kurtul, A. & Acikgoz, S. K. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients With ST-segment elevation myocardial infarction. Am. J. Cardiol. 120(4), 534–541 (2017).
Balta, S. et al. The relation between monocyte to HDL ratio and no-reflow phenomenon in the patients with acute ST-segment elevation myocardial infarction. Am. J. Emerg. Med. 34(8), 1542–1547 (2016).
Kotani, J. et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation 106(13), 1672–1677 (2002).
Wagdy, S., Sobhy, M. & Loutfi, M. Neutrophil/lymphocyte ratio as a predictor of in-hospital major adverse cardiac events, new-onset atrial fibrillation, and no-reflow phenomenon in patients with ST elevation myocardial infarction. Clin. Med. Insights Cardiol. 10, 19–22 (2016).
Karabag, Y. et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur. J. Clin. Invest. 48(6), e12928 (2018).
Gao, S. et al. Allergic asthma aggravated atherosclerosis increases cholesterol biosynthesis and foam cell formation in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 519(4), 861–867 (2019).
Niccoli, G. et al. Allergic inflammation is associated with coronary instability and a worse clinical outcome after acute myocardial infarction. Circ. Cardiovasc. Interv. 8(8), e002554 (2015).
Niccoli, G., Montone, R. A., Sabato, V. & Crea, F. Role of allergic inflammatory cells in coronary artery disease. Circulation 138(16), 1736–1748 (2018).
Marx, C. et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 134(21), 1859–1872 (2019).
Gao, S. et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 286, 128–134 (2019).
Ali, D., Snead, D., Dhakshinamurthy, V. A. & Banerjee, P. Rise and fall of the eosinophils in heart failure: A rare but important phenomenon seen with cardiomyopathy. BMJ Case Rep. 2018, bcr-2017 (2018).
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39(2), 119–177 (2018).
Jiang, P., Wang, D. Z., Ren, Y. L., Cai, J. P. & Chen, B. X. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron. Artery Dis. 26(2), 101–106 (2015).
Shah, A. D., Denaxas, S., Nicholas, O., Hingorani, A. D. & Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A CALIBER cohort study. Open Heart 3(2), e000477 (2016).
Rios-Navarro, C. et al. Characterization and implications of the dynamics of eosinophils in blood and in the infarcted myocardium after coronary reperfusion. PLoS ONE 13(10), e0206344 (2018).
Liu, J. et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 11(1), 6396 (2020).
Toor, I. S. et al. Eosinophil deficiency promotes aberrant repair and adverse remodeling following acute myocardial infarction. JACC Basic Transl. Sci. 5(7), 665–681 (2020).
Zhao, X. et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur. J. Prev. Cardiol. 26(8), 872–882 (2019).
Toor, I. S., Jaumdally, R., Lip, G. Y., Millane, T. & Varma, C. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb. Res. 130(4), 607–611 (2012).
Funding
This work was supported by (1) School-level scientific research projects in 2021: Tracheal intubation training system based on virtual simulation technology (2021LZYK06); (2) School-level scientific research projects in 2021: Nasal feeding teaching system based on virtual simulation technology (2021LZYK05); and (3) National Vocational Education Teacher Teaching Innovation Team Project Research Project (YB2020130103).
Author information
Authors and Affiliations
Contributions
T.L. conceived and designed the study; C.W. performed the study; J.L. help to the study; D.M. analyzed the data and draft the paper; All authors read, critically revised the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mo, DG., Wang, CS., Liu, JH. et al. The predictive value of eosinophil levels on no-reflow in patients with STEMI following PCI: a retrospective cohort study. Sci Rep 12, 17862 (2022). https://doi.org/10.1038/s41598-022-22988-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-22988-2