Abstract
Spinach has been suggested as a potential rotation crop for increasing crop yield by enhancing beneficial fungal microbes in continuous monocropping. However, no research on the use of spinach as a green manure has been reported. Thus, we tested the effects of spinach and Korean mustard cultivars (green and red mustards) (10 g pot −1) as green manure on soil chemical properties, pepper productivity, and soil microbiome of long-year pepper-monocropped soil. Spinach improved the soil nutrition (e.g., pH, SOM, TN, NH4+, and K), weed suppression, and pepper growth. Spinach had by far the highest fruit yield, over 100% pepper fruit yield increment over the mustard green manures and control. Our study showed that the major influencing factors to cause a shift in both bacterial and fungal community assemblies were soil pH, TC TN, and K. Following green manure amendment Bacillota, especially Clostridium, Bacillus and Sedimentibacter, were enriched, whereas Chloroflexi and Acidobacteriota were reduced. In addition, spinach highly reduced the abundance of Leotiomycetes and Fusarium but enriched Papiliotrema. FAPROTAX and FUNGuild analysis revealed that predicted functional profiles of bacterial and fungal communities in spinach-amended soil were changed. Spinach-treated soil was differentially abundant in function related to hydrocarbon degradation and functional guilds of symbiotrophs and ectomycorrhizal. This study contributes significantly to our understanding of how the soil fertility and soil microbiome alteration via spinach green manure application as a pre-plant soil treatment might help alleviate continuous cropping obstacles.
Similar content being viewed by others
Introduction
Continuous monocropping is a modern agricultural practice in many parts of the world to increase yield on limited land1,2,3. To achieve and maintain high yields and economic benefits, high-value crop production is managed intensively year-round by applying high input chemical fertilizers and agricultural pesticides2,3. However, as a result of these practices, the deterioration of soil quality year after year as a result of soil acidification and imbalance of soil nutrient and the microbiome4, which ultimately affect plant growth and causes continuous cultivation obstacles, has raised concerns about the sustainability of agroecosystems5,6. Similarly, soil nutrient imbalance and soil contamination are serious soil threats in South Korea, for which the government has devised action plans for sustainable soil management7. Several measures have been proposed to overcome continuous cultivation obstacles, including crop rotation, chemical fumigation, soil solarization, and organic amendment8,9,10.
Green manuring is an eco-friendly agricultural practice that improves soil fertility and crop productivity while alleviating impediments to continuing cultivation1,11. The use of green manures is widely practiced as a sustainable agricultural soil management option because it improves the biological, physical and chemical properties of soil11,12,13. Green manures scavenge nutrients from the soil, prevent nutrient leaching, and slowly release the nutrients that they have absorbed and locked in during decomposition14. Incorporating green manures into the soil increases organic matter in the soil, which improves soil structure and fertility, allowing for better plant growth15. In addition, many beneficial microbes that play a major role in soil nutrient cycling, soil health, and crop productivity have been found to be stimulated by incorporating green manures into the soil13,16,17. The modification of soil nutrients, which ultimately alter soil microbe growth and colonization is partly responsible for the change in soil microbial community structure following addition of green manure18,19,20. For instance, after green manuring, a nutrient-rich environment favors copiotrophs4,21,22. while a similar environment discourages slow-growing oligotrophic bacteria23. The strong positive link between the alteration of soil microbial community and suppression of soil-borne pathogens24. suggests that green manure not only improves soil nutrition but also enriches beneficial microbes with bio-control potential17. However, depending on the type of green manure utilized, the efficacy of green manuring might vary greatly25.
Spinach, a cool-season vegetable that matures quickly, has been suggested as a potential rotation crop for increasing cucumber yield by enhancing beneficial fungal microbes in continuous monocropping26. Nevertheless, to our knowledge, there has not been any recorded research on the usage of spinach as a green manure. Furthermore, Brassica species, when used as a green manure, also contain glucosinolates (GSLs), which are hydrolyzed into isothiocyanates and become toxic to soil-borne pests and weeds. Thus, GSL-containing brassicas, such as mustard cultivars, would have better effects on green manuring to effectively alleviate the continuous cultivation obstacles27. Nevertheless, the effect of green manuring with Korean mustard cultivars (green and red mustard) and spinach on the productivity of chili pepper (Capsicum annum) and the taxonomic and functional diversity of the soil bacterial and fungal communities remains unknown. Chili pepper is a highly profitable crop grown in many parts of the world, including South Korea. However, a recent study found that long-term pepper monoculture made the soil more acidic, causing a significant effect on soil microbial communities28. Given that spinach matures quickly, grows in autumn and spring when pepper cultivation does not overlap, and is also nutrient-rich with the potential to increase soil suppressiveness and plant productivity through soil microbiota modification26, we hypothesize that spinach would be a suitable alternative to other green manures for tackling soil sickness brought on by long-term monocropping. Thus, we aimed to the investigate the effects of spinach and Korean mustard cultivars as green manures on soil chemical properties, weed suppression, pepper productivity, and soil microbiome.
Results
Effect of green manures on soil chemical properties, weed emergence and pepper performance
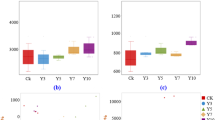
The impact of green manures on soil chemical properties is shown in Table 1. Although the soil in all treatments was initially taken from a single composite soil sample, the addition of green manures significantly (p ≤ 0.05) increased soil pH, NH4+, and K, but not AP compared to the non-amended control. Spinach also strongly increased EC, SOM, and TN content when compared to control. The highest NO3− and K contents were recorded in green mustard- and spinach-amended soils, which were 1.95- and 2.8-times higher than those in control, respectively. Furthermore, green mustard showed the highest C:N ratio while spinach had the lowest. The highest Overall, the nutritional status of the soil was improved by green manures.
The addition of green manures had a remarkable effect on weed emergence reduction and pepper productivity (Table 2). Spinach and green mustard incorporation showed a significant reduction in the emergence of weed populations, particularly monocots, compared with control. In addition, similar to the results of soil nutritional status, control showed the lowest pepper fruit yield whereas spinach had by far the highest fruit yield, over 100% yield increment over control and mustard cultivars. Similarly, spinach improved pepper growth, including plant height, stem diameter, chlorophyll content, canopy diameter, and primary branch diameter. Green mustard also significantly (p ≤ 0.05) increased pepper growth compared to control, but showed insignificant (p > 0.05) differences in terms of fruit yield.
Changes in soil microbial diversity and composition structure after green manuring
Alpha diversity indices of bacterial and fungal communities were estimated for all green manure amendments, as indicated in Fig. 1a–d and Table S1. Most diversity indices showed that green manuring had a strong negative effect on bacterial diversity than on fungal diversity. Red mustard significantly (p ≤ 0.05) increased fungal diversity compared to control and other treatments. Spinach containing no GSL, however, remarkably reduced microbial diversity, implying that the impact of green manuring on soil microbial diversity is highly dependent not only on the type of GSL content but also on other nutritional aspects of the type of green manures.
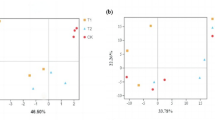
Changes in soil microbial diversity and community structure after green manuring. Alpha diversity of bacteria (a,c) and fungi (b,d) in green manure treatments measured by observed and Shannon indices. Mean values (n = 3) followed by different letter (s) in each parameter represent significant differences at p ≤ 0.05, DMRT test. Principal coordinate analysis (PCo1 and PCo2) of bacteria (e) and fungi (f) communities based on Bray–Curtis distance. Taxonomic composition (> 0.1%) of bacterial (g) and fungal communities (h).
Green manures had a remarkable impact on the taxonomic composition and structure of bacterial and fungal communities (Fig. 1e,f, Table S2). In comparison to control, all green manures significantly (p ≤ 0.05) enriched Bacillota population, while Acidobacteriota and Chloroflexi abundances were greatly reduced (Fig. 1g, Table S2). Bacteroidota abundance was also slightly elevated with green manure amendments. At the class level, Clostridia was the dominant class at all green manure-amended soils, whereas, in control, it was rare group. Spinach also highly reduced the relative abundance of Acidobacteriae (Fig. 1g). Ascomycota was the dominant phylum in the fungal community, accounting for more than 90% of the total across all treatments (Fig. 1h, Table S2). With the exception of spinach, fungal family Chaetomiaceae dominated the phylum Ascomycota. On the other hand, Stachybotryaceae was the most abundant family in spinach-amended soil. Among the other phyla, Basidiomycota, particularly Rhynchogastremataceae, increased in relative abundance with spinach application (Fig. 1h).
Differential abundant taxa after green manuring
Potential microbial biomarkers following green manuring were identified using four differential abundance testing tools, namely metastat, metagenomeSeq, LEfSe analysis, and the random forest model (Fig. 2, Supplementary file 2). We identified 160 bacterial and 35 fungal taxa as the key taxa that were differentially abundant between the treatment groups. Significant number of members from p_Acidobacteriota and p_Chloroflexi, such as c_Ktedonobacteria and o_Gaiellales, were reduced in green manured soil compared to control. On the other hand, members of Bacillota including Clostridium and Bacillus were the most highly stimulated genera in green manure-amended soils and were consistently detected by all the microbiome differential abundance methods (Fig. 2a,c, Supplementary file 2). In addition, several members of f__Sphingomonadaceae, and o__Xanthomonadales, such as Luteimonas and Sphingomonas were considerably more abundant in spinach-treated soil when compared to control and the other mustard green manures. On the other hand, Sedimentibacter was found especially abundant only in mustard-amended soils.
Analysis of differentially abundant taxa after green manuring. Linear discriminatory analysis Effect size (LEfSe) analysis of differentially abundant bacterial (a) and fungal (b) taxa among the green manures. Random forest graph displaying the most predictive bacterial (c) and fungal (d) taxa indicators after green manuring. Taxon names are abbreviated as p: phylum, c: class, o: order, f: family, g: genus. s: species.
In the fungal community, most tools used in the current study indicated members of Rhynchogastremataceae, such as Papiliotrema were the most markedly enriched fungal genera in spinach whereas Chaetomium and Fusarium were most reduced by the same treatment (Fig. 2, Supplementary file 2). This implies that the stated genera can be considered key fungal biomarkers for spinach. The relative abundance of f_Aspergillaceae and Emericellopsis were enriched in red mustard amendment. Furthermore, most biomarker detection tools identified Chaetomium, Fusarium, and c_Leotiomycetes as the differentially abundant taxa in control (Fig. 2b,d).
Relationships between soil chemical properties and soil microbial communities
The impact of soil chemical properties changes following green manuring on microbial community structure (bacteria and fungi) was determined using the Mantel test (Table S3). Soil pH, K, TN, and TC were significantly (p ≤ 0.05) correlated with both bacterial and fungal community assemblies (Fig. 3, Table S3). Furthermore, RDA analysis exhibited that the soil chemical properties explained 46.9 and 79.0% of the total bacterial and fungal variation, respectively (Fig. 3a,b). The first two RDA components separated the bacterial and fungal communities in the treatments into three clusters. Bacterial and fungal communities of mustard cultivars-treated soils were clustered together and were separated from control and spinach-treated soils. In the case of bacterial alpha diversity, K, pH and NH4+ were significantly (p ≤ 0.05) negatively correlated with almost all indices of bacterial diversity, whereas soil AP showed a significant positive correlation (Fig. 3c). On the other hand, soil EC was significantly (p ≤ 0.05) negatively correlated with fungal diversity (Fig. 3d). Spearman correlation analysis at the phylum level also showed that Acidobacteriota, Nitrospirota and Armatimonadota had a significant (p ≤ 0.05) negative correlation with soil pH, NH4+ and K but a positive correlation to AP (Fig. 3e). Basidiomycota and Ascomycota showed an inverse relationship with soil chemical properties, including the SOM (Fig. 3f).
Distance-based redundancy analysis (dbRDA) exhibiting the relationship between soil chemical properties and microbial communities of green manure treatments based on Bray–Curtis distance similarities: bacteria (a) and fungi (b). The contribution of each soil chemical properties to the soil microbial community structure variation was indicated by the length of arrows. Spearman correlation between soil chemical properties with alpha diversity indices and phylum relative abundance of bacteria (c,e) and fungi (d,f). Refer Table 1 for the abbreviation of soil chemical properties.
The SEM analysis also demonstrated that changes in soil chemical properties had a significant (p < 0.05) impact on the soil microbiota (Fig. S1). Furthermore, a SEM analysis was performed to determine whether changes in the soil chemical properties affected pepper yield directly or indirectly (through microbiota shift). The results showed that the model was fit and that soil chemical properties, particularly TN and K alteration, had a greater impact pepper fruit yield than soil microbiota (Fig. S1).
Functional diversity after green manuring
According to FAPROTAX analysis, a total of 41 predicted bacterial functions were identified from all treatments, with chemoheterotrophy and aerobic chemoheterotrophy being the most functionally redundant predicted functions. Bacterial communities in spinach-amended soil were clustered separately based on their predicted functional profiles (Fig. 4a). LEfSe analysis was performed to identify bacterial functions highly associated with green manures. Among the treatments, spinach-treated soil was differentially abundant in function related to hydrocarbon degradation, while the same treatment had the lowest predicted abundance of nitrate respiration, nitrogen respiration, predatory/exoparasitic, photoheterotrophy, and phototrophy (Fig. 4a). Furthermore, spinach had no predicted denitrification function, whereas green mustard treated-soil showed the highest rates of nitrate and nitrogen respiration. Control, however, showed the lowest abundant functions of anaerobic chemoheterotrophy (Fig. 4a,b). Further correlation analysis showed that anaerobic chemoheterotrophy, fermentation and hydrocarbon degradation were strongly positively associated (p ≤ 0.05) with soil pH and K. The soil C:N ratio was positively associated with predicted cellulolysis function. Nitrite and nitrate denitrification showed a significant negative correlation (p ≤ 0.05) with K. Soil EC increased the process of functions related to chemoheterotrophy but not with sulfur, fumarate, or manganese respiration (Fig. S2a).
FUNGuild is used to predict changes in the ecological functions of the fungal communities following green manuring. The results showed that the most dominant functional guilds in all treatments were saprotrophs (Fig. 5a). The functional guilds of symbiotrophs and ectomycorrhizal were positively impacted by spinach green manure; however, soil amendments with mustard cultivars did not enhance such predicted functions (Fig. 5b,c). These functions are beneficial to plants because symbiotrophs and ectomycorrhizal fungi make beneficial relationship with plants. Furthermore, some of the predicted functions, including symbiotroph were significantly (p > 0.05) associated with soil EC (Fig. S2b).
Predicted ecological functions of soil fungal communities in different green manure-amended soils. Heatmap depicting the predicted ecological functions of soil fungal communities in different green manures based on the FUNGuild database (a). Spinach-amended soil enriched with symbiotroph (b) and ectomycorrhizal (c) predicted functions when compared to other green manures and control.
Discussion
Soil acidification, soil nutrient depletion, weeds, and soil-borne diseases are major problems in the continuous monocropping of intensive cultivation production systems28,29. Thus, alternative solutions are required to tackle these problems5. Green manuring is an eco-friendly traditional agricultural practice that improves soil fertility and crop productivity while alleviating impediments to continuing cultivation1,11,30. Our study showed that the incorporated spinach green manures resulted in an improvement in soil nutritional conditions (e.g. pH, SOM, TN, NH4+, and K). Previous reports noted that increased ammonification may contribute to the pH increase in green manure-amended soils31. Increased mineralization of organic matter, as seen in spinach with the lowest C:N ratio, enhances the hydroxyl group consumption of H+, increasing soil pH32. The low pH soil condition might cause high soil AP because of the higher solubility of AP in acidic soil conditions33. Thus, addressing the problem of soil acidification would help improve nutrient balance in soil-degraded monocropping agroecosystems32. In addition, increased nutrient availability in the green manure-amended soil was observed in our study. Previous studies have revealed that soil nitrogen and exchangeable potassium, following green manuring were the determinant factors for yield enhancement, which is consistent with our work30.
Weeds are a major crop production constraint that increases production costs, which necessitates efficient and sustainable weed control. The most effective green manure for suppressing weed populations in our study was spinach followed by mustard. This is consistent with previous reports on weed suppression by various green manures33. Even while it is predicted that soil amendment with brassicas will suppress weeds34, it was interesting to find that spinach, a non-brassica with no GSL (Table S4), had the highest weed suppression effect. Notably, high fermentation and hydrocarbon degradation (as seen in FAPROTAX predicted function, Fig. 4b) by the presence of abundant Clostridium may increase the conversion of carbohydrates to organic acids, which may aid in the suppression of weed growth35. Nevertheless, further research is needed to determine why spinach is effective in weed control. Furthermore, spinach had by far the highest fruit yield among green manures, with a yield increase of more than 100% over control. Including spinach in crop rotation increased cucumber yield considerably by increasing beneficial microbes, according to a recent study26. The government of South Korea has developed action plans to address challenges with monocropping through sustainable soil management programs7. Our study thus raises prospect of encouraging the use of spinach as a green manure preplant soil treatment in the pepper growing regions.
Changes in soil mineral composition following agricultural practices are known to cause a shift in soil microbial community structure and functional diversity30,36,37. This can lead to improved crop yield owing to enhanced soil suppressiveness, nutrient cycling, and availability38,39 The shift in soil bacterial and fungal community structure after green manure amendment18,19 has been previously reported partly because the incorporated substrate modifies the soil nutrient for soil microbe growth and colonization20. Our results supports the previous studies that soil pH, K, and TN are major influencing factors in both bacterial and fungal community assemblies30. Soil pH, which is associated with cation release during decomposition40, is an important factor that markedly influences the bacterial and fungal community structure assembly41. Furthermore, the reduced microbial diversity following green manuring can be linked to intra- and inter-kingdom competition caused by changes in soil chemical properties30,42,43. In our study, where green manures altered soil nutritional status, bacterial diversity was significantly negatively associated with NH4+. Previous research has also found that soil bacterial diversity is highly negatively associated with N application23. Green manuring with low GSL-containing red mustard cultivar had higher microbial diversity than green mustard cultivar with high GSL content. Although GSL is known to have negative effect on microbial diversity, more research is required to confirm it34.
Our results support previous reports that many members of Bacillota, including Clostridium, Bacillus and Sedimentibacter, were enriched in response to green manure soil amendments. Bacillota are copiotrophs in which substrate-amendment enhances nutrient-rich environment for their growth4,21,22. The genus Clostridium are diazotrophs capable nitrogen fixation10,44 and the production of toxic organic acids that could suppress soil-borne pathogens and weeds29,45,46. On the other hand, Chloroflexi and Acidobacteriota were reduced with green manure addition. These findings comply with the previous studies that such oligotrophic bacteria are adapt to low available soil nutrients23 and low pH conditions3,20,47. Chloroflexi are often strongly associated with low crop productivity3,48, and some studies label them as disease inducible49. In addition, the majority of Chloroflexi members do not fix nitrogen; instead, they compete with other beneficial microbes and the host plant itself for nitrogen resources4,50. Basidiomycota and Ascomycota had an inverse relationship with soil chemical properties as reported in the previous study51. The positive correlation between Basidiomycota and soil organic matter supports the previous report that Basidiomycota are the primary decomposers of soil debris51. Fusarium was differentially more abundant in control as opposed to the spinach-amended soil. Fusarium is serious soil-borne pathogens that affect a variety of crops, including peppers, and are well adapted to pepper monoculture24,52. The reduction in Fusarium abundance with organic amendment complies with the previous finding24, suggesting the potential of spinach as green manure for the suppression of Fusarium-incited diseases. Furthermore, the enrichments of beneficial soil fungi, such as Papiliotrema53,54, that have the potential to engage in biocontrol activities following spinach amendment shows that spinach as green manure not only improves soil nutrition but also promotes resident soil microbes with biocontrol potential to flourish.
In summary, spinach improved soil nutrition (e.g., pH, SOM, TN, NH4+, and K), pepper growth, pepper fruit yield and suppressed weed population. Green mustard also increased soil nutrition and suppressed weed growth but had no significant effect on pepper yield. The major influencing factors in both bacterial and fungal community assemblies were soil pH, TC, TN, and K. All green manures highly stimulated members of Bacillota, including Clostridium and Bacillus. Spinach also highly reduced the abundance of members of Acidobacteriota and Chloroflexi while enriching fungal members of Rhynchogastremataceae, such as Papiliotrema. Overall, spinach outperformed other treatments in terms of weed control and yield improvement, whereas red mustard exceeded for positive effect on soil fungal diversity. This study contributes significantly to our understanding of how the soil microbiome and soil fertility alteration via green manure application as a pre-plant soil treatment might help alleviate continuous cropping obstacles.
Materials and methods
Materials, study design and sampling
Seeds of mustard cultivars (green and red mustard) were acquired from the National Institute of Crop Science (NICS), Rural Development Administration, South Korea. Spinach seeds were obtained from Jeilseed Company in Doan-myeon, Chungcheongbuk-do, South Korea. Seeds of these Brassica cultivars and spinach were planted in a polyhouse at Kyungpook National University, South Korea, and plant biomass was collected two months after planting. The soil for this study pot experiment was collected in January 2021 from a long-year pepper-monocropped soil in Gunwi-gun, Gyeongsangbuk-do province, South Korea (36°10′09′′N,128°38′24′′E), whose productivity had declined substantially (Fig. S3). The soil was sieved through an 8-mm sieve and completely homogenized. The initial soil chemical properties are indicated in the Table S5.
The fresh harvested biomass of green manures, which contained a variable range of total GSL concentrations (Table S4), was mix homogeneously and separately with the soil at 0.5% (w/w) on a dry weight basis. Soil with no green manure amendment served as the non-amended control. The soil from each treatment group was placed in plastic containers with three replicates. Each treatment, including control, was watered (sterile distilled water) to 70% field capacity and covered for 30 days (Start date: June 6, 2021 End date: February 5, 2021) with a plastic transparent polythene film in the polyhouse. The polythene film was uncovered and the soil was air-drained for 60 days (Start date: February 5, 2021 End date: April 9, 2021). Pots (15 cm diameter, 31 cm height, aerated with holes at the bottom) were filled with 2 kg green manure-amended soil (10 g pot −1). One pepper (cultivar Dongmudae) seedling, one-month-old, was transplanted into each pot (April 9, 2021). Four different treatments were used in the current study: control, spinach, red mustard and green mustard. All treatments were replicated three times and laid out in a completely randomized experimental design, with each replicate containing five pots (15 pots per treatment). Pepper plants were grown in a polyhouse for three months (Start date: April 2021 End date: July 2021) and watered twice a week. Soil samples for chemical property analysis and DNA extraction were collected after soil treatment immediately before transplantation. Soil samples were collected at three points within each pot and the samples were pooled to yield three pooled samples (replicates). The soil samples were kept at − 80 °C until the DNA extraction.
Soil chemical analysis
The soil chemical properties were analyzed from dried soil samples. Using a pH and EC meter (SP2000, Skalar BV, Netherlands), the electrical conductivity (EC) and pH of the soil were determined in a 1:5 (w/v) soil: deionized distilled water suspension. A titrando automatic titrator (Metrohm 888, Switzerland) was used to analyze soil organic matter (SOM). The BaCl2-H2SO4 exchange method55 was used to determine the soil cation exchange capacity (CEC). The ammonium-nitrogen (NH4+) nitrate-nitrogen (NO3−) concentration in the soil were measured colorimetrically by salicylate method56 and cadmium reduction method57, respectively, using BLTEC QuAAtro (BLTEC KK, Japan). The soil total nitrogen (TN) concentration was determined by the method described by Dumas58 with S832DR (Leco, USA). Soil exchangeable potassium (K) concentration was analyzed using a PerkinElmer® Optima 8300 ICP-OES (PerkinElmer, Inc., MA, USA). The soil available P2O5 (AP) concentration was analyzed using a SKALAR San + + system autoanalyzer (Skalar Analytical B.V., Breda, Netherlands).
Weed emergence and pepper performance
The reduction in the emergence counts of monocot and dicot weeds following green manure application was determined before pepper transplanting. Pepper growth parameters, such as plant height, stem diameter, primary branch length and diameter, and chlorophyll content, were measured at the end of the experiment (July 2021), three months after transplanting. Chlorophyll content (SPAD unit) was measured using a chlorophyll-meter (Konica Minolta, Japan). Fully shiny matured green fruits with over 5 cm were collected three times.
DNA extraction, library preparation and sequencing
The DNeasy® PowerSoil® Pro Kit (Qiagen, Hilden, Germany) was to used extract microbial DNA from soil samples (0.5 g) according to the manufacturer’s protocol. The extracted DNA quantity and purity were measured using a Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and NanoDrop™ OneC spectrophotometer (Thermo Fisher Scientific). The extracted DNA was stored at − 80 °C until it was used for Illumina MiSeq sequencing.
The fungal internal transcribed spacer 1 (ITS1) region and the bacterial V4-V5 hypervariable region of the 16S rRNA gene were PCR-amplified with ITS86F/ITS4R59,60 and the universal primers 515F/907R61, using an Eppendorf Mastercycler® Nexus PCR Cycler (Eppendorf, Hamburg, Germany). The 50 µl PCR reaction mixture included 25 µl EmeraldAmp® PCR Master Mix (Takara, Shiga, Japan), 1 µl DNA template, 1 µl (0.5 µM/µl) per primer, and 22 µl of double-distilled water. The PCR reaction conditions and primer sequences are shown in Table S6. The Nextera®XT Index Kit (Illumina, San Diego, CA, USA) was used to ligate the Illumina sequence adapters to the PCR products, according to the manufacturer’s protocol. The final PCR products were purified using AMPure XP beads (Beckman Coulter Life Sciences, CA, USA) and kept at − 20 °C until use. The size variation in the amplicon product was considered while pooling samples of both 16S rRNA and ITS2 indexed amplicons at equimolar concentration. The libraries were checked for their concentration and size using an Agilent Bioanalyzer (Santa Clara, Ca, USA), and the pooled library with a final loading concentration of 20 pM was sequenced using the Illumina MiSeq platform (Illumina) at Kyungpook National University’s NGS Core Facility Center in South Korea.
Bioinformatics analysis
Bacterial and fungal raw sequences were demultiplexed using the QIIME2 pipeline (https://qiime2.org), and the reads were denoised in QIIME2 using DADA2, and chimeric sequences and singletons were removed62. Reads were truncated, and the ones with quality scores of ≥ 25 were retained. Non-chimeric representative sequences that made up amplicon sequence variants (ASVs) were aligned using MAFFT63 and taxonomy was assigned using a classify-sklearn-based qiime feature-classifier trained on the reference SILVA 99% full-length database (version 138.1)64 and UNITE database (version 8.3)65 for bacteria and fungi, respectively. ASVs assigned as mitochondria, chloroplasts, and unclassified taxa at the kingdom level were excluded. The sample reads were rarefied to equal size to enable a similarity comparison between treatments. The normalized data set contained 1786 and 202 ASVs of bacteria and fungi, respectively. FAPROTAX, functional annotation of prokaryotic taxa, was used to predict the ecological functions of bacterial communities66,67,68. Fungal functional guild (FUNGuild)69 was used to predict the functional changes in fungal communities following BF treatment.
Statistical analysis
All downstream statistical data analyses were conducted using the R statistical software (v4.1.3)70. Data visualization was performed using different R packages: ggplot71 and ComplexHeatmap (neatmap v2.1.0)72. Homogeneity of variance and multivariate homogeneity of dispersion were checked using Levene’s test and PERMDISP73,74, respectively. The data normality assumption was tested using the Shapiro–Wilk test. ANOVA with Duncan’s multiple range test with dplyr package were used to compare the statistical difference between treatments in soil chemical properties, plant phenotype and alpha diversity indices (at ASVs-level). The overall statistical difference in microbial community composition between treatments was determined using permutational multivariate analysis of variance (PERMANOVA) (Adonis; vegan, version 2.5.7)75. The association between soil chemical properties and abundance of soil microbial communities was assessed using dbRDA in R. LEfSe76, metastat77, metagenomeSeq78, and Random forest79 in R were used to identify potential microbial biomarkers that were statistically differentially abundant between control and green manure-amended treatments.
Using the vegan and sem packages in R (v4.1.3)70, structural equation modeling (SEM) analysis was carried out to comprehend how a change in the soil chemical properties and microbial community following the addition of green manure effects pepper yield. Additionally, the of first PCOA values were served as a representation of the bacterial and fungal community structures in the SEM analysis80. Microbial diversity was a representation of bacterial and fungal diversities. Low chi-square (X2) value/degree of freedom (< 2), non-significant X2 test (p > 0.05), a low root mean squared error of approximation (RMSEA < 0.05), high comparative fit index (CFI > 0.9) and low standard root mean square residual (SRMR < 0.05) were used to determine the model’s fit.
Data availability
Under the PRJNA857858 BioProject, all raw sequences of bacteria and fungi are available at the NCBI Sequence Read Archive (SRA) repository (SRX Accessions SRX16121282-SRX16121301).
References
Xie, Z., Shah, F. & Zhou, C. Combining rice straw biochar with leguminous cover crop as green manure and mineral fertilizer enhances soil microbial biomass and rice yield in South China. Front. Plant Sci. 13, 1–12 (2022).
Zhang, H. et al. Accumulation, sources and health risks of trace metals in elevated geochemical background soils used for greenhouse vegetable production in southwestern China. Ecotoxicol. Environ. Saf. 137, 233–239 (2017).
Liang, B., Ma, C., Fan, L., Wang, Y. & Yuan, Y. Soil amendment alters soil physicochemical properties and bacterial community structure of a replanted apple orchard. Microbiol. Res. 216, 1–11 (2018).
Li, J. et al. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 195, 104366 (2019).
Sun, R., Zhang, X. X., Guo, X., Wang, D. & Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 88, 9–18 (2015).
Chen, D. et al. Acidic amelioration of soil amendments improves soil health by impacting rhizosphere microbial assemblies. Soil Biol. Biochem. 167, 108599 (2022).
Jeon, S. et al. Geoderma regional soil management priorities in Korea. Geoderma Reg. 29, e00516 (2022).
Tubeileh, A. M. & Stephenson, G. T. Soil amendment by composted plant wastes reduces the Verticillium dahliae abundance and changes soil chemical properties in a bell pepper cropping system. Curr. Plant Biol. 22, 100148 (2020).
Guerrero, M. del M. et al. Low temperature biodisinfection effectiveness for Phytophthora capsici control of protected sweet pepper crops in the Southeast of Spain. Front. Sustain. Food Syst. 5, 1–8 (2021).
Gao, Y. et al. Pepper-maize intercropping affects the occurrence of anthracnose in hot pepper. Crop Prot. 148, 105750 (2021).
Sharma, S. et al. Tillage, green manure and residue retention improves aggregate-associated phosphorus fractions under rice–wheat cropping. Sci. Rep. 12, 1–13 (2022).
Ansari, M. A. et al. Green manuring and crop residue management: Effect on soil organic carbon stock, aggregation, and system productivity in the foothills of Eastern Himalaya (India). Soil Tillage Res. 218, 105318 (2022).
Zhou, W. et al. The effect of organic manure or green manure incorporation with reductions in chemical fertilizer on yield-scaled N2O emissions in a citrus orchard. Agric. Ecosyst. Environ. 326, 107806 (2022).
He, H. B. et al. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass (Lolium multiflorum L.)-rice (Oryza sativa L.) rotation. Soil Tillage Res. 196, 104487 (2020).
Frøseth, R. B. et al. Effects of green manure herbage management and its digestate from biogas production on barley yield, N recovery, soil structure and earthworm populations. Eur. J. Agron. 52, 90–102 (2014).
Longa, C. M. O. et al. Soil microbiota respond to green manure in organic vineyards. J. Appl. Microbiol. 123, 1547–1560 (2017).
LeBlanc, N. Green manures alter taxonomic and functional characteristics of soil bacterial communities. Microb. Ecol. https://doi.org/10.1007/s00248-022-01975-0 (2022).
Zhou, X. et al. Control of Fusarium wilt of Lisianthus by reassembling the microbial community in infested soil through reductive soil disinfestation. Microbiol. Res. 220, 1–11 (2019).
Zhao, J. et al. Reductive soil disinfestation incorporated with organic residue combination significantly improves soil microbial activity and functional diversity than sole residue incorporation. Appl. Microbiol. Biotechnol. 104, 7573–7588 (2020).
Ye, G. et al. Manure application increases microbiome complexity in soil aggregate fractions: Results of an 18-year field experiment. Agric. Ecosyst. Environ. 307, 107249 (2021).
Li, T. et al. Changes in soil bacterial community structure as a result of incorporation of Brassica plants compared with continuous planting eggplant and chemical disinfection in greenhouses. PLoS ONE 12, e0173923 (2017).
Gao, W., Wang, L. & Jia, Z. Heterotrophy-coordinated diazotrophy is associated with significant changes of rare taxa in soil microbiome. Pedosphere 32, 402–413 (2022).
Wang, C., Liu, D. & Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 120, 126–133 (2018).
Ma, Y. et al. Impact of brassicaceous seed meals on the composition of the soil fungal community and the incidence of Fusarium wilt on chili pepper. Appl. Soil Ecol. 90, 41–48 (2015).
Dong, N. et al. Effects of green-manure and tillage management on soil microbial community composition, nutrients and tree growth in a walnut orchard. Sci. Rep. 11, 1–13 (2021).
Ali, A. et al. Cover plants-mediated suppression of Fusarium wilt and root-knot incidence of cucumber is associated with the changes of rhizosphere fungal microbiome structure-under plastic shed system of North China. Front. Microbiol. 13, 1–17 (2022).
Campanella, V., Mandalà, C., Angileri, V. & Miceli, C. Management of common root rot and Fusarium foot rot of wheat using Brassica carinata break crop green manure. Crop Prot. 130, 105073 (2020).
Chen, W., Guo, X., Guo, Q., Tan, X. & Wang, Z. Long-term chili monoculture alters environmental variables affecting the dominant microbial community in rhizosphere soil. Front. Microbiol. 12, 1–15 (2021).
Wang, T. et al. Rhizosphere microbial community diversity and function analysis of cut Chrysanthemum during continuous monocropping. Front. Microbiol. 13, 1–16 (2022).
Tao, J., Liu, X., Liang, Y., Niu, J. & Xiao, Y. Maize growth responses to soil microbes and soil properties after fertilization with different green manures. Appl. Microbiol. Biotechnol. https://doi.org/10.1007/s00253-016-7938-1 (2017).
Serrano-Pérez, P., Rosskopf, E., De Santiago, A. & del Carmen Rodríguez-Molina, M. Anaerobic soil disinfestation reduces survival and infectivity of Phytophthora nicotianae chlamydospores in pepper. Sci. Hortic. Amsterdam. 215, 38–48 (2017).
Wang, F., Wang, X. & Song, N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 315, 107425 (2021).
Sjursen, H., Brandsæter, L. O. & Netland, J. Effects of repeated clover undersowing, green manure ley and weed harrowing on weeds and yields in organic cereals. Acta Agric. Scand. Sect. B Soil Plant Sci. 62, 138–150 (2012).
Tagele, S. B., Kim, R. H. & Shin, J. H. Interactions between Brassica biofumigants and soil microbiota: Causes and impacts. J. Agric. Food Chem. 69, 11538–11553 (2021).
Tazawa, J. et al. Suppressive activity of volatile fatty acids and aromatic carboxylic acids on the germination of Monochoria vaginalis. Plant Prod. Sci. 24, 505–511 (2021).
Carson, J. K., Rooney, D., Gleeson, D. B. & Clipson, N. Altering the mineral composition of soil causes a shift in microbial community structure. FEMS Microbiol. Ecol. 61, 414–423 (2007).
Dai, Z. et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 14, 757–770 (2020).
Qi, G., Chen, S., Ke, L., Ma, G. & Zhao, X. Cover crops restore declining soil properties and suppress bacterial wilt by regulating rhizosphere bacterial communities and improving soil nutrient contents. Microbiol. Res. 238, 126505 (2020).
Bonilla, N. et al. Organic amendments to avocado crops induce suppressiveness and influence the composition and activity of soil microbial communities. Appl. Environ. Microbiol. 81, 3405–3418 (2015).
McCarty, D. G. et al. Field evaluation of carbon sources for anaerobic soil disinfestation in tomato and bell pepper production in Tennessee. HortScience 49, 272–280 (2014).
Jia, T., Guo, T., Yao, Y., Wang, R. & Chai, B. Seasonal microbial community characteristic and its driving factors in a copper tailings dam in the Chinese loess plateau. Front. Microbiol. 11, 1574 (2020).
Hu, X. et al. Long-term manure addition reduces diversity and changes community structure of diazotrophs in a neutral black soil of northeast China. J. Soils Sediments 18, 2053–2062 (2018).
Vida, C., de Vicente, A. & Cazorla, F. M. The role of organic amendments to soil for crop protection: Induction of suppression of soilborne pathogens. Ann. Appl. Biol. 176, 1–15 (2020).
Liao, H., Li, Y. & Yao, H. Biochar amendment stimulates utilization of plant-derived carbon by soil bacteria in an intercropping system. Front. Microbiol. 10, 1–13 (2019).
Huang, X. et al. Toxic organic acids produced in biological soil disinfestation mainly caused the suppression of Fusarium oxysporum f. sp. cubense. Biocontrol 60, 113–124 (2015).
Mazzola, M., Hewavitharana, S. S. & Strauss, S. L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 105, 460–469 (2015).
Eo, J. & Park, K. C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 231, 176–182 (2016).
Wang, L. et al. Long-term application of bioorganic fertilizers improved soil biochemical properties and microbial communities of an apple orchard soil. Front. Microbiol. 7, 1–12 (2016).
Niu, J. et al. The succession pattern of soil microbial communities and its relationship with tobacco bacterial wilt. BMC Microbiol. 16, 1–10 (2016).
Ren, G. et al. Soil bacterial community was changed after Brassicaceous seed meal application for suppression of Fusarium wilt on pepper. Front. Microbiol. 9, 1–15 (2018).
Fernandes, M. L. P. et al. Functional soil mycobiome across ecosystems. J. Proteom. 252, 104428 (2022).
Liang, Y., Gao, Y., Wang, R. & Yang, X. Fungal community characteristics and driving factors during the decaying process of Salix psammophila sand barriers in the desert. PLoS ONE 16, 1–17 (2021).
Palmieri, D. et al. Complete genome sequence of the biocontrol yeast Papiliotrema terrestris strain LS28. G3 Genes, Genomes, Genet. 11, 0–4 (2021).
Liu, Z., Li, X., Sun, Z., Wang, Z. & Li, G. Papiliotrema flavescens colonized in biochars inhibits wheat crown rot and Fusarium head blight. Biochar 3, 625–639 (2021).
Hendershot, W. H. & Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 50, 605–608 (1986).
Kempers, A. J. & Zweers, A. Ammonium deteminaation in soil extracts by salicylate method. Commun. Sol. Sci. Plant Anal. 17, 715–723 (1986).
Huffman, S. A. & Barbarick, K. A. Soil nitrate analysis by cadmium reduction 1. Commun. Sol. Sci. Plant Anal. 12, 79–89 (1981).
Saint-Denis, T. & Goupy, J. Optimization of a nitrogen analyzer based on the Dumas method. Anal. Chim. Acta. 515, 191–198 (2004).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. https://doi.org/10.1016/b978-0-12-372180-8.50042-1 (1990).
Pryce, T. M., Palladino, S., Kay, I. D. & Coombs, G. W. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med. Mycol. 41, 369–381 (2003).
Parada, A. E., Needham, D. M. & Fuhrman, J. A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 18, 1403–1414 (2016).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. https://doi.org/10.1093/nar/gks1219 (2013).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264 (2019).
Louca, S., Parfrey, L. W. & Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 353, 1272–1277 (2016).
Sansupa, C. et al. Can we use functional annotation of prokaryotic taxa (FAPROTAX) to assign the ecological functions of soil bacteria?. Appl. Sci. 11, 1–17 (2021).
Djemiel, C. et al. Inferring microbiota functions from taxonomic genes: A review. Gigascience 11, 1–30 (2022).
Nguyen, N. H. et al. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248 (2016).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2022).
Wickham, H. ggplot2: Elegant graphics for data analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4 (2016).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Oksanen, J. et al. Vegan: ccommunity ecology package. (2020).
Anderson, M. J. et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28 (2011).
Dixon, P. Computer program review VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
White, J. R., Nagarajan, N. & Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 5, e100352 (2009).
Paulson, J. N., Colin Stine, O., Bravo, H. C. & Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 10, 1200–1202 (2013).
Beck, D. & Foster, J. A. Machine learning techniques accurately classify microbial communities by bacterial vaginosis characteristics. PLoS ONE 9, e87830 (2014).
Sun, C., Liu, G. & Xue, S. Interaction between plant competition and rhizospheric bacterial community influence secondary succession of abandoned farmland on the loess plateau of China. Front. Plant Sci. 9, 1–12 (2018).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea (NRF-2020R1I1A3074522 and NRF-2022R1I1A3071893) and Korea Basic Science Institute (National Research Facilities and Equipment Center) (NRF-2021R1A6C101A416) financed by Ministry of Education, Republic of Korea. This research was also supported by a project to train professional personnel in biological materials by the Ministry of Environment.
Author information
Authors and Affiliations
Contributions
S.B.T., R.-H.K., and J.-H. S planned and designed the research study; S.B.T., R.-H.K., D-K.L., T-H.P., B.F.T., K-M.L., D.-R.J., Y.-J.P. and M.K. performed the research; S.B.T., R.-H.K., M-S.J., and J.-H.S. analyzed the data; S.B.T., R.-H.K., prepared figures and tables; S.B.T., R.-H.K., and J.-H. S wrote the main manuscript.
Corresponding author
Ethics declarations
The use of plants in our experimental research complies with all local, national, and international guidelines and legislations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, RH., Tagele, S.B., Jeong, M. et al. Spinach (Spinacia oleracea) as green manure modifies the soil nutrients and microbiota structure for enhanced pepper productivity. Sci Rep 13, 4140 (2023). https://doi.org/10.1038/s41598-023-31204-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-31204-8
This article is cited by
-
Combined use of green fertilizer and soil conditioner improves soil physicochemical properties, microbial community structure and tobacco quality in continuous cropping
Annals of Microbiology (2025)
-
The Application of Orychophragmus violaceus as a Green Manure Relieves Continuous Cropping Obstacles in Peanut Cultivation by Altering the Soil Microbial Community and Functional Gene Abundance
Journal of Soil Science and Plant Nutrition (2024)
-
Co-application of Green Manure and Trichoderma spp. Induced Plant Growth Promotion by Nutrient Improvement and Increased Fungal Biomass in Soil
Agricultural Research (2024)
-
The effect of winter crop incorporation on greenhouse gas emissions from double rice-green manure rotation in South China
Environmental Science and Pollution Research (2023)