Abstract
We aimed to perform superpixel segmentation of ocular blood flow maps obtained using laser speckle flowgraphy (LSFG) and investigate the effects of systemic parameters such as body weight, height, and sex on ocular blood flow resistivity. We studied 757 healthy participants (583 men, 174 women). We calculated the average beat strength over mean blur rate (BOM) as a LSFG resistivity index, as a function of age and sex using ordinary regions of interest (ROI) centered on the optic nerve head (ONH), the retinal vessels region and tissue around the ONH, and the choroid (CHD). We compared the ROI and superpixel-based methods, which are segmented based on image processing, for calculating the BOM. The sex differences in the BOM for the ONH, retinal-vessels region and tissue region of the ONH and CHD were significant for individuals aged ≤ 50 years (P < 0.01) but not those > 50 years old (P > 0.05). The average BOMs calculated using the ROI and superpixel methods were strongly correlated in the ONH (coefficient = 0.87, R2 = 0.8, P < 0.0001, n = 5465). In summary, a superpixel-segmented BOM map is suitable for two-dimensional visualization of ocular blood flow resistivity.
Similar content being viewed by others
Introduction
Laser speckle flowgraphy (LSFG) is a non-invasive and quantitative method1 for determining ocular blood flow2,3. We previously studied the relationship between blood flow resistivity and aging in healthy adults using LSFG4, with the aim of estimating age based on ocular blood flow measurement during a health check or complete physical examination. LSFG monitors the movement of erythrocytes in the retina, choroid (CHD)5, and optic nerve head (ONH), and the mean blur rate (MBR) is used as an indicator of ocular blood flow6,7. Several studies have revealed that age8,9,10,11 and sex8,9,10,11,12 are significantly correlated with the average MBR in the ONH. The change in MBR throughout the cardiac cycle is represented by waveform parameters such as blowout score (BOS)13,14,15,16,17,18 and blowout time (BOT)14,19,20,21,22,23,24,25, which are used to quantify age-dependent increases in resistivity8 and to assess the relationship between blood flow and resistivity26.
BOS and BOT partly reflect vascular resistance27,28, but their calculation requires averaging from separate images acquired for each heartbeat, and these parameters are thus strongly influenced by heart rate. Beat strength (BS) over the temporal average of the MBR (BOM) parameter, which does not depend on heart rate, was therefore devised to overcome this drawback. The BOM is an LSFG waveform parameter obtained from frequency analysis and is similar to the pulsatility index (PI) used in the Doppler method to detect the resistivity of blood flow in systemic vascular disease29,30. Like BOT and BOS18, the BOM parameter is a measure of vascular resistance, with the additional advantage of not requiring the detection of separating heartbeats. The BOM can also be applied to the relative flow volume (RFV) parameter31, to assess resistivity in retinal and choroidal vessel segments.
Vascular resistance is a key factor in the pathogenesis of retinal vein occlusion (RVO), which is a common retinal vascular disease32,33. It is important to identify the location of the vascular occlusion in patients with vascular occlusive diseases, such as central RVO (CRVO) and branch RVO (BRVO), but it is difficult to determine the location of the increased vascular resistance using blood flow maps alone. Until now, a single BOM value has been calculated from time-series blood flow values by drawing a region of interest (ROI), known as a ‘rubber band’, around the vessel with the suspected vascular occlusion34. This rubber band used in LSFG can be a rectangle, ellipse, or other shape. In fundamental analysis, the average values of the parameters within the ROI enclosed by the rubber band are calculated and used as representative values for that region. However, it would be clinically useful to automatically map the vascular resistance before the rubber band is placed, to enable the location of the vascular occlusion to be easily identified.
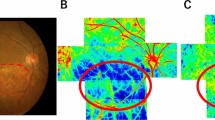
Recent improvements in image analysis software have made it possible to visualize superpixel-segmented BOM maps34 as blood flow maps (Fig. 1). The fully automated superpixel segmentation method reduces computation time by treating multiple pixels as a single cluster, which enables better visualization of blood flow35,36. Pixel-wise calculation and mapping of the BOM is possible but visually complex (Fig. 1A-2), time-consuming, and unsuitable for clinical use. For example, the pixel-wise computation time for one measured set of LSFG images used in this study was 15,795 s (4.4 h), compared with a corresponding computation time of 10 s using the superpixel method. In addition, superpixel segmentation attenuates the speckle noise in the LSFG blood flow map, making the structural boundaries clearer. However, one disadvantage is that the BOM values obtained using the superpixel method are higher than those obtained using the standard rubber band method, and the BOM thresholds for vascular occlusion obtained with the standard rubber band method in previous studies therefore cannot be used.
Example of superpixel-segmented BOM maps. Representative maps of average blood flow and resistivity (BOM) obtained using laser speckle flowgraphy and simple linear iterative clustering. Images show the same healthy subject at ages 40 and 48 years. The average blood flow maps show no discernable change (cf. panels B-1 and C-1), but the BOM maps show an age-dependent increase in retinal blood vessel resistivity and choroidal resistivity (cf. panels B-2 and C-2). (A-1) is the fundus image of the same eye. (A-2) is a pixel-wise BOM map. BOM, average beat strength over mean blur rate; y.o., years old.
Regarding the threshold of BOM for vascular occlusion, Matsumoto et al. reported that the total capillary resistance (TCR), defined as the BOM in the major vessels (arteries and veins) in the ONH, was higher for CRVO patients than for normal subjects, irrespective of sex and age37, while Yanagida et al. reported a sex-based difference between resistance values in normal subjects11. However, these reports did not elaborate on the association with systemic parameters such as body weight, height, or ocular perfusion pressure (OPP). The first aim of this study was to clarify the relationship between BOS and BOM as a measure of resistivity. The second aim was to assess the systemic parameter- and sex-based differences in BOM values in the ONH, retinal vessels, and CHD in healthy adults. The third aim was to visualize superpixel-segmented BOM maps by averaging their superpixel intensities and compare them with a threshold value, to detect blockages in patients. A clear relationship between the average BOM values of multiple superpixels and the BOM values obtained using the standard rubber band method would make it possible to determine by inspection if the BOM map was normal.
Results
Relationship between BOS and BOM
A total of 325 of the 1082 participants were excluded, leaving 757 participants (583 men, 174 women) who met the study criteria. A total of 514 participants were ≤ 50 years old (393 men, 121 women) and 243 were > 50 years old (190 men, 53 women). We used univariate regression analysis to determine the associations between BOS and BOM in the ONH (r = − 0.95, P < 0.001), retinal vessels (r = − 0.92, P < 0.001), the tissue surrounding the vessels (r = − 0.96, P < 0.001) and in the CHD (r = − 0.84, P < 0.001).
Characteristic of BOM in healthy adults based on systemic parameters
Table 1 shows the systemic parameters and BOM values for the entire ONH (BOM.A), CHD (BOM.CHD), retinal vessels (TCR37), and the tissue surrounding the vessels in the ONH (BOM.T) in relation to sex and age.
We performed multiple regression analysis to compare BOM parameters and sex, weight, height, and OPP between the two age groups (Table 2). Sex and OPP showed strong correlations (P < 0.001) with all BOM parameters in participants ≤ 50 years, and BOM.A and BOM.T were significantly correlated (P < 0.05) with weight and height. In participants > 50 years, TCR was correlated with OPP (P < 0.05) and was strongly correlated (P < 0.01) with weight, and BOM.A and BOM.T were correlated (P < 0.05) with weight.
Comparison of BOM values calculated using rubber band and superpixel methods
In this study, we attempted to acquire data three times per eye for both eyes of all 1082 healthy participants. Some eyes were difficult to measure and, even if they could be measured, the rubber band could not be set due to poor fixation. As a result, 2004 eyes were measured, and rubber bands were set on the data that measured well, so a total of 5465 data were included in this analysis. Scatter plots of BOM (n = 5465) in the ONH (Fig. 2A) and CHD (Fig. 2B) were analyzed by linear regression. Significant correlations were identified for the ONH (coefficient = 0.870, R2 = 0.80, P < 0.001) and CHD (coefficient = 0.852, R2 = 0.81, P < 0.001). For the ONH, the linear relationship was:
where the SP and RB refer to the values determined using the superpixel and rubber band methods, respectively. The corresponding relationship for the CHD was:
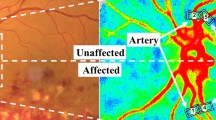
Scatter plots of BOM, (A) Optic nerve head (BOM.ONH) and (B) choroid (BOM.CHD), calculated using the rubber band method and the superpixel method. These scatter plots do not include BOM.T or TCR. The blue line on each graph was fitted using univariate regression. There were good correlations between the parameters calculated using the rubber band method and superpixel method for both regions (ONH: R2 = 0.8, CHD: R2 = 0.81). (C-1) is the fundus image of the same eye. Examples of average blood flow maps and BOM maps that showed high correlation (C-1,C-2) between the rubber band method and the supperpixel method are shown in panels (C-1) and (C-2); maps with low correlation are shown in (D-1) and (D-2). The blue arrow in (C-2) indicates an artifact caused by a shadow of the eyelash present during measuring.
Shown in Fig. 2 are examples of average blood flow and BOM maps with high correlation (Fig. 2C-1, C-2) and low correlation (Fig. 2D-1, D-2) between BOM.ONHRB and BOM.ONHSP. The superpixel structures of the BOM in the CHD and ONH tissue areas changed significantly, possibly because of changes in the distribution of blood.
The coefficients of variation, as measures of repeatability, were 5.7 ± 4.0% (superpixel) and 8.7 ± 6.9% (rubber band) in the ONH, and 6.1 ± 4.3% (superpixel) and 8.7 ± 7.1% (rubber band) in the CHD.
Discussion
Our previous study showed that women had a lower BOS across the entire ONH than men4. A low BOS is associated with high resistivity38, and given that BOM is considered to be the inverse property of BOS4, a high BOM value corresponds to high resistivity. We showed a significant correlation between BOS and BOM, thus indicating that BOM represents the resistivity value in each region.
The resistivity value in each region depended on sex, weight, height, and OPP in participants aged ≤ 50 years. Height was positively correlated with BOM.A and BOM.T in participants ≤ 50 years old. However, there was no significant correlation to resistivity values in participants > 50 years old. Weight affected resistivity in each region, and OPP and sex had significant impacts on resistance values. Regarding the higher resistivity in women, one of the reasons may be functional, as the artery compliance of the retina is significantly lower in women than in men and blood flow is faster. The greater volume of blood flowing through lower vessels results in higher resistivity. In fact, it has been reported that the elasticity of small arteries is significantly lower and systemic vascular resistance (SVR) is higher in young healthy women than in men39. Another report referred to mechanisms by which estrogen reduces vasoconstriction40. These earlier results match our findings. Another study reported a decrease in vascular compliance in women older than 50 years41. We believe that premenopausal women maintain vascular compliance by reducing vasoconstriction due to the effects of female hormones, and that such women have the robustness to maintain blood flow in the circulatory system even with high resistance. Decreased resistance can be interpreted as an increased ease of flow. One situation that may facilitate flow is an increase in the total cross-sectional area of capillaries. This is equivalent to an increase in the number of capillaries. Since the number of capillaries is thought to increase with increasing body weight, resistance and body weight may be negatively correlated. OPP is the effective intraocular return pressure, so a decrease in resistance will result in an increase in OPP due to easier flow, leading to a negative correlation between resistance and OPP. There were significant differences in height and weight in relation to sex in the different age groups, because the absolute value of the standardized partial regression coefficient for sex was higher than for the other parameters. The BOM.A value for women was higher than that for men up to the age of 50 years, indicating that women had higher ocular blood flow resistivity in the ONH than men in the same age group, consistent with our previous findings4. However, there were no significant sex-based differences in BOM.A, BOM.T, or TCR in participants over the age of 50 years. The reduced effect of sex on BOM values as a function of age may be largely related to the menopause. Iwase et al. suggested that the sex hormone estrogen was associated with sex-based differences in blood flow and resistance10. Cardiovascular disease is also common in men, but its prevalence in women becomes similar to that in men after the menopause42. However, the occurrence of BRVO and retinal artery emboli do not differ between the sexes43,44. A different paper reported that women have a higher incidence of systolic hypertension45. Additionally, ocular blood flow and kidney damage have a strong relationship46. Glycosylated hemoglobin A1c contributed independently to the choroid MBR with a negative correlation between each other47. Therefore, peripheral vascular resistance is greater in women, and peripheral organ damage, such as that caused by ocular macrovascular and microvascular diseases, may therefore require more systemic management, including maintenance of suitable blood pressure, in women. These findings may help to clarify the effect of sex on the development of these macrovascular and microvascular diseases.
In clinical studies of CRVO37,48, measurement of TCR in addition to MBR enabled threshold TCR values to be established for the presence (0.93) and absence (0.68 ± 0.20) of CRVO. We calculated TCR values of 0.56 ± 0.28 for men and 0.59 ± 0.27 for women in participants > 50 years old, suggesting the absence of CRVO in our cohort. Because the systemic parameter- and sex-based differences in this age group were much smaller than the increase in TCR required to reach the threshold for CRVO diagnosis, our findings also supported the proposal that the TCR threshold does not depend on sex37,48.
As for comparison of rubber band and superpixel methods of BOM calculation,our data identified a good correlation between the BOMs calculated using the superpixel and rubber band methods. As for the reproducibility of BOMs, in general, the reliable reproductivity of the COV was less than 10%. This study's results by both methods were acceptable because they were less than 10%. The superpixel images maintained the edges of the retinal vessels because the methods were based on assembling a similar MBR. For both methods, the blood flow in the CHD measured using LSFG was 92% of the average MBR, as previously reported3,49, with the BOM value obtained using the rectangular rubber band dominated by the choroidal blood flow. Retinal blood flow in the CHD region enclosed within this rubber band was therefore not expected to affect the measurement of average blood flow value. The BOM values in the CHD region including retinal vessels also had little effect on the measured retinal blood flow, possibly indicating resistance to choroidal blood flow through medium and large vessels.
The intercepts obtained from linear regression analyses showed that the superpixel-derived BOM values in the ONH and CHD regions were approximately 0.2 units higher than those derived using the rubber band method. This discrepancy appears to result from different averaging of the BS, which was calculated from the time-average of many more pixels using the rubber band method compared with the superpixel method. Blood flow data includes a lot of noise caused by unrelated biological signals. The BS measured using the rubber band method is expected to be lower than that determined using the superpixel method because the rubber band method smooths noise in the BS by averaging many data points. For the CHD, each rubber band has 40,000 samples, whereas each superpixel has approximately 200 samples. The variance of blood flow values including speckle noise in LSFG images was unbiased when the number of samples was > 1000 using the rubber band method; speckle noise granularity remained if the number of samples was < 1000. The spatial mean therefore did not converge as the number of samples in the superpixel method increased, resulting in bias. These observations can be described by:
where ai, bi are biased Fourier coefficients of the power spectrum, aunbias_i and bunbias_i are their corresponding biased values, and their differences are given by Δai and Δbi, respectively. In the case of superpixels, substituting Eqs. (3) into Eq. (12) in the Methods section yields:
The superpixel method averages n superpixels, so:
Because the values aunbias_i and bunbias_i are unbiased and can be considered elements of the rubber band method, the following inequality holds:
where Pss and Prb are the power spectra of the superpixel and rubber band methods, respectively. The BS of the superpixel method is therefore greater than that of the rubber band method:
and this difference causes an offset of the BS. This may be understood by considering that the BS of a biased sample contains noise, so its deviation will be higher. As result:
The BS calculated using the superpixel method contains more bias; by invoking the concept of noise power, we estimated that this noise contributes an additional offset of approximately 0.2 units to the BOM, as indicated by the intercept factor in Eq. (1) or (2). The method-dependent difference in BOM values may also be caused by differences in the occupation rate between vessels and tissue and in the statistical distributions of the BS and average MBR values, which in turn depend on the sample size. The scatter plots in Fig. 2 show some outliers in the low BOM region, where BOM.ONHRB was < 0.2 and BOM.ONHSP was > 0.5. However, the regression line was not significantly altered after repeat analysis excluding these outliers, and the intercept and slope only decreased by approximately 0.05 units, respectively. Although there was a slight difference between the BOM values derived using the superpixel and rubber band methods, we suggest that superpixel-segmented resistivity maps of the ONH and CHD regions are suitable for quantifying changes in local resistivity associated with diseases such as CRVO, BRVO, and aging.
This study had some major limitations. First, confirmation of the participants’ systemic status, such as their medical history, was only obtained by interview, and the possibility that asymptomatic or systemic disease was present in some participants thus cannot be ruled out. Second, we did not record menopausal status and did not measure levels of sex hormones (estrogen and androgen). Finally, all the participants were Japanese, and it is unclear if our conclusions also apply to non-Japanese populations.
Using LSFG, we identified age- and sex-based differences in the ocular blood flow resistivity parameter BOM in the ONH, retinal vessels, non-vessel tissue in the ONH, and CHD. There were sex-based differences in BOM among individuals ≤ 50 years old, but not in those aged > 50 years; notably, the sex-based difference in the retinal vessels was much smaller than the increase in BOM required to reach the threshold value for a diagnosis of CRVO, supporting previous assertions that this threshold does not depend on sex. We also identified a strong correlation between the BOM values determined using the superpixel and rubber band methods, indicating that superpixel segmentation is a suitable method for visualizing two-dimensional resistivity maps.
Methods
Participants
We enrolled healthy 1082 Japanese participants who participated in a medical checkup program at the Department of Health Care Center of the Japan Community Health Care Organization, Tokyo Kamata Medical Center, between December 2016 and December 2018. Participants were excluded if they had any of the following: atherosclerotic disease, such as hypertension, dyslipidemia, diabetes mellitus, cardiovascular or cerebrovascular events, and arrhythmia; ophthalmic disease, such as glaucoma, uveitis, optic neuropathy, vitreous or retinal disease; retinal or choroidal vascular disease; best corrected visual acuity < 40/50; and previous intraocular surgery. Blood pressure measurements and LSFG were performed after patients had rested for 10 min in a quiet, air-conditioned room maintained at 24 °C. All participants abstained from smoking, alcohol, and caffeine for ≥ 24 h prior to the measurements. All evaluations were performed between 9:00 and 11:00 a.m. after the participants had fasted overnight.
Examinations of the ONH and CHD using LSFG
LSFG images were obtained using an LSFG-NAVI system (Softcare Co., Fukutsu, Japan) and the BOM values were calculated using LSFG Analyzer software (V3.8.0.4) running on a Windows-based PC with an AMD Ryzen 7 5800X 8-core processor (3.8 GHz, 32 GB RAM). Briefly, the LSFG system consisted of a fundus camera equipped with a diode laser (wavelength 830 nm) and a CCD camera. For evaluation of the ONH circulation, an elliptical rubber band was placed around the ONH (Fig. 3A). The software delineated the ONH vessels and tissue using an automated definitive threshold with a histogram method (Fig. 3B). The BOM.A parameter was calculated using the BOM in the entire ONH, BOM.T was calculated using the BOM in tissue surrounding the vessels in the ONH, and the TCR was calculated using the BOM in the retina vessels in the ONH.
Example ROI, regions of BOM analysis using LSFG. (A) Color map of total measurement area. An elliptical rubber band enclosed the entire optic nerve head (ONH) and a rectangular rubber band enclosed a region in the choroid (CHD). The ellipse surrounds the area of the ONH. (B) Software was used to demarcate the retinal vessels using an automated definitive threshold throughout the ONH, within the ONH vessels (shown in white) and the ONH tissue (black).
To evaluate CHD circulation, a rectangular rubber band (200 × 200 pixels) was placed on the temporal side approximately one ONH diameter away from the ONH or as far away as possible, avoiding large retinal vessels (Fig. 3A).
All rubber bands were placed semi-automatically using auto-alignment software (Cobitos V1.0.58.0) with representative rubber bands placed manually by one experienced examiner. All measurements were made with the participants in the seated position, without the use of pupil-dilating eye drops.
Calculation of BOM to assess the relationship BOS and BOM (first aim)
The amplitude of blood flow in the cardiac cycle divided by the average blood flow defines the resistivity of blood flow quantified by the PI50 in Doppler ultrasound. On the basis of the above definition of the PI, we proposed that BOM was an equivalent resistivity parameter in LSFG.
Using LSFG, the MBR, which is the spatial average of the blur rate measured within the region enclosed by the rubber band, can be captured in 118 frames over a 4-s period and tuned to the cardiac cycle. The amplitude of changes in the MBR can then be used to detect peak-to-peak blood flow. Notably, the BS in LSFG is proportional to the peak-to-peak blood flow and is based on the predicted Fourier spectrum, which, unlike BOS and BOT, does not require heartbeat detection. The predicted Fourier spectrum was calculated using time–frequency analysis based on sparse modeling, and generated a dynamic index describing the strength of heartbeats. BS was defined as the square root of the power of the frequency at the point where the maximum power was observed. Figure 4 illustrates the procedure for obtaining BOM using the standard rubber band method. MBRavg was first calculated as the temporal average of the MBR in the region enclosed by an elliptical rubber band (Fig. 4A-1). The time-series blood flow data Y = (y1, y2,…, y118) were then normalized by centering on MBRavg (Fig. 4B-1). In general, Y can be obtained from a Fourier series expansion of the time information T(t1, t2,…, t118):
where
Diagram showing BOM calculation using standard rubber band selection method, representative example using an elliptical rubber band enclosing the entire ONH is shown. Functions Y and P are defined in Eqs. (1) and (4), respectively. MBR, mean blur rate; MBRavg, temporal average of the MBR; BS, beat strength.
M is the frequency division number of the power spectrum (in this case, M = 100), and f1, f2, .., fM are frequency elements for the power spectrum (in this case, 0.1, 0.2, ..,10 Hz). In Eq. (11), X is the vector of 2 M Fourier coefficients ai, bi (i = 1, M) corresponding to the ith frequency element of the power spectrum. After Fourier transformation of Eq. (9) (Fig. 4B-2), the elements of the square root of the power spectrum for each frequency (fi) are given as:
and BS (Fig. 4B-3) is defined as:
where C is a constant scaling factor.
The detailed method for calculating the BS is described in the following patent application: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2018003139.51
An advantage of measuring BS is the ability to apply a band-pass filter when monitoring cardiac frequency. It is therefore possible to evaluate the resistivity in an observation region peripheral to the heart. The resistivity parameter is defined as:
Because BS is proportional to the peak-to-peak blood flow within a given region, BOM represents the corresponding resistivity in that region.
We evaluated the systemic- and sex-dependence of the resistivity in the ONH, retinal vessels, and CHD, using previously described statistical and graphical methods for BOM analysis with a standard rubber band selection method (Fig. 4).
When assessing the relationship between BOM and BOS in the ONH, retinal vessels, the tissue surrounding the vessels and the CHD, both eyes were included.
Calculation of BOM to assess systemic parameter- and sex-based differences in BOM values in the ONH, retinal vessels, and CHD (second aim)
To assess our second aim (systemic parameter- and sex-based differences in BOM values in the ONH, retinal vessels, and CHD), only the right eye was included. The participants were divided into two groups with a cut-off age of 50 years, to examine differences in systemic parameters between these groups.
Application of simple linear iterative clustering (SLIC) to LSFG (Third aim)
Recent image processing studies have identified SLIC as an excellent algorithm for clustering proximal pixels with similar colors into superpixels52. We proposed that this algorithm could be used to cluster pixels based on the average MBR instead of color, allowing us to reduce the computation time and speckle noise in each frame and thus allow the rapid generation of BOM maps for clinical use. Occluded vessels and those becoming occluded can be identified by their high resistivity34. Using the superpixel method will result in loss of spatial resolution with increasing superpixel size, and we therefore adjusted the superpixel size to preserve the resolution of large retinal vessels. Below we describe the clustering of pixels on the LSFG map into superpixels. Figure 5A,B shows the initial placement and final segmentation, respectively, of clusters on the LSFG map. The cluster centers were initially placed S pixels apart horizontally and vertically, with each row of clusters staggered by S/2 pixels. The final placement of each cluster center was determined after the superpixel grouping was completed. Figure 5C shows the initial superpixel cluster boundary containing S × S pixels and the search region containing 2S × 2S pixels, with each region enclosing a cluster center. To maintain the shape and structure of the blood vessels, we decided to use S = 14. The distance from the cluster center to each pixel was calculated based on LSFG parameters, such as the average MBR, and actual pixel length. The cluster to which each pixel belonged was then updated and the above procedure was repeated until no more such updates occurred. The only difference between the SLIC procedure described here and the standard SLIC method was the use of LSFG parameters instead of color to calculate the distance. Finally, the superpixels on the LSFG map were created by grouping similar pixels in each region to delineate those containing retinal vessels or peripheral tissue.
In this study, we performed pixel-averaging using the above superpixel method and by tracing an ordinary rubber band and clustering the pixels within it. In the standard rubber band method, the average BOM of the pixels enclosed within each rubber band was calculated (Fig. 4); in the superpixel method, the average BOM of the superpixels enclosed within each rubber band was calculated (Fig. 6).
LSFG data from the ONH and CHD regions in all participants were analyzed using both methods, and the correlation between the BOM output for each method was calculated using univariate regression. Additionally, the coefficients of variation of each method were calculated.
Statistical analyses
To address the first aim, we used univariate regression analysis to assess the relationship between BOS and BOM.
To address the second aim, data for continuous variables are presented as the mean ± standard deviation. Wilcoxon’s rank sum test was used to compare systemic parameters such as body weight, and height, and sex-based differences in parameters. In addition, multiple regression analysis was performed for participants aged ≤ 50 and > 50 years, with sex, weight, height, and OPP as explanatory variables, and BOM parameters such as TCR as response variables. Each parameter was normalized to estimate the standardized partial regression coefficient. Values of P < 0.05 were considered to indicate statistical significance.
For the third aim, we compared BOM calculated using the superpixel method (response variable) and rubber band method (explanatory variable) by linear regression. All statistical analyses were carried out using R software (V3.5.2, www.r-project.org).
Ethical approval
This cross-sectional study was approved by the Ethics Committee of Toho University School of Medicine (No. A16062), and all participants provided informed consent for their participation in accordance with the tenets of the Declaration of Helsinki. This study was registered in the University Hospital Medical Information Network clinical trials registry (ID: UMIN000026778).
Data availability
The datasets analyzed in the current study are available from the corresponding author on request.
References
Fujii, H. Visualisation of retinal blood flow by laser speckle flowgraphy. Med. Biol. Eng. Comput. 32, 302–304 (1994).
Tamaki, Y., Araie, M., Kawamoto, E., Eguchi, S. & Fujii, H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp. Eye Res. 60, 373–383 (1995).
Isono, H. et al. Observation of choroidal circulation using index of erythrocytic velocity. Arch. Ophthalmol. 121, 225–231 (2003).
Kobayashi, T. et al. Influence of age and gender on the pulse waveform in optic nerve head circulation in healthy men and women. Sci. Rep. 9, 17895 (2019).
Calzetti, G. et al. Assessment of choroidal blood flow using laser speckle flowgraphy. Br. J. Ophthalmol. 102, 1679–1683 (2018).
Sugiyama, T. Basic technology and clinical applications of the updated model of laser speckle flowgraphy to ocular diseases. Photonics 1, 220–234 (2014).
Takahashi, H. et al. Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp. Eye Res. 108, 10–15 (2013).
Kobayashi, T., Shiba, T., Kinoshita, A., Matsumoto, T. & Hori, Y. The influences of gender and aging on optic nerve head microcirculation in healthy adults. Sci. Rep. 9, 15636 (2019).
Yanagida, K. et al. Sex-related differences in ocular blood flow of healthy subjects using laser speckle flowgraphy. Invest. Ophthalmol. Vis. Sci. 56, 4880 (2015).
Iwase, T. et al. Investigation of causes of sex-related differences in ocular blood flow in healthy eyes determined by laser speckle flowgraphy. Sci. Rep. 7, 13878 (2017).
Aizawa, N. et al. Age- and sex-dependency of laser speckle flowgraphy measurements of optic nerve vessel microcirculation. PLoS ONE 11, 1 (2016).
Luft, N. et al. Ocular blood flow measurements in healthy white subjects using laser speckle flowgraphy. PLoS ONE 11, e0168190 (2016).
Takeshima, S. et al. Effects of trabeculectomy on waveform changes of laser speckle flowgraphy in open angle glaucoma. Invest. Ophthalmol. Vis. Sci. 60, 677–684 (2019).
Rina, M., Shiba, T., Takahashi, M., Hori, Y. & Maeno, T. Pulse waveform analysis of optic nerve head circulation for predicting carotid atherosclerotic changes. Graefes Arch. Clin. Exp. Ophthalmol. 253, 2285–2291 (2015).
Tsuda, S. et al. Pulse-waveform analysis of normal population using laser speckle flowgraphy. Curr. Eye Res. 39, 1207–1215 (2014).
Masai, S., Ishida, K., Anraku, A., Takumi, T. & Tomita, G. Pulse waveform analysis of the ocular blood flow using laser speckle flowgraphy before and after glaucoma treatment. J.Ophthalmol. 2019, e1980493 (2019).
Sato, T. et al. Longitudinal changes of ocular blood flow using laser speckle flowgraphy during normal pregnancy. PLoS ONE 12, e0173127 (2017).
Kobayashi, T., Shiba, T., Okamoto, K., Usui, T. & Hori, Y. Characteristics of laterality in the optic nerve head microcirculation obtained by laser speckle flowgraphy in healthy subjects. Graefes Arch. Clin. Exp. Ophthalmol. https://doi.org/10.1007/s00417-022-05631-8 (2022).
Shiba, T., Takahashi, M. & Maeno, T. Pulse-wave analysis of optic nerve head circulation is significantly correlated with kidney function in patients with and without chronic kidney disease. J. Ophthalmol. 2014, 291687 (2014).
Shiba, T., Takahashi, M., Hori, Y. & Maeno, T. Pulse-wave analysis of optic nerve head circulation is significantly correlated with brachial-ankle pulse-wave velocity, carotid intima-media thickness, and age. Graefes Arch. Clin. Exp. Ophthalmol. 250, 1275–1281 (2012).
Miyaji, A., Ikemura, T. & Hayashi, N. Effect of aging on the blowout time in various ocular vessels. J. Aging Sci. 4, 1–6 (2016).
Ismail, A. et al. Pulse waveform analysis on temporal changes in ocular blood flow due to caffeine intake: A comparative study between habitual and non-habitual groups. Graefes Arch. Clin. Exp. Ophthalmol. 256, 1711–1721 (2018).
Shiba, T., Takahashi, M., Hori, Y., Maeno, T. & Shirai, K. Optic nerve head circulation determined by pulse wave analysis is significantly correlated with cardio ankle vascular index, left ventricular diastolic function, and age. J. Atheroscler. Thromb. 19, 999–1005 (2012).
Kunikata, H. et al. Relationship of ocular microcirculation, measured by laser speckle flowgraphy, and silent brain infarction in primary aldosteronism. PLoS ONE 10, e0117452 (2015).
Bhatti, M. S., Tang, T. B. & Laude, A. Effects of water drinking test on ocular blood flow waveform parameters: A laser speckle flowgraphy study. PLoS ONE 12, e0181512 (2017).
Pappelis, K., Choritz, L. & Jansonius, N. M. Microcirculatory model predicts blood flow and autoregulation range in the human retina: In vivo investigation with laser speckle flowgraphy. Am. J. Physiol.-Heart Circul. Physiol. 319, H1253–H1273 (2020).
Shiba, T., Takahashi, M., Matsumoto, T. & Hori, Y. Pulse waveform analysis in ocular microcirculation by laser speckle flowgraphy in patients with left ventricular systolic and diastolic dysfunction. J. Vasc. Res. 55, 329–337 (2018).
Shiba, T., Takahashi, M., Hashimoto, R., Matsumoto, T. & Hori, Y. Pulse waveform analysis in the optic nerve head circulation reflects systemic vascular resistance obtained via a Swan-Ganz catheter. Graefes Arch. Clin. Exp. Ophthalmol. 254, 1195–1200 (2016).
Aburahma, A. F. Noninvasive Vascular Diagnosis: A Practical Textbook for Clinicians. Springer, Berlin. https://doi.org/10.1007/978-3-319-54760-2 (2017).
de Riva, N. et al. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocrit Care 17, 58–66 (2012).
Calzetti, G. et al. Short-term changes in retinal and choroidal relative flow volume after anti-VEGF treatment for neovascular age-related macular degeneration. Sci. Rep. 11, 23723 (2021).
Michelson, G. & Harazny, J. Increased vascular resistance for venous outflow in central retinal vein occlusion. Ophthalmology 104, 659–663 (1997).
Keyser, B. J. et al. Color Doppler imaging of arterial blood flow in central retinal vein occlusion. Ophthalmology 101, 1357–1361 (1994).
Tomita, R. et al. Elevated retinal artery vascular resistance determined by novel visualized technique of laser speckle flowgraphy in branch retinal vein occlusion. Sci. Rep. 11, 20034 (2021).
Wang, J.-K., Tamplin, M. R., Garvin, M. K., Grumbach, I. M. & Kardon, R. H. A superpixel-histogram method to analyze retinal, optic nerve, and choroidal blood flow using laser speckle flowgraphy. In Medical Imaging 2022: Biomedical Applications in Molecular, Structural, and Functional Imaging (eds. Gimi, B. S. & Krol, A.) 77 (SPIE, 2022). doi:https://doi.org/10.1117/12.2611850.
Tamplin, M. R. et al. Reduced blood flow by laser speckle flowgraphy after 125I-plaque brachytherapy for uveal melanoma. BMC Ophthalmol 22, 285 (2022).
Matsumoto, M. et al. Retinal microvascular resistance estimated from waveform analysis is significantly higher with a threshold value in central retinal vein occlusion. Trans. Vis. Sci. Tech. 9, 4 (2020).
Fukami, M. et al. Diurnal variation of pulse waveform parameters determined by laser speckle flowgraphy on the optic nerve head in healthy subjects. Medicine 96, e8312 (2017).
Winer, N., Sowers, J. R. & Weber, M. A. Gender differences in vascular compliance in young, healthy subjects assessed by pulse contour analysis. J. Clin. Hypertens. (Greenwich) 3, 145–152 (2001).
Sowers, J. R. Diabetes mellitus and cardiovascular disease in women. Arch Intern Med 158, 617 (1998).
Glasser, S. P. et al. Vascular Compliance and Cardiovascular Disease. 10.
Kannel, W. B., Hjortland, M. C., McNamara, P. M. & Gordon, T. Menopause and risk of cardiovascular disease: The Framingham study. Ann Intern Med 85, 447–452 (1976).
Shin, Y. U. et al. Prevalence and associated factors of retinal vein occlusion in the Korean National Health and Nutritional Examination Survey, 2008–2012: A cross-sectional observational study. Medicine (Baltimore) 95, e5185 (2016).
Yasuda, M. et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: The Hisayama Study. Invest. Ophthalmol. Vis. Sci. 51, 3205 (2010).
Burt, V. L. et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: Data From the Health Examination Surveys, 1960 to 1991. Hypertension 26, 60–69 (1995).
Iwase, T., Ueno, Y., Tomita, R. & Terasaki, H. Relationship between retinal microcirculation and renal function in patients with diabetes and chronic kidney disease by laser speckle flowgraphy. Life 13, 424 (2023).
Shiba, C., Shiba, T., Takahashi, M., Matsumoto, T. & Hori, Y. Relationship between glycosylated hemoglobin A1c and ocular circulation by laser speckle flowgraphy in patients with/without diabetes mellitus. Graefes Arch. Clin. Exp. Ophthalmol. 254, 1801–1809 (2016).
Matsumoto, M. et al. Retinal vascular resistance significantly correlates with visual acuity after 1 year of anti-VEGF therapy in central retinal vein occlusion. Trans. Vis. Sci. Tech. 10, 19 (2021).
Watanabe, G., Fujii, H. & Kishi, S. Imaging of choroidal hemodynamics in eyes with polypoidal choroidal vasculopathy using laser speckle phenomenon. Jpn J Ophthalmol 52, 175–181 (2008).
Holwerda, S. W. et al. Aortic stiffness is associated with changes in retinal arteriole flow pulsatility mediated by local vasodilation in healthy young/middle-age adults. J. Appl. Physiol. 129, 84–93 (2020).
FUJII, H. et al. Blood Flow Dynamic Imaging Diagnosis Device and Diagnosis Method. https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2018003139 (2018).
Achanta, R. et al. SLIC Superpixels Compared to State-of-the-Art Superpixel Methods. IEEE Trans. Pattern Anal. Mach. Intell. 34, 2274–2282 (2012).
Acknowledgements
This work was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C) awarded to T.S. (#16K11275). The authors thank Ayako Kinoshita, who collected data in this study. We also thank Susan Furness, PhD, from Edanz (https://jp.edanz.com/ac) for editing drafts of this manuscript.
Author information
Authors and Affiliations
Contributions
Design and conduct of the study (K.O, T.K. and T.S.); collection of data (T.K.), management, analysis, and interpretation of the data (K.O, N.T and T.S.); preparation, review, or approval of the manuscript (K.O, T.K., T.S., H.F. and Y.H.).
Corresponding author
Ethics declarations
Competing interests
KO, NT, and HF are employed by Softcare Co., Ltd. TK, TS and YH declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okamoto, K., Takahashi, N., Kobayashi, T. et al. Novel superpixel method to visualize fundus blood flow resistivity in healthy adults. Sci Rep 13, 6171 (2023). https://doi.org/10.1038/s41598-023-33450-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-33450-2