Abstract
Sericinus montela, a globally threatened butterfly species, feeds exclusively on Aristolochia contorta (Northern pipevine). Field surveys and glasshouse experiments were conducted to obtain a better understanding of the relationship between the two species. Interviews with the persons concerned with A. contorta were conducted to collect information about the site management measures. We found that management practices to control invasive species and manage the riverine areas might reduce the coverage of A. contorta and the number of eggs and larvae of S. montela. Our results indicated that the degraded quality of A. contorta may result in a decrease in S. montela populations by diminishing their food source and spawning sites. This study implies that ecological management in the riverine area should be set up to protect rare species and biodiversity.
Similar content being viewed by others
Introduction
Butterflies significantly contribute to ecosystem biodiversity1,2,3, providing various ecological benefits such as pollination and natural pest control. Furthermore, butterflies can serve as indicator species to assess and interpret the status of an ecosystem4,5,6,7. However, butterflies are currently highly threatened due to habitat degradation and loss as a result of human activity8,9,10. Humans are encroaching on and destroying wildlife habitats for their own needs at an alarming rate11,12,13,14. Weeds and pests are some of the main targets for elimination in a human-centered ecosystem15. In agroforestry industries, pesticides have been extensively used to control pests on crops, resulting in ecological collapse16,17,18. Butterflies are sensitive to human activities19, 20, as they lay their eggs on their host plants or nearby in areas populated by people. The presence, abundance, and vitality of host plants can influence the survival and diversity of butterfly populations21. In the larval stage, butterflies are in danger if host plants are disturbed by human activities such as applying pesticides, mowing, and weeding, or grazing and trampling by livestock22. Understanding the quality of host plants is an important step in estimating the abundance and status of butterflies and establishing management practices for butterfly conservation.
The dragon swallowtail butterfly (Sericinus montela) is designated as a vulnerable species on a regional scale in South Korea according to IUCN criteria23. S. montela is known for the beauty of its unique wings, which have a pair of slender tails elongated from each hindwing24, 25. These butterflies have a life cycle of 36–54 days including the egg stage (5–7 days), larval stage (15–25 days), pupal stage (10–12 days), and adult stage (7–12 days)25. Its larvae are monophagous, feeding exclusively on Northern pipevine (Aristolochia contorta), a perennial herbaceous vine species. This plant is a stem-twiner, but is ground-rooted, non-parasitic, and non-epiphytic26. Usually, A. contorta grows on the edges of forests, rivers, and agricultural fields in East Asia (Korea, Japan, China, and Russia)27,28,29. It has low sexual reproductivity and forms small populations30. One of the reasons why A. contorta populations are in decline may be the transition from traditional agricultural practices to modern methods, including the use of pesticides, herbicides, and weeding27,28,29,30,31,32. In addition, river improvement works, human-made changes to improve navigation, drainage, irrigation, or flood control, may have also contributed to the decline of its population33. These activities could have disrupted the natural habitat of A. contorta, making it difficult for this species to survive and reproduce in time. The decline of the A. contorta population may result in a decrease of the S. montela population, since S. montela relies on the plant as the only food source for its larvae.
There are studies on the ecological importance of A. contorta and S. montela focusing on functional aspects of the plant (i.e., its secondary metabolite)34,35,36,37, as well as the mitochondrial genome, development, and metapopulation dynamics of the butterfly38,39,40. Recently, the optimal habitat of A. contorta29 and the interactive effects of CO2 on A. contorta for S. montela41, 42 were reported. Currently, none of the studies have examined the cumulative effect of anthropogenic disturbances on A. contorta and S. montela beyond one-year life cycle. To address this gap, we investigated the anthropogenic factors that can damage the habitat of A. contorta using a four-year interval field survey. It involved interviews with stakeholders such as government officers, laborers, and farmers who were directly involved in land management practices that may affect the survival of S. montela populations. Furthermore, we performed a glasshouse experiment to examine the effects of the disturbed A. contorta on S. montela. We hypothesized that (1) mowing and pesticides will negatively impact the growth and reproduction of A. contorta, and (2) the population of S. montela will be negatively affected by the decline in growth and reproduction of A. contorta resulting from human activities. By identifying the factors contributing to the population decline of S. montela, this study can suggest management practices that promote the survival and conservation of the species. The findings of this study could be used to develop management strategies that reduce the negative impact of human activities on the habitat of A. contorta and the consequent survival of S. montela.
Results
Human activity and A. contorta
We interviewed stakeholders and summarized the mowing and weeding information into six categories (Table 1). Toward the management of various sites including measures such as removing alien species and preserving ecosystem diversity, information was obtained through a series of interviews. PC was managed by Cheongju City and herbaceous plants were removed around the river three times a year by subcontractors. At MA, all plants except the common hibiscus (Hibiscus syriacus), a beloved flower in Korea, and some trees, such as the false acacia (Robinia pseudoacaci), and the tree of heaven (Ailanthus altissima) were removed, while at JW, the A. contorta habitat was managed under financial support from Pyeongtaek City. A. contorta was considered for removal at GC, where the plant community was separated by a low fence to protect Solomon’s seal (Polygonatum stenophyllum). Selective weeding was performed to remove only invasive plants such as Japanese hop (Humulus japonicus) and bur cucumber (Sicyos angulatus) at YU, but pesticide use was reported by farmers at JP.
According to interviews conducted to investigate the anthropogenic activities affecting A. contorta, invasive alien plants were removed through government-led conservation programs at sites PC, MA, JW, GC, and YU; however, the removal involved not only invasive exotic plants but also all other native plants and A. contorta. At site JP, private mowing machines and herbicides were used for road maintenance.
The coverage of A. contorta in 2021 was reduced significantly compared to that observed in 2017 at site PC and GC. In 2021, the coverage were 11.86 ± 0.30% at site PC, 18.50 ± 2.80% at site GC and 32.70 ± 7.67% at site PC, 40.80 ± 8.35% at site GC in 2017 (Fig. 1a). The height of A. Contorta increased significantly only at site PC (Fig. 1b). The height of A. contorta was uniform at approximately 150 cm at site JW in 2017 and 2021 because they were winding around artificial structures, with a height of 150 cm. When considering A. contorta at site MA, they were winding around shrubs (79.50 ± 12.17 cm, H. syriacus) in 2017, but only a few vines were left due to mowing (58.10 ± 11.56 cm) in 2021. The number of leaves per quadrat decreased significantly at sites PC, MA, and GC, but it slightly increased at site JW with an artificial planting area for A. contorta and at site YU, where only invasive species were cut. For instance, at site PC, the number of leaves per quadrat was 5.1, the lowest in 2021, which showed a steep decline from 90 in 2017, while the study site with the most leaves in 2021 was JP (188.3 ± 28.7). A positive correlation was found between the coverage and the number of leaves per quadrat (r = 0.678, p < 0.01).
Growth characteristics and reproductive traits of A. contorta at study sites in 2017 and 2021 (JP only has data for 2021). (a) Coverage, (b) Height, (c) Leaf number per quadrat, (d) Flower number per quadrat, (e) Fruit number per quadrat. Bars indicate standard error. *p < 0.05, **p < 0.01, ***p < 0.001.
In 2021, A. contorta displayed higher coverage, height (with the exception of at site JW which had artificial supports), and number of leaves and flowers in well-maintained areas and lower in areas that had intensive mowing or weeding. Coverage was the highest at site JP (56.43 ± 3.64%), which was newly investigated in 2021, followed by at site YU (34.00 ± 4.66%), JW (29.80 ± 1.36%), MA (19.10 ± 3.45%), GC (18.50 ± 2.80%), and PC (56.43 ± 3.64%) (Fig. 1a). In 2021, the highest A. contorta was observed at site YU (208.5 ± 32.54 cm), followed by at site PC (192.86 ± 30.84 cm) and then at site JP (182.86 ± 22.44 cm) which were winding around R. pseudoacacia and Morus alba, respectively (Fig. 1b). However, there were many withered A. contorta leaves at site PC, so their coverage was low. The number of leaves was the highest at site JP (188.29 ± 18.68), followed by at site JW (172.50 ± 16.09%), YU (47.03 ± 7.76%), MA (32.00 ± 2.84%), GC (21.20 ± 4.35%), and PC (5.00 ± 0.82%) (Fig. 1a).
The values of the reproductive traits of A. contorta were significantly different depending on the years and sites (Fig. 1). At sites PC and GC, there was a 100% decrease in the number of flowers (2017 PC, 38.0 ± 11.0; 2021 PC, 0; 2017 GC, 109.0 ± 27.2; 2021 GC, 0). The number of fruits in 2021 significantly decreased at site GC compared to 2017. The number of flowers per quadrat was the highest at site JP (111.86 ± 28.95) and the lowest at sites PC and GC in 2021 (PC, 0; MA, 21.6 ± 7.5; JW, 26.6 ± 9.3; GC, 0; YU, 33.5 ± 14.2; JP, 111.9 ± 28.9; Fig. 1d). A positive correlation was found between the number of flowers per quadrat and the height (r = 0.340, p < 0.05). The number of fruits per quadrat was the highest at site PC and the lowest at site JW in 2021 (PC, 14.3 ± 3.5; JP, 5.3 ± 2.4; GC, 0; YU, 0.3 ± 0.1; JW, 2.1 ± 0.9) (Fig. 1e).
In 2021, the number of larvae decreased at all sites other than YU compared to 2017. In particular, the number of egg clusters was reduced compared to that of 2017 (Fig. 2). At site PC, S. montela larvae were distributed in large numbers at a density of 16.9 individuals/m2 in 2021 (Fig. 2a). Larvae ate fresh leaves or buds at most sites except for at site PC in 2021, where most leaves had turned brown in August, therefore the larvae gnawed at the surface of fruits. Dead larvae were found dried on the leaves of the host plants (6.6 ± 0.4 individuals/m2, Fig. 2a). Due to the lack of leaves, sufficient shade was not provided, therefore the larvae exposed to direct sunlight were likely to die. Also, people, bicycles, and cars killed larvae that reached ground level (8.3 ± 0.2 individuals/m2, Fig. 2a).
Effects of feeding organ and pesticides on the growth of S. montela
Before examining the effect of the feeding organ, the C/N ratio of the feeding organ was as follows. The C/N ratio of fruits used in the experiment was 34.02 ± 2.99 (JP), 32.84 ± 3.12 (PC), and the C/N ratio of leaves was 15.82 ± 0.23 (JP), 16.53 (PC), respectively. Among a total of 120 larvae, 75 died before pupation (62.50% larval mortality), eight did not emerge from pupae (6.67% of the initial number of larvae), while 37 emerged (30.83% of the initial number of larvae).
The morphological traits of S. montela responded differently according to the food types (leaves or fruits) and the presence or absence of pesticides (Fig. 3). The S. montela group fed w/o-Pesticides/Fruits (w/o-P/F) plants had a longer pupal length than those fed with-Pesticides/Fruits (with-P/F). The group fed with-P/L had a longer wingspan than those fed with-Pesticides/Fruits (with-P/F) (Fig. 3). Pupal length and wingspan were the longest in the group of w/o-P/L and were the shortest in the group of w-P/F (Fig. 3). In weight change (%), which is an increase or decrease compared to the original weight of the larva, S. montela with-P/F lost more weight than w/o-P/F (Fig. 4). Furthermore, w/o-P/L gained more weight than with-P/L (Fig. 4). In the case of S. montela with-P/L, the average weight was temporarily decreased as many large larvae died, but the average weight finally increased (Fig. 4). Furthermore, larvae that fed on fruits showed higher mortality than those fed on leaves (Fruits, 76.67% mortality; Leaves, 61.67% mortality; Kaplan–Meier test Chi-square = 4.596, df = 3, p-value = 0.004). In addition, the group fed with-Pesticides food showed higher mortality (80.00%) than those fed on plant matter w/o-Pesticides groups (58.33%; Fig. 5). Larvae fed on fruits with pesticides showed the highest mortality (83.33%). In comparison, those which fed on leaves without pesticides showed the lowest mortality (46.67%; Fig. 6). The comparison of the fractions of dead and emerged individuals from w/o-P/F was 21 vs. 9, while w/o-P/L was 14 vs. 16. In the case of -P/F the comparison between dead and emerged individuals was 25 vs. 5, and in P/L is was 23 vs. 7 with a higher proportion of larvae reaching the adult stage on w/o-P/L than others (Fig. 6). Some larvae did not reach the pupal stage (20 out of 30 w/o-P/F; 11 out of 30 w/o-P/L; 23 out of 30 with-P/F; 21 out of 30 with-P/L). In addition, some pupae did not reach the adult stage (1 out of 10 w/o-P/F; 3 out of 19 w/o-P/L; 2 out of 7 with-P/F; 2 out of 9 with-P/L). There was no mating event observed, but only a few eggs (w/o-P/F, 19, two clusters; w/o-P/L, 35, two clusters; with-P/F, 14, one cluster; with-P/L, 22, one cluster) were laid in the cages.
Variations of S. montela morphological traits among treatments (w/o-Pesticides: feeding on foods without pesticides, with-Pesticides: feeding on foods with pesticides, Fruits: fruit-eating group, Leaves: leaf-eating group, (a) Pupal length, (b) Wingspan. (Duncan test, p < 0.05). Bars indicate standard error.
Pupal length, wingspan, larval weight change, and the number of survival days of S. montela depended on the food types and pesticide (Table 2). The pupal length was only affected by pesticides, and the number of survival days was only affected by food types. The wingspan and the larval weight change were affected by the food types and pesticide, respectively. However, there were no interactive effects on pupal length, wingspan, larval weight change, or the number of survival days (Table 2). The differences according to the food types were not apparent with the presence of pesticide and vice versa.
Discussion
Response of A. contorta to human activities and subsequent effects on the S. montela population
Aristolochia contorta, the exclusive food source of S. montela larvae, mainly occurred along the river bank and in the ecotone between forest and agricultural areas at the surveyed sites. The population size tends to be constantly limited and grows faster as age increases43. Furthermore, A. contorta grows taller in the presence of trees29. The more suitable habitat for S. montela is when coverage is higher, stems are more extended, and more leaves are present on A. contorta. The coverage and number of A. contorta leaves are more significantly correlated with available food resources for butterfly larvae21. Consequently, site JP would be the most suitable habitat for A. contorta among the study sites (Fig. 1). However, site JP would not be a good habitat for S. montela due to pesticide application (Figs. 2, 6).
Aristolochia contorta was easily removed during mowing or weeding. In the process of weeding invasive species, A. contorta instead of being protected, was also being removed. This occurred through various mechanisms, such as the use of non-selective herbicides, mechanical removal methods, or changes in ecosystem processes resulting from the removal of invasive species. The negative impacts of invasive species management on non-target species can be mitigated through a systematic approach to conservation planning and management that prioritizes biodiversity conservation44. This approach involves identifying and prioritizing areas that are critical for biodiversity conservation, including the habitats of vulnerable and threatened species, and incorporating this information into the planning and management of invasive species44.
Furthermore, the removal of invasive alien trees such as R. pseudoacacia in the same period, resulted in a loss of support structures which is one of the critical factors of A. controta growth and reproduction29. A management strategy that promotes the restoration of support structures may be necessary. One potential approach could be the planting of native trees or shrubs with similar branching structures as R. pseudoacacia in the area that can provide similar support structures29. Additionally, artificial support structures such as stakes or trellises could be used to provide support for individual plants until they are able to develop their own support structures29. Moreover, the mowing period overlapped with the flowering period of A. contorta. After mowing, the roots sprout again, but they may not grow high enough to flower. Because the higher the A. contorta, the better the flowers bloom29. Consequently, the mowing could affect reproductive traits, inhibiting sexual reproduction by A. contorta. However, the rate of asexual reproduction increased due to regular mowing, and new aboveground parts of A. contorta regenerated after sustaining damage from mowing. Perennial plants generally tend to reproduce new individuals through asexual reproduction from underground rhizomes or root shoots if the aboveground parts are damaged45. If there are human activities during the transitional period before flowering, the reproduction rate of A. Contorta could plummet and asexual reproduction could be active30. Thus, anthropogenic activities may facilitate asexual reproduction, thereby reducing the genetic diversity of A. Contorta. In fact, currently, the genetic diversity of A. contorta in South Korea is very low compared to A. contorta in Russia, China, and Japan30, 46.
In the places where mowing did not occur, other factors might have affected A. contorta growth. For instance, high stress resulting from interspecies competition could also bring changes in flowering and fruiting timing in plants47. Our results at site GC might indicate that interspecies competition (in this case with an invasive vine plant, S. angulatus) can affect the growth of the A. contorta population. Interspecies competition may encourage the deterioration of the growth condition of A. contorta.
Unexpectedly, the population of A. contorta at site PC showed more vigorous growth than at the other study sites: Flowering and fruiting were completed in early August 2021. In Korea, A. contorta blooms from July to August, and fruits appear from September to October48. Stressed plants eaten by herbivorous insects may engage in rapid flowering and fruiting49. High foraging pressure caused by crowding S. montela larvae may increase the stress of A. contorta individuals. As such, plant stress resulting from insect herbivory could be a factor accelerating the flowering period of plants50. Stress may have stimulated the early flowering and fruiting of plant, so the leaves were wilted early in the A. contorta population at site PC.
Larvae generally do not leave their host plants before reaching the third instar, except for early-stage larvae swept away by heavy rain22. It can be inferred that the larvae at site PC were looking for food due to the lack of fresh leaves by cutting the stem (personal observation of PSH). In this way, the changes in the vegetation of A. contorta may affect the population of S. montela (Fig. 3). Studies on invertebrates demonstrated a strong correlation between the abundance of phytophagous insects and edible biomass for larval resource21, 51, 52. Curtis et al.21 found that butterfly abundance was determined by food availability and was mediated by species traits. Especially, monophagous species with narrow diet breadth are far more likely to be resource-limited than polyphagous species53, 54. In addition, monophagous species are vulnerable to serving as a buffer for the lack of preferred plants, so the abundance of monophagous S. montela could be directly affected by host plant A. contorta availability. Management of host plants of target butterfly species can increase population abundance55. This, in turn, may allow individual species to attain higher population densities, thereby reducing the risk of extinction and consequently increasing the species abundance of the region56, 57.
An interview revealed that most of the growth and reproduction changes in A. contorta compared to those observed 4 years ago were due to anthropogenic activities. Because most interviewees were unaware of A. contorta and S. montela, selective weeding was performed frequently but not discriminately except at sites JW and YU. The selective weeding of invasive plants may positively affect the growth of A. contorta. Therefore, it is necessary to notify the weeders of the existence of A. contorta and S. montela and to tell them not to remove these plants because they should be protected.
The life history variation of S. montela under feeding conditions
In this study we investigated whether the types of plant organs the butterflies feed on and the application of pesticides on the food resources can affect the growth of S. montela. Wingspan, weight change, and survival rate of S. montela varied depending on the sort of available food (leaves or fruit), which may be related to the C/N ratio. The C/N ratio is considered an essential factor in plant–herbivore interaction. The leaves are usually rich in nitrogen, which is beneficial for the development of herbivorous insects58,59,60,61,62 and may consequently be preferred by herbivores. Furthermore, the high-carbon compounds in fruits significantly reduce the larvae's preference63. Therefore, when larvae ate fruits with higher carbon, they could not grow properly. When leaves are lacking, S. montela may look for some more leaves, requiring more nutrients to reach the adult stage64. However, the larvae are likely to be killed while looking for leaves like at site PC (personal observation of PSH).
The pesticides applied to the leaves and fruits used in the experiment were tricyclazole, benfuracarb, and buprofezin. Tricyclazole inhibits pentaketide-derived melanin biosynthesis in fungi and shows AF production–inhibitory activity65. Benfuracarb is a broad-spectrum benzofuranyl methylcarbamate insecticide used for crop protection, and it biochemically inhibits acetylcholinesterase activity66. Buprofezin, a thiadiazine insecticide, is effective against hemipteran pests and other insects67. Buprofezin inhibits chitin synthesis, resulting in molting disruption and abnormal deposition of endocuticles, causing premature death68, 69. They are also highly toxic to target and non-target organisms, including insects, mammals, birds, and aquatic organisms70,71,72,73,74. The larvae, which had been gaining weight by feeding on with-P/L, suddenly increased their mortality five days after the experiment started (Fig. 5). Because the larvae that fed on a little amount of pesticide-stained leaves and had a smaller weight change survived, the average growth curves for larvae reared in with-P/L suddenly decreased (Fig. 4). Many studies revealed that various pesticides could change the morphological characteristics of insects, such as body length, duration of juvenile development, survival rate, emergence rate, copulation rate, and hatchability75,76,77. Although the molecular mechanism of action of the pesticides could not be confirmed directly in our experiment, it obviously affected the phenotype of S. montela.
Conclusion
This study identified specific threatening factors which were a decrease of feeding organs due to weeding and pesticide spraying to A. contorta and S. montela. The riverine habitats of A. contorta and S. montela are likely to get damaged due to inattentive management methods. We suggest systematic conservation planning and management centered on biodiversity conservation to protect and conserve vulnerable species and their habitats even while managing invasive species.
Methods
Field observations and survey
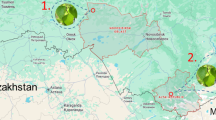
We investigated A. contorta habitats through literature and media reports and selected six sites on roadsides near rivers or rice paddies in South Korea (Fig. 7). Field surveys and observations were conducted from August to November in 2017 and 2021. We added site JP in 2021. Interviews related to habitat management were conducted from August 2021 to February 2022 with 11 stakeholders (1 government officer in each site of PC, MA, JW, GC, YU, 1 research center in JW, 1 subcontractor in GC, 2 laborers in MA, YU, 2 farmers in JP). Environmental properties (presence of accompanying invasive species and coverage of the invasive plant) and plant growth were surveyed with ten quadrats of 1 m × 1 m at each site in the same location in the years 2017, and 2021. The area of the population was different for each site, but each quadrat was at least 5 m apart. The coverage of all plant species was calculated by dividing the area of each species by the area of the quadrat (Coverage (%) = The covered area of each species/The area of the quadrat). Height was measured using the tallest A. contorta individual in the quadrat. The number of leaves and flowers of A. contorta was counted in each quadrat in August 2017 and 2021, and the number of fruits was counted in each quadrat in November 2017 and 2021 (the same site was investigated on a similar date). The number of eggs, larvae, and dead larvae of S. montela present on the stem and leaves of A. contorta and on the ground was recorded. Field observations were conducted to record any disturbances on host plants, such as mowing, weeding, trampling, and pesticide application. In association with direct observation, we interviewed laborers, city officials, and local people on the sites to record information on the past and present status of the habitat, threats, and conservation of the study areas.
We identified plants with illustrated plant books of Lee48 and Flora of Korea Editorial Committee78. S.-H.P took pictures of all the plants for certification according to the national regulation. There is no regulation for the collection of A. contorta in Korea and A. contorta is regarded as a weed by farmers.
Experimental setting
Based on field observations, factors that could threaten A. contorta were identified. At site JP, the condition of the A. contorta habitat was mainly suitable for S. montela, but mowing was performed and we captured farmers applying pesticides there. According to the farmers, the pesticides included tricyclazole, benfuracarb, and buprofezin. We collected A. contorta leaves and fruits at site JP for the experiment to investigate the effects of mowing and pesticides on the growth of S. montela. The C/N ratio is related to food quality, which in turn affects S. montela survival rate, and was analyzed with the part of the leaves and fruits using an Elemental Analyzer (Flash EA 1112, Thermo Electron, USA) by NICEM at Seoul National University. Site PC, whose C/N ratio of leaves and fruits was similar to site JP, was selected as the control. In order to exclude the predecessor feeding experience of the larvae in site PC and JP, 120 third-instar larvae, which have a low mortality rate from other causes22, were taken from site GC. Experimental settings were established in a test room at Seoul National University from August 2021 to September 2021. Three larvae were put into an insect breeding dish (10 cm diameter and 4 cm depth). Four experimental treatments (30 larvae per treatment) were performed with a factorial array of leaves × fruits of A. contorta, and pesticides sprayed × not sprayed. Feeding, molting, and development of larvae were observed in insect breeding dishes at 25 ± 2 °C. Larvae were weighed every 3–5 days, and larval weight change (increase or decrease) compared to their original weight was calculated. The body length of pupae and wingspan were measured using vernier calipers. For eclosions the pupae, glued to sticks were placed in the Butterfly Habitat Terrarium (60 × 60 × 90 cm). The number of deaths from the larval stage and eclosions from the pupae stage were recorded, and mortality rates as well as the number of days S. montela survived were calculated. After eclosions, we observed whether they mated and counted the number of eggs. All the experiments were performed in accordance with relevant guidelines (ethical guidelines, animal welfare, data management) and safety regulations.
Data analysis
To compare the growth and reproductive characteristics of A. contorta, and the average number of larvae and egg clusters of S. montela in 2017 and 2021, t-test at the 5% significance level were conducted after the homogeneity of variance test using SPSS ver.23.0 software (SPSS, Inc., Chicago, IL). Correlation analysis was performed to examine the relationships between variables (the coverage and the number of leaves per quadrat, the number of flowers per quadrat and the height) using SPSS. Pearson's correlation coefficient was used to assess the strength and direction of association between variables. The effects of feeding organs (leaves, fruits) and the effects of pesticides on morphological and reproductive traits of S. montela were also analyzed with the same process. The interactions between feeding on leaves × fruits and feeding on foods with pesticides × without pesticides were analyzed with a two-way analysis of variance (ANOVA) after homogeneity of variance was confirmed. Duncan’s test was used for post-hoc analysis.
Data availability
The article contains all of the data that were created and used in this investigation, and the corresponding authors can provide you with these data upon request.
References
Kunte, K. I. Lifescape: Butterflies of Peninsular India. (Universities, 2000).
Aluri, J. S. R. & Rao, S. P. Psychophily and evolutionary considerations of Cadaba fruticosa L. (Capparaceae). J. Bombay Nat. Hist. Soc. 99, 59–63 (2002).
Thomas, J. A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360(1454), 339–357. https://doi.org/10.1098/rstb.2004.1585 (2005).
Gatehouse, A. M., Ferry, N. & Raemaekers, R. J. The case of the monarch butterfly: A verdict is returned. Trends Genet. 18, 249–251. https://doi.org/10.1016/s0168-9525(02)02664-1 (2002).
Kremen, C. & Chaplin-Kramer, R. Insects as providers of ecosystem services: Crop pollination and pest control in Insect Conservation Biology. In Proceedings of the Royal Entomological Society’s 23rd Symposium 349–382. (CABI, 2007).
Crowder, D. W., Northfield, T. D., Strand, M. R. & Snyder, W. E. Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112. https://doi.org/10.1038/nature09183 (2010).
Wratten, S. D., Gillespie, M., Decourtye, A., Mader, E. & Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 159, 112–122. https://doi.org/10.1016/j.agee.2012.06.020 (2012).
van Swaay, C., Warren, M. & Loïs, G. Biotope use and trends of European butterflies. J. Insect Conserv. 10, 189–209. https://doi.org/10.1007/s10841-006-6293-4 (2006).
Hill, G. M., Kawahara, A. Y., Daniels, J. C., Bateman, C. C. & Scheffers, B. R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev. Camb. Philos. Soc. 96, 2113–2126. https://doi.org/10.1111/brv.12746 (2021).
Warren, M. S. et al. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proc. Natl. Acad. Sci. U. S. A https://doi.org/10.1073/pnas.2002551117 (2021).
Plieninger, T. Habitat loss, fragmentation, and alteration–quantifying the impact of land-use changes on a Spanish Dehesa landscape by use of aerial photography and GIS. Landsc. Ecol. 21, 91–105. https://doi.org/10.1007/s10980-005-8294-1 (2006).
Li, X. S., Zhang, Y., Fang, J., Schweiger, O. & Settele, J. A butterfly hotspot in western China, its environmental threats and conservation. J. Insect Conserv. 15, 617–632. https://doi.org/10.1007/s10841-010-9361-8 (2011).
Meng, H. H. et al. Conflict between biodiversity conservation and economic growth: Insight into rare plants in tropical China. Biodivers. Conserv. 28, 523–537. https://doi.org/10.1007/s10531-018-1661-4 (2019).
Matović, S. Habitat loss. In Climate Action. Encyclopedia of the UN Sustainable Development Goals (eds Leal Filho, W. et al.) (Springer, Cham, 2020). https://doi.org/10.1007/978-3-319-95885-9_57.
Nag, P. K., & Gite, L. P. Human-Centered Agriculture: Ergonomics and Human Factors Applied. (Springer Nature, 2020).
Revellin, C., Giraud, J. J., Silva, N., Wadoux, P. & Catroux, G. Effect of some granular insecticides currently used for the treatment of maize crops (Zea mays) on the survival of inoculated Azospirillum lipoferum. Pest Manag. Sci. 57, 1075–1080. https://doi.org/10.1002/ps.398 (2001).
Kilani-Morakchi, S. et al. Cuticular hydrocarbon profiles in Blattella germanica: Effects of halofenozide, boric acid and benfuracarb. Commun. Agric. Appl. Biol. Sci. 71(2 Pt B), 555–562 (2006).
Yi, J. H., Perumalsamy, H., Sankarapandian, K., Choi, B. R. & Ahn, Y. J. Fumigant toxicity of phenylpropanoids identified in Asarum sieboldii aerial parts to Lycoriella ingenua (Diptera: Sciaridae) and Coboldia fuscipes (Diptera: Scatopsidae). J. Econ. Entomol. 108, 1208–1214. https://doi.org/10.1093/jee/tov064,Pubmed:26470247 (2015).
Maes, D. & Van Dyck, H. V. Habitat quality and biodiversity indicator performances of a threatened butterfly versus a multispecies group for wet heathlands in Belgium. Biol. Conserv. 123, 177–187. https://doi.org/10.1016/j.biocon.2004.11.005 (2005).
Sax, D. F., Early, R. & Bellemare, J. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol. Evol. 28, 517–523. https://doi.org/10.1016/j.tree.2013.05.010 (2013).
Curtis, R. J., Brereton, T. M., Dennis, R. L. H., Carbone, C. & Isaac, N. J. B. Butterfly abundance is determined by food availability and is mediated by species traits. J. Appl. Ecol. 52, 1676–1684. https://doi.org/10.1111/1365-2664.12523 (2015).
Li, X. et al. On the ecology and conservation of Sericinus montelus (Lepidoptera: Papilionidae)–Its hreats in Xiaolongshan orests area (China). PLoS ONE 11, e0150833. https://doi.org/10.1371/journal.pone.0150833 (2016).
National Institute of Biological Resources. Red Data Book of Endangered Insects in Korea I. (Ministry of Environment, 2012).
Ackery, P. A guide to the geneara and species of Parnassiinae (Lepidoptera: Papilionidae). Bull. Br. Mus. Nat. Hist. (Ent.) 31, 16 (1975).
New, T. R. & Collins, N. M. Swallowtail Butterflies: an Action Plan for Their Conservation (i-iv, 1–36). (Gland, 1991).
Lim, M. S., Lee, B. H., & Lee, S. J. (Eds.). Endemic Species of Korea. National Institute of Biological Resources. Incheon, Korea (in Korean). (2012).
Sviridov, A. V. Sericinus montela Gray 1853. Red Book of Russian Soviet Federative Socialist Republic: Animals. (Rosagropromizdat, 1983).
Matsuka, H. An epoch of Sericinus montela. Yadoriga 103, 15–21 (1981).
Park, S. H., Nam, B. E. & Kim, J. G. Shade and physical support are necessary for conserving the Aristolochia contorta population. Ecol. Eng. 135, 108–115. https://doi.org/10.1016/j.ecoleng.2019.05.019 (2019).
Nakonechnaya, O. V., Kholina, A. B., Koren, O. G. & Zhuravlev, Y. N. Genetic diversity of a rare species Aristolochia contorta Bunge (Aristolochiaceae) in Primorsky Krai. Russ. J. Genet. 48, 152–162. https://doi.org/10.1134/S1022795411120088 (2012).
Stoytcheva, M. (Ed.). Pesticides in the Modern World: Risks and Benefits. (IntechOpen, 2011).
Altieri, M., & Nicholls, C. I. Ecological impacts of modern agriculture in the United States and Latin America. Glob. Rural Environ. 121–135 (2001).
Gurnell, A. et al. A multi-disciplinary frameworkfor assessing river channel sensitivity to environmental change. Wiley Interdiscip. Rev. Water 3, 513–532. https://doi.org/10.1002/wat2.1156 (2016).
Hashimoto, K. et al. Quantitative analysis of aristolochic acids, toxic compounds, contained in some medicinal plants. J. Ethnopharmacol. 64, 185–189. https://doi.org/10.1016/s0378-8741(98)00123-8 (1999).
Cheung, T. P., Xue, C., Leung, K., Chan, K. & Li, C. G. Aristolochic acids detected in some raw Chinese medicinal herbs and manufactured herbal products—A consequence of inappropriate nomenclature and imprecise labelling?. Clin. Toxicol. (Phila) 44, 371–378. https://doi.org/10.1080/15563650600671712 (2006).
Heinrich, M., Chan, J., Wanke, S., Neinhuis, C. & Simmonds, M. S. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2—A global assessment based on bibliographic sources. J. Ethnopharmacol. 125, 108–144. https://doi.org/10.1016/j.jep.2009.05.028 (2009).
Nakonechnaya, O. V., Gorpenchenko, T. Y., Voronkova, N. M., Kholina, A. B. & Zhuravlev, Y. N. Embryo structure, seed traits, and productivity of relict vine Aristolochia contorta (Aristolochiaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 208, 293–297. https://doi.org/10.1016/j.flora.2013.03.010 (2013).
Kim, D. S. & Kwon, Y. J. Metapopulation dynamics of the oriental long-tailed swallow Sericinus montela (Lepidoptera: Papilionidae) in Korea. Korean J. Appl. Entomol. 49, 289–297. https://doi.org/10.5656/KSAE.2010.49.4.289 (2010).
Ji, L. W. et al. The complete mitochondrial genome of the dragon swallowtail, Sericinus montela Gray (Lepidoptera: Papilionidae) and its phylogenetic implication. Acta Entomol. Sin. 55, 91–100 (2012).
Hong, S. J. et al. Temperature-dependent development of the Swallowtail Butterfly, Sericinus montela Gray. Int. J. Ind. Entomol. 29, 153–161. https://doi.org/10.7852/ijie.2014.29.2.153 (2014).
Park, H. J. et al. Reduced host plant growth and increased tyrosine-derived secondary metabolites under climate change and negative consequences on its specialist herbivore. Sci. Total Environ. 759, 143507. https://doi.org/10.1016/j.scitotenv.2020.143507 (2021).
Park, H. J. et al. Ontogeny-dependant effects of elevated CO2 and watering frequency on interaction between Aristolochia contorta and its herbivores. Sci. Total Environ. 838, 156065. https://doi.org/10.1016/j.scitotenv.2022.156065 (2022).
Dewalt, S. J., Schnitzer, S. A. & Denslow, J. S. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. J. Trop. Ecol. 16, 1–19. https://doi.org/10.1017/S0266467400001231 (2000).
Sandlund, O. T., Schei, P. J., & Viken, Å. (Eds.). Invasive Species and Biodiversity Management, vol. 24. (Springer Science & Business Media, 2001).
Cook, R. E. Asexual reproduction: A further consideration. Am. Nat. 113, 769–772. https://doi.org/10.1086/283435 (1979).
Nam, B. E. et al. An analysis of the genetic diversity of a riparian marginal species, Aristolochia contorta. J. Wetlands Res. 22, 100–105 (2020).
Weiner, J. A. C. O. B. The influence of competition on plant reproduction. Plant Reprod. Ecol. Patterns Strateg. 228–245 (1988).
Lee, T. B. Colored Flora of Korea. (Hyangmunsa, 2003).
Trumble, J. T., Kolodny-Hirsch, D. M. & Ting, I. P. Plant compensation for arthropod herbivory. Annu. Rev. Entomol. 38, 93–119. https://doi.org/10.1146/annurev.en.38.010193.000521 (1993).
Brody, A. K. Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78, 1624–1631. https://doi.org/10.1890/0012-9658(1997)078[1624:EOPHAS]2.0.CO;2 (1997).
Strong, D. R., Lawton, J. H. & Southwood, S. R. Insects on Plants. Community Patterns and mechanisms. (Blackwell Scientific Publicatons, 1984).
Marques, E. S. D. A., Price, P. W. & Cobb, N. S. Resource abundance and insect herbivore diversity on woody fabaceous desert plants. Environ. Entomol. 29, 696–703. https://doi.org/10.1603/0046-225X-29.4.696 (2000).
Dennis, R. L. H., Shreeve, T. G., Arnold, H. R. & Roy, D. B. Does diet breadth control herbivorous insect distribution size? Life history and resource outlets for specialist butterflies. J. Insect Conserv. 9, 187–200. https://doi.org/10.1007/s10841-005-5660-x (2005).
Mattila, J. et al. Increased habitat structure does not always provide increased refuge from predation. Mar. Ecol. Prog. Ser. 361, 15–20. https://doi.org/10.3354/meps07392 (2008).
Ellis, S., Bourn, N. A. & Bulman, C. R. (ed.). Landscape-Scale Conservation for Butterflies and Moths: Lessons from the UK (2012). (Butterfly Conservation, Wareham, 2012).
Hurlbert, A. H. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 7, 714–720. https://doi.org/10.1111/j.1461-0248.2004.00630.x (2004).
Evans, K. L., Greenwood, J. J. & Gaston, K. J. Dissecting the species–energy relationship. Proc. Biol. Sci. 272(1577), 2155–2163. https://doi.org/10.1098/rspb.2005.3209 (2005).
Clancy, K. M. & Price, P. W. Rapid herbivore growth enhances enemy attack: Sublethal plant defenses remain a paradox. Ecology 68, 733–737. https://doi.org/10.2307/1938479 (1987).
Lambers, H. A. N. S. & Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences Adv. Ecol. Res. 23(187), 261. https://doi.org/10.1016/S0065-2504(08)60148-8 (1992).
Cornelissen, J. H. C., Werger, M. J., Castro-Díez, P., van Rheenen, J. W. & Rowland, A. P. Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia 111, 460–469. https://doi.org/10.1007/s004420050259 (1997).
Garnier, E., Cordonnier, P., Guillerm, J. L. & Sonié, L. Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 111, 490–498. https://doi.org/10.1007/s004420050262 (1997).
Awmack, C. S. & Leather, S. R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844. https://doi.org/10.1146/annurev.ento.47.091201.145300 (2002).
Coley, P. D. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74, 531–536. https://doi.org/10.1007/BF00380050 (1988).
Mattila, A. L. & Kaitala, A. Effects of food quality on the life-history traits of the Glanville fritillary butterfly. Oecologia 157, 119–126 (2008).
Mander, L., & Liu, H. W. Comprehensive Natural Products II: Chemistry and Biology, vol. 1. (Elsevier, 2010).
European Food Safety Authority (EFSA) et al. Cumulative dietary risk assessment of chronic acetylcholinesterase inhibition by residues of pesticides. EFSA J. 19, e06392 (2021). https://doi.org/10.2903/j.efsa.2021.6392.
Ishaaya, I., Mendel, Z. & Blumberg, D. Effect of buprofezin on California Red scale, Aonidiella aurantii (Maskell), in a citrus orchard. Isr. J. Entomol. 25, 67–71 (1992).
Meola, S. M. & Mayer, R. T. Inhibition of cellular proliferation of imaginal epidermal cells by diflubenzuron in pupae of the stable fly. Science 207, 985–987. https://doi.org/10.1126/science.207.4434.985 (1980).
Prabhaker, N. & Toscano, N. C. Toxicity of the insect growth regulators, buprofezin and pyriproxyfen, to the glassy-winged sharpshooter, Homalodisca coagulata Say (Homoptera: Cicadellidae). Crop Prot. 26, 495–502. https://doi.org/10.1016/j.cropro.2006.04.019 (2007).
Iesce, M. R. et al. Transformation and ecotoxicity of carbamic pesticides in Water. Environ. Sci. Pollut. Res. Int. 13, 105–109. https://doi.org/10.1065/espr2005.10.285,Pubmed:16612899 (2006).
Sánchez-Moreno, S., Alonso-Prados, E., Alonso-Prados, J. L. & García-Baudín, J. M. Multivariate analysis of toxicological and environmental properties of soil nematicides. Pest Manag. Sci. 65, 82–92. https://doi.org/10.1002/ps.1650 (2009).
Abass, K., Reponen, P., Mattila, S., Rautio, A. & Pelkonen, O. Comparative metabolism of benfuracarb in vitro mammalian hepatic microsomes model and its implications for chemical risk assessment. Toxicol. Lett. 224, 290–299. https://doi.org/10.1016/j.toxlet.2013.08.009 (2014).
Eren, Y., Erdoğmuş, S. F., Akyıl, D. & Özkara, A. Mutagenic and cytotoxic activities of benfuracarb insecticide. Cytotechnology 68, 637–643. https://doi.org/10.1007/s10616-014-9811-3 (2016).
Lewis, R. A. Hawley's Condensed Chemical Dictionary. (Wiley, 2016).
Tiwari, S., Clayson, P. J., Kuhns, E. H. & Stelinski, L. L. Effects of buprofezin and diflubenzuron on various developmental stages of Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 68, 1405–1412. https://doi.org/10.1002/ps.3323 (2012).
Wang, Z., Zhou, C., Long, G., Yang, H. & Jin, D. Sublethal effects of buprofezin on development, reproduction, and chitin synthase 1 gene (SfCHS1) expression in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia Pac. Entomol. 21, 585–591. https://doi.org/10.1016/j.aspen.2018.03.009 (2018).
Guo, S., Wu, Y., Xiao, P. & Li, W. Benfuracarb inhibits body growth and causes oxidative stress in zebrafish (Danio rerio). Chemosphere 291, 132955. https://doi.org/10.1016/j.chemosphere.2021.132955 (2022).
Flora of Korea Editorial Committee. The Genera of Vascular Plants of Korea. (Hongreung Publishing Company, 2018).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A2041895) and by Korea Environment Industry & Technology Institute (KEITI) through 'Wetland Ecosystem Value Evaluation and Carbon Absorption Value Promotion Technology Development Project, funded by Korea Ministry of Environment (MOE) (2022003640003).
Author information
Authors and Affiliations
Contributions
J.G.K. and S.-H.P. designed and directed the studies. S.-H.P. conducted a field survey and experiment, took pictures of all the plants for certification, and wrote the first manuscript. J.H.K. determined the structures, performed calculations, and fix the manuscript. All the authors analyzed the data, discussed the results, and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, SH., Kim, J.H. & Kim, J.G. Effects of human activities on Sericinus montela and its host plant Aristolochia contorta. Sci Rep 13, 8289 (2023). https://doi.org/10.1038/s41598-023-35607-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-35607-5