Abstract
This study aims to clarify the effects of different concentrations of sodium chloride on the carbon and nitrogen metabolism and yield of Tartary buckwheat. The salt-sensitive cultivar Yunqiao 2 was pot-grown and treated with four salt concentrations including 0, 2, 4, and 6 g kg−1. The root morphology index increased from seedling stage to maturate stage. The content of soluble protein in the leaves reached the maximum at the anthesis stage, and the other substances content and the enzymes activity related to carbon and nitrogen metabolism reached the maximum at the grain filling stage. The root morphology index, root activity; invertase, amylase, sucrose synthase, and sucrose phosphate synthase activities; nitrate-nitrogen, ammonium nitrogen, and soluble protein content; and nitrate reductase and glutamate synthase activities increased first and reached the maximum at 2 g kg−1 treatment and then decreased with increasing salt stress concentration. The content of soluble sugars and sucrose and the activity of glutamate dehydrogenase increased continuously with increasing salt concentration, and reached the maximum in the 6 g kg−1 treatment. The grain number per plant, 100-grain weight, and yield per plant increased first and reached the maximum at 2 g kg−1 treatment and then decreased with increasing salt stress concentration. In summary, moderate salt stress (2 g kg−1) can promote the root growth, increase the content of carbon and nitrogen metabolism-related substances and enzyme activity, and increase the yield per plant of Tartary buckwheat.
Similar content being viewed by others
Introduction
Saline-alkali soil in China has an extensive distribution. The total area of saline soil is 3.69 × 107 hm2, accounting for about 5% of the total cultivated land area in China. The area of saline soil ranks third in the world, and the area of saline soil are still increasing year by year1. Soil salinization is one of the major types of abiotic stress limiting agricultural development2. The excessive accumulation of sodium chloride (NaCl) in saline soil result in osmotic stress, ion toxicity, and oxidative stress in plants, resulting in a sharp decrease in seed germination, chlorophyll degradation, and photosynthetic rates and changes in osmotic pressure. The absorption of salt by transporters from soil induces ion toxicity and disrupts ion absorption and its metabolic balance, thus affecting the normal growth of plants3,4,5.
Nitrogen and carbon metabolism are the two most important metabolic processes in plants and play a vital role in the whole life activities of plants6,7. Nitrogen is one of the basic nutrients required for plant growth and development8. It is the main component of amino acids and proteins and can regulate nitrogen assimilation and carbohydrate metabolism. Plants use nitrogen in the form of nitrate and ammonium, which are converted into various amino acids9. Apart from the legume family, higher plants fix nitrogen by first reducing its inorganic form NO3− to NH4+ and then incorporating it into an organic form10,11. This process mainly occurs through the glutamine synthetase/glutamate synthase (GS/GOGAT) cycle12,13. Nitrogen metabolism is regulated by external factors, such as heat, drought, and salinity14. Salt stress inhibits nitrogen uptake and metabolism, especially nitrate uptake by plants15. Sha15 found that nitrogen content in rice leaves and sheath decreases significantly under salt stress, nitrate-nitrogen content decreases, and ammonium nitrogen content increases. Yang16 found that nitrate reductase (NR) activity decreases with nitrate content in plants under salt stress. Lutts et al.17 found that salt stress significantly reduces the activity of GS in rice leaves and the GS activity of salt-sensitive varieties is lower than that of salt-tolerant varieties.
In plants, carbon metabolism is the most important and basic physiological metabolism, which is of great significance to plant growth and development, transformation of various chemical components, and final yield8. Invertase, sucrose synthase (SS), sucrose phosphate synthase (SPS), and amylase are important enzymes in the carbon metabolism pathway of plants, and their activities directly affect the accumulation of carbohydrates in plants. Invertase is a hydrolase that can convert sucrose into monosaccharide and facilitate the remobilization of sugar in plants. A series of glucose metabolism disorders occurs in plants under stress. Salt stress has significant effects on carbon metabolism in plants. Peng et al.18 reported that salt stress increases the activities of SS and SPS in cotton leaves. The content of sucrose and fructose in plant cells increases with salt concentration19. Strong salt-tolerant wheat alleviates salt stress by synthesizing large amounts of soluble sugars and soluble protein20. Salt stress promotes the synthesis and accumulation of soluble sugars in leaves of different salt-tolerant rice cultivars21.
Tartary buckwheat (Fagopyrum tataricum Gaertn) belongs to genus Fagopyrum Mill. It has remarkable functions, such as lowering blood sugar, blood pressure, and blood lipid level22 and has great market demand globally, especially in Europe, America, and Southeast Asia23. To date, research on Tartary buckwheat and salt stress mainly focuses on seed germination24, seedling physiological characteristics25, genomics and transcriptomics26. The effects of salt stress on the carbon and nitrogen metabolism of Tartary buckwheat are rarely explored. Therefore, this experiment used the salt-sensitive Tartary buckwheat variety Yunqiao 2 as the experimental material in studying the effects of salt stress on the root morphology and physiology, leaf carbon and nitrogen metabolism, and yield formation of Tartary buckwheat to reveal the relationship between the salt tolerance and carbon and nitrogen metabolism of Tartary buckwheat and provide a reference for the high-yield cultivation of Tartary buckwheat.
Materials and methods
Plant materials and growing condition
The seeds of salt-sensitive Tartary buckwheat variety Yunqiao 2 used in this study were collected from the Yunnan Academy of Agricultural Sciences, China (25° 05′ N, 102° 71′ E), and complied with relevant institutional, national, and international guidelines and legislation. We have obtained the permission to collect seeds. The experiment was conducted from September to November 2021 at the growth chamber and outdoor potting test site of the Research Center of Buckwheat Industry Technology of Guizhou Normal University (106° 27′ E, 26° 44′ N). A canopy was built on top of the outdoor potted plant test site to prevent rain. The monthly average temperatures and monthly average sunshine from September to November were 16.9 °C and 193.8 h in 2021 (data obtained from the Guiyang Meteorological Bureau website of China; http://gz.cma.gov.cn/dsqxj/gy/index.html; accessed on May 5, 2022). The walls and roof of the growth chamber were made of translucent polyurethane material. Sunlight was projected, and temperature could not be controlled. The monthly average temperature from September to November of the growth chamber was 17.0 °C and the average relative humidity was 50.0%.
Treatment
New soil (pH 6.29) containing 10.82 g kg−1 organic matter, 2.97 mg kg−1 available nitrogen, 14.71 mg kg−1 available phosphorus, and 103.76 mg kg−1 available potassium was collected from the experiment station. Soil nutrient contents were determined using a multichannel intelligent soil nutrient meter (OK-V24, China).
According to the quality of soil in the porcelain pot, sodium chloride (NaCl) was added for the preparation of low-salt (S2), medium-salt (S4), and high-salt (S6) soil with salt content of 2, 4, and 6 g kg−1, respectively. New soil without NaCl was used as the control (CK, NaCl 0 g kg−1). Each porcelain pot (25 cm in diameter and 18 cm in height) was filled with 5 kg of soil with a different salt concentration.
On September 8, 2021, the seeds of Tartary buckwheat with the same size were selected and disinfected with 1.2% HgCl2 for 30 min, washed with distilled water, and air-dried, and sowed. Fifteen seeds were sowed in each porcelain pot, and 10 seedlings were set after emergence. Instead of compound fertilizer (N: P: K = 15:15:15) dosage of 600 kg ha−1 (usually used by local farmers), 2.9 g of compound fertilizer as the base fertilizer was applied once per pot before sowing and fully mixed with saline soil. Fertilization was not performed in the whole growth period. Every 10 days, 1.5 L of water was added to each porcelain pot, and leaching caused by salt reduction was prevented by returning water from the bottom tray to the porcelain pot immediately. Each treatment was performed on 20 porcelain pots.
Sampling and measurements
Root morphology and root activity
Five Tartary buckwheat plants with similar growth were randomly selected from porcelain pots treated in the growth chamber and outdoor pot experiment field at the seedling stage (19 days after sowing), flowering stage (40 days after sowing), grain filling stage (55 days after sowing), and maturation stage (outdoor, 77 days after sowing; growth chamber, 80 days after sowing), and the whole plants were dug. After carefully scooping up soil near the plant with a shovel, the plant was gently pulled up, and the integrity of the root system was preserved as much as possible. After washing with clean water, the Tartary buckwheat roots were cut. After surface water in the roots was removed with absorbent paper, all roots were image-scanned using a root scanner (GXY-A, Zhejiang Tuopu Instrument Co., Ltd.)27. Root length, surface area, volume, and mean diameter were obtained with the root analysis system WinRHIZO (version 4.0b, Regent Instruments Inc.). Root activity was determined using the 2,3,5-triphenyl-tetrazolium chloride method28.
Substance content and enzyme activity related to carbon and nitrogen metabolism
At the seedling stage, flowering, grain filling, and mature stages, fresh leaves on the one to four sections at the top of the main stem of Tartary buckwheat were collected from the growth chamber and outdoor potting test field, respectively. Some of the leaves were frozen in liquid nitrogen for 30 s and quickly stored in a refrigerator at − 80 °C. The other part was deactivated at 105 °C for 15 min, dried to constant weight in an oven at 80 °C, crushed, passed through a 40-mesh sieve, and stored.
The content of soluble sugars was determined by anthrone colorimetry. 0.2 g of dry Tartary buckwheat leaves was weighed, ground evenly with 4 mL of 80% ethanol, and kept in a water bath at 80℃ for 30 min. After centrifugation at 5000 r/min for 10 min, the supernatant was transferred, and the residue was added with 2 mL of 80% ethanol for repeated extraction. Then, the supernatant was combined. The activated carbon was added, decolored in a water bath for 30 min, diluted to 10 mL, and filtered to obtain soluble sugars and sucrose solution. 0.5 mL of the solution to be measured was mixed with an equal volume of distilled water, and 5 mL of anthrone sulfate solution was added to shake quickly. The mixture was boiled for 10 min, and the light absorption value at 620 nm was measured after cooling28.
The sucrose content was determined by using the resorcinol method. 0.4 mL of the above mentioned test solution and 0.2 mL of 2 mol/L NaOH were collected and boiled in water for 5 min. After cooling, 2.8 mL of 30% HCl and 0.8 mL of 0.1% resorcinol reagent were added. The mixture was shaken, mixed, and kept at 80℃ for 10 min. The optical density value at 480 nm was measured after cooling28.
The method of He29 was used to determine invertase activity. 0.5 g of fresh Tartary buckwheat leaves was ground with pre-cooled distilled water and diluted to 10 mL. After centrifugation at 4000 r/min for 15 min, the supernatant was collected for analysis. After mixing 1 mL of crude enzyme solution, 2.5 mL of pH6.0 phosphate-buffered saline, and 0.5 mL of 10% sucrose solution, the mixture was incubated in a water bath at 37 °C for 0.5 h. One milliliter of the above mentioned mixture was collected and added with 0.75 mL of 3,5-dinitrosalicylic acid, and the mixture was boiled for 5 min. After cooling in an ice-water bath, the mixture was diluted to 10 mL with distilled water, and the OD value at 540 nm was determined.
The SS and SPS activities were determined in accordance with the method of Guo30. 0.5 g of fresh Tartary buckwheat leaves was ground evenly with 3 mL of Hepes–NaOH extraction buffer and centrifuged at 10000 r/min for 10 min. The supernatant was taken as the enzyme solution to be tested. The reaction mixture system 50 μL of Hepes–NaOH buffer (pH7.5), 20 μL of 0.05 mol/L MgCl2, 20 μL of 0.1 mol/L UDPG, 20 μL of 0.1 mol/L fructose, and 50 μL of crude enzyme extract. The control was replaced with the inactivated enzyme solution. The above mentioned mixture was bathed in water at 30 °C for 0.5 h, and 0.2 mL of 2 mol/L NaOH solution was added to terminate the reaction. The mixture was bathed in boiling water for 10 min. After cooling, 1.5 mL of 30% HCl and 0.5 mL of 0.1% resorcinol were added, and the mixture was bathed in water at 80 °C for 10 min. The optical density value at 480 nm was recorded.
The extraction method of SPS activity was the same as that of SS. In the reaction mixture system, fructose 6-phosphate was used instead of fructose, and the other methods were the same as those of SS.
Amylase activity was determined in accordance with the method of Tang and Luo28. First, 2–5 g of fresh leaves was collected, added with 2–4 mL of pH5.6 citric acid buffer in the mortar, ground into homogenate, added with 6 mL of buffer, transferred to a 50 mL volumetric flask, and extracted in a 40 °C water bath for 1 h. Afterward, the mixture was shaken, stand, and centrifuged. Ten millimeters of the supernatant was absorbed. Subsequently, 2 mL of 8% oxalic acid solution was added to each tube and bathed in boiling water for 15 min. Then, two drops of 0.1% phenolphthalein reagent were added to each tube and neutralized with 1 mol · L−1 NaOH solution at a constant volume of 25 mL. One milliliter of the aforementioned reagent was added into a 10 mL test tube, added with 1 mL of DNS reagent, boiled for 5 min, and quickly cooled.Then, distilled water was used to scale, and the OD value was measured at a wavelength of 520 nm.
The content of nitrate nitrogen was determined using the method of Tang and Luo28. Three milliliters of distilled water was added to a mortar containing 0.5 g of fresh Tartary buckwheat leaves for grinding. The grinding solution was extracted in a 100 °C water bath for 0.5 h, and the volume was adjusted to 5 mL after filtration. 0.4 mL of salicylic acid–sulfuric acid solution was added to 0.1 mL of test solution. The mixture was stand for 20 min and added with 9.5 mL of 0.08 g/mL NaOH solution. The light absorption value was measured at a wavelength of 410 nm.
The content of ammonium nitrogen was determined using the method of Tang and Luo28. 0.5 g of fresh Tartary buckwheat leaves was ground into a homogenate with 5 mL of 10% acetic acid solution, diluted with distilled water to 100 mL, and filtered for later use. Two milliliters of the test solution was added with 3 mL of ninhydrin hydrate and 0.1 mL of 1% ascorbic acid and heated at 100 °C for 15 min. After cooling, the liquid was diluted to 10 mL with anhydrous ethanol, and the optical density value was measured at OD580 nm after shaking.
The soluble protein content was determined by using the Coomassie brilliant blue method28. 0.3 g of fresh Tartary buckwheat leaves was added to 5 mL of distilled water, ground, and centrifuged at 3000 r/min for 10 min. 0.5 mL of the supernatant and equal volume of distilled water were added to 5 mL of Coomassie brilliant blue solution, and the light absorption value was measured at 595 nm after 2 min.
The activity of NR was determined in accordance with the method of Tang and Luo28. 0.5 g of fresh Tartary buckwheat leaves was ground with 4 mL of extract and centrifuged at 4000 r/min for 15 min. The supernatant contained the test solution. 0.4 mL of enzyme solution was added to 1.2 mL of 0.1 mol/L KNO3 phosphate buffer and 0.4 mL of NADH solution, and the solution was bathed in water at 25 °C for 0.5 h. One milliliter of sulfonamide solution was added to terminate the reaction, and then 1 mL of naphthyl vinylamine was added to develop the color for 15 min. After centrifugation, the supernatant was collected, and the OD value was measured at 540 nm.
GOGAT activity was determined in accordance with the method of Yu31. The extraction method of crude enzyme solution was the same as that of GS. The reaction mixture consisted of 1.5 mL of Tris–HCl buffer (pH 7.6), 0.1 mL of 10 mmol/L KCl, 0.5 mL of 20 mmol/L α-ketoglutarate, 0.2 mL of 3 mmol/L NADH, 0.3 mL of enzyme solution, and 0.4 mL of 20 mmol/L L-glutamine. After the reaction was started, the change in OD value was recorded every 20 s at 340 nm for 10 consecutive times, and the change in enzyme activity was measured by decreasing the optical density value.
The activity of glutamate dehydrogenase was determined in accordance with the method of Loulakakis and Roubelakis-Angelakis32. The extraction method of crude enzyme solution was the same as that of GS. The reaction system contained 2.6 mL of crude enzyme solution, 0.1 mL of ddH2O, 0.1 mL of 30 mmol/L CaCl2, 0.1 mL of 6 mmol/L NADH, and 0.1 mL of crude enzyme extract. The change in OD value was measured at 340 nm. The OD value was recorded every 20 s and continuously measured 10 times. A section with a stable decrease in OD value was taken to measure the change in enzyme activity.
Yield
Grains were harvested from each porcelain plot when at least 70–80% of the grains were mature (Outdoor, November 24; growth chamber, November 27). After the seeds were naturally dried, the number of grains per plant and yield per plant were determined, and the average of three replicates was calculated7,27.
Statistical analyses of experimental data
Data were processed using Microsoft Excel 2003 and SPSS 22.0. One-way ANOVA was performed, and means were tested by least significant difference at P = 0.05 (LSD 0.05). The results of the growth chamber and outdoor potting test site were similar. Therefore, the outdoor potting test site data were presented in this study, and the data from the growth chamber were deposited as supplementary data.
Results
Effects of salt stress on root morphology and root activity
The root length, root surface area, and root volume of Tartary buckwheat were increased from seedling stage to maturity stage (except the root length of S4 and S6 treatments; Table 1). The average root diameter reached a peak at the grain filling stage. Root activity decreased gradually with growth period. The root length, root surface area, root volume, average root diameter, and root activity in the S6 treatment were the lowest. Compared with CK, the S2 treatment increased the root length, root surface area, root volume, average root diameter, and root activity by 6.41%, 13.04%, 8.33%, 15.36%, and 17.50%, respectively.
Effects of salt stress on contents of carbon metabolism related substances
The content of soluble sugars and sucrose in the leaves of Tartary buckwheat reached the maximum at the grain filling stage and the minimum at the seedling stage (Table 2). The content of soluble sugars and sucrose in leaves increased continuously with increasing salt concentration. Compared with CK, the S6 treatment increased the soluble sugars and sucrose content by 1.36 and 1.40 times.
Effects of salt stress on enzyme activities related to carbon metabolism
The activities of invertase, amylase, SS, and SPS in the leaves reached a peak at the grain filling stage (Table 3). The activities of invertase, amylase, SS, and SPS in the S2 treatment were 1.02, 1.08, 1.07, and 1.09 times higher than those of CK. The leaves under each treatment were sequenced according to enzyme activities as follows: S2 > CK > S4 > S6.
Effects of salt stress on contents of nitrogen metabolism related substances
The content of nitrate-nitrogen and ammonium nitrogen reached the maximum at the grain filling stage, and the content of soluble protein reached a peak at the anthesis stage (Table 4). The S6 treatment had the lowest content of nitrate-nitrogen, ammonium nitrogen, and soluble protein. Compared with CK, the S2 treatment increased the nitrate-nitrogen, ammonium nitrogen, and soluble protein content by 5.84%, 6.65%, and 2.20%, respectively.
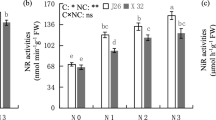
Effects of salt stress on activities of nitrogen metabolism related enzymes
The activities of NR, GOGAT, and GDH in the leaves reached a peak at the grain filling stage (Table 5). Compared with CK, the S2 treatment increased the NR and GOGAT activities by 1.61% and 2.10%, respectively. The S6 treatment had the highest GDH activity.
Effect of salt stress on the yield of Tartary buckwheat
The yield per plant of the S2 treatment was significantly higher than the yield of the CK, S4, and S6 treatments (Table 6). The 100-grain weight and grain number per plant of the S2 and CK treatments were significantly higher than those of the S4 and S6 treatments. Compared with CK, the S2 treatment increased the number of grains per plant, 100-grain weight, and yield per plant by 7.06%, 2.13%, and 3.16%, respectively.
Discussion
Effects of salt stress on the carbon metabolism of Tartary buckwheat
The activity of carbon metabolism-related enzymes in plants is an important index for measuring the intensity of carbon metabolism. SS and SPS are soluble enzymes in the cytoplasm. SS mainly plays a role in cell wall and starch synthesis process and a key role in sucrose degradation33. SPS plays an active role in inducing sucrose synthesis in plants, and its enzymatic activity can directly determine the content of soluble sugars and reflect the “source” supply capacity to some extent. In plants, sugars serve as metabolic resources and structural components of cells, and sugars undergo osmotic adjustment under various stress conditions34,35,36. Sucrose is the main source of carbon and energy for plant metabolism.
Plants initiate regulatory mechanisms under stress conditions, adjust sucrose content by changing SS and SPS activities, and promote carbohydrate redistribution to adapt to stress37. Low salt stress induces SS and SPS activities in plants, thereby enhancing the ability of crops to synthesize sucrose and soluble sugars, and the accumulation of carbohydrates maintains cell osmotic pressure and exerts a positive effect, thereby enhancing resistance and ensures normal growth. By contrast, high salt stress decreases SS and SPS activities and inhibits the synthesis and transport of sucrose in the leaves and plant growth and development38,39. Wang et al.40 found that exogenous glucose and sucrose contribute to the growth of triticale seedlings under salt stress and low salt stress can induce increases in SPS and SS activities. In the present study, low salt stress promoted increases in SPS and SS activities in Tartary buckwheat leaves (Table 3), consistent with the above results.
Liu et al.3 pointed out that salt stress causes an increase in soluble sugars content. Gao41 found that soluble sugars content in rice leaves gradually increases with the level of saline-alkali stress. The results of this experiment showed that salt stress promoted the increase of soluble sugars and sucrose content in Tartary buckwheat leaves, consistent with the above research results. The reason may be related to self-regulation under stress. Salt stress changes ion concentration in leaves, thereby increasing SS and SPS activities and directly promoting the accumulation of sucrose and soluble sugars. The accumulation of large amounts of sucrose inhibits SS activity by negative feedback, weakening the inhibitory ability of SS reverse regulation on soluble sugars synthesis pathway. SS activity directly promotes the accumulation of total soluble sugars, and the accumulation of soluble sugars content have a significant effect on improving plant resistance. In the results of the present experiment, the activity of SS and SPS decreased significantly with salt stress treatment period and salt concentration. Therefore, sucrose in the leaves tended to degrade sugar and glucose. However, it only accounted for a small part of soluble sugars14. Therefore, the hydrolysis of sucrose into small molecular carbohydrates may not be the main mechanism by which Tartary buckwheat resists salt stress.
Effects of salt stress on nitrogen metabolism in Tartary buckwheat
Nitrogen is a macro-essential element in plants and participates to structure of chlorophyll, proteins, nucleic acids, and rubisco involved in CO2 assimilation and quaternary ammonium compounds related to stress tolerance42. Stress restricts assimilation and translocation by limiting uptake and transport of NO3− to plants and impairs the activities and synthesis of enzymes involved in assimilation43. Depending on these changes, stress limits the development, growth, and fertility of plants44. Katiyar and Dubey45 showed that the endogenous NR activity of salt-tolerant rice varieties increases significantly in response to salt stress, whereas the endogenous NR activity of salt-sensitive varieties decreases significantly in response to salt stress. GOGAT and GS constitute to the GS/GOGAT pathway, which catalyzes NH4+ assimilation reaction. This pathway is the center of the whole nitrogen metabolism, and most of the nitrogen content in plants is assimilated by this pathway46. Liu et al.47 found that the activities of NR, GS, and GOGAT increases under mild saline-alkali stress and short stress time, which stimulates the absorption of nitrogen by sugar beet, thereby reducing the harm of stress. However, it decreases significantly with further increase in saline-alkali stress level or stress time. The results of the present study showed that the activities of NR and GOGAT in Tartary buckwheat leaves first increased and then decreased with increasing salt concentration, reaching the maximum in the S2 treatment and the minimum in the S6 treatment. These results are basically consistent with the results of Liu et al.47 and indicate that moderate salt stress can increase the activities of enzymes related to nitrogen metabolism in Tartary buckwheat leaves which through nitrogen metabolism enzymes that can reduce the oxidative stress response of Tartary buckwheat under salt stress and can thus improve the stress resistance of plants2.
Soluble protein is an important organic osmotic adjustment substance in plants and plays an important role in alleviating damage. Its content is closely related to plant nitrogen metabolism and senescence. Cai et al.48 found that the soluble protein content of sweet potato seedlings under salt stress increases first and then decreases with increasing salt concentration. Similar results were obtained in the current experiment. When S2 was treated, the soluble protein content in the leaves of Tartary buckwheat reached the maximum and then decreased with further increase in salt concentration. Decrease in soluble protein content in leaves under high salt stress may be related to the fact that the Tartary buckwheat samples were in a state of nitrogen deficiency for a long time under salt stress and the activity of proteolytic enzymes in plants increased and promoted proteolysis.
Nitrate-nitrogen and ammonium nitrogen are the two most important inorganic nitrogen compounds absorbed and utilized by plants. The results of this experiment showed that the content of nitrate-nitrogen and ammonium nitrogen in the leaves increased first and then decreased with increasing salt concentration, reached the maximum in the S2 treatment, decreased with further increase in salt concentration, and was lower than that in the control treatment. The results indicated that salt stress had a “low promotion and high inhibition” effect on the content of nitrate-nitrogen and ammonium nitrogen possibly due to the competitive absorption of Cl− and NO3− under salt stress or the inhibition of nitrate on transport.
Effects of salt stress on yield of Tartary buckwheat
Meng et al.49 showed that the spike number, grain number per spike, and 1000-grain weight of wheat decreased under salt stress, and the decrease rate increased with salt concentration. Chen et al.50 showed that the spike number and 1000-grain weight of oats were greatly affected by salt stress, and the yield decreased. Zhang et al.51 found that salt stress significantly decreased 1000-grain weight of rice. Consistent with the results mentioned above, the present study showed that the grain number per plant, grain weight per plant, and 100-grain weight of Tartary buckwheat first increased and then decreased with the increasing salt concentration, indicating that low concentration of salt stress can promote the growth and yield of Tartary buckwheat. This may be due to promoted root growth and root activity of Tartary buckwheat under low salt stress (Table 1). These effects facilitate the absorption of water and nutrients in the soil tillage layer by Tartary buckwheat roots, improve the net photosynthetic rates of the above-ground leaves, prolong the photosynthesis time of leaves, and improve dry matter production52,53, thereby increasing final yield per plant (Table 6). The possible reason also is that because low salt stress can increase the activities of key enzymes in carbon and nitrogen metabolism in Tartary buckwheat and increase the synthesis, transport, and accumulation of organic compounds in plants, resulting in increased yield per plant. High salt stress inhibits photosynthesis and Tartary buckwheat growth and thus affects dry matter production, accumulation, transfer, and distribution and leads to a decrease in yield per plant.
Conclusions
Low salt stress treatment (2 g kg−1) promoted root growth, improved root activity, significantly increased the content of carbon and nitrogen metabolites and related enzyme activities in the leaves of Tartary buckwheat, ensured the metabolic balance of cells, and improved the osmotic adjustment ability by accumulating osmotic adjustment substances, such as soluble sugars. As result, the tolerance of Tartary buckwheat and the yield per plant under salt stress increased. High concentration of salt treatment reduced the osmotic adjustment substances such as soluble sugars, destroyed the structure and function of cell membrane, accelerated senescence, affected the physiological metabolism and photosynthesis, and thus affects dry matter production and accumulation and leads to inhibited the growth of Tartary buckwheat. The effects of salt stress on the growth of Tartary buckwheat were low promotion and high inhibition. The tolerance of Tartary buckwheat to salt stress has a threshold, that is, 2 g kg−1.
Data availability
The data that support this study are available in the article and accompanying online Supplementary material.
References
Zhu, J. F. et al. Research advances and prospect of saline and alkali land greening in China. World For. Res. 31, 70–75 (2018).
Rezayian, M. & Zarinkamar, F. Nitric oxide, calmodulin and calcium protein kinase interactions in the response of Brassica napus to salinity stress. Plant Biol. 25, 411–419 (2023).
Liu, L. et al. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 9, 275 (2018).
Navada, S. et al. Biofilms remember: Osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res. 176, 115732 (2020).
Rezayian, M., Ebrahimzadeh, H. & Niknam, V. Metabolic and physiological changes induced by nitric oxide and its impact on drought tolerance in soybean. J. Plant Growth. Regul. 42, 1905–1918 (2023).
Khalil, R., Yusuf, M., Bassuony, F., Haroun, S. & Gamal, A. Alpha-tocopherol reinforce selenium efficiency to ameliorates salt stress in maize plants through carbon metabolism, enhanced photosynthetic pigments and ion uptake. S. Afr. J. Bot. 144, 1–9 (2022).
Zhou, Q., Tang, J., Liu, C., Huang, K. & Huang, X. Effects of phosphate fertilizer application on the growth and yield of tartary buckwheat under low-nitrogen condition. Agronomy 13(7), 1886. https://doi.org/10.3390/agronomy13071886 (2023).
Singh, M., Singh, V. P. & Prasad, S. M. Nitrogen modifies NaCl toxicity in eggplant seedlings: Assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocatal. Agric. Biotechnol. 7, 76–86 (2016).
Giagnoni, L. et al. Availability of different nitrogen forms changes the microbial communities and enzyme activities in the rhizosphere of maize lines with different nitrogen use efficiency. Appl. Soil Ecol. 98, 30–38 (2016).
Beatty, P. H. et al. Understanding plant nitrogen metabolism through metabolomics and computational approaches. J. Exp. Bot. 68, 2501–2512 (2017).
Oliveira, H. C., Justino, G. C., Sodek, L. & Salgado, I. Amino acid recovery does not prevent susceptibility to Pseudomonas syringae in nitrate reductase double-deficient Arabidopsis thaliana plants. Plant Sci. 176, 105–117 (2009).
Liu, Y. & Von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 68, 2581–2592 (2017).
Miflin, B. J. & Habash, D. Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 53, 979–987 (2002).
Zandalina, S. I., Mittler, R., Balfagón, D., Arbona, V. & Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plantarum. 162, 2–12 (2018).
Sha, H.J. Effects of salicylic acid, proline and γ-aminobutyric acid on nitrogen metabolism, yield, and quality of rice under salt stress. Doctor’s thesis, Northeast agricultural university (2018).
Yang, C. W. Mechanisms of alkali tolerance in Chlorisvirgata and rice (Oryza sativa). Doctor’s thesis, Northeast Normal University (2010).
Lutts, S., Majerus, V. & Kinet, J. M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plantarum 105, 450–458 (1999).
Peng, J. et al. Effects of soil salinity on sucrose metabolism in cotton leaves. PLoS ONE 11, e0156241 (2016).
Wang, X. M. Analysis of physiological effects on four rice germplasms genotype to salinity stress. Master’s thesis, Guangdong Ocean University (2019).
Zhang, Y., Shi, S. Q., Li, Y. P., Gao, T. P. & Yang, Y. L. Osmotic regulation and chlorophyll fluorescence characteristics in leaves of wheat seedlings under different salt stress. Chin. J. Appl. Ecol. 32, 4381–4390 (2021).
Xu, C. Research of physiological response mechanism in different rice varieties under salt stress. Master’s thesis, Jilin Agricultural University (2013).
Luthar, Z., Golob, A., Germ, M., Vomberger, B. & Kreft, L. Tartary Buckwheat in human nutrition. Plants 10, 700 (2021).
Huang, K. F. et al. Research progress on physiology of buckwheat under high-yield cultivation. J. Guizhou Norm. Univ. (Nat. Sci. Ed.) 37, 115–120 (2019).
Qin, P. Y. et al. Low concentration of sodium bicarbonate improves the bioactive compound levels and antioxidant and α-glucosidase inhibitory activities of tartary buckwheat sprouts. Food Chem. 224, 124–130 (2017).
Lei, X. H., Leng, J. J., Tao, J. C., Wan, C. Q., Wu, Y. X., Wang, J. L., Wang, P. K., Feng, B. L. & Gao, J. F. Effects of foliar spraying selenium on photosynthetic characteristics, yield, and selenium accumulation of common buckwheat (Fagopyrum esculentum M.). Acta Agron. Sin. https://kns.cnki.net/kcms/detail/11.1809.S.20221019.1039.002.html. (2022)
Zhao, J. L. et al. FtNAC31, a Tartary buckwheat NAC transcription factor, enhances salt and drought tolerance in transgenic Arabidopsis. Plant Physiol. Bioch. 191, 20–33 (2022).
Zhang, Y., He, P., Huang, X. & Huang, K. Removal of apical dominance in common buckwheat improves grain fill and yield. Agron. J. 115(3), 1308–1319. https://doi.org/10.1002/agj2.21287 (2023).
Tang, S. H. & Luo, C. Experimental Course of Plant Physiology (Southwest Normal University Press, 2012).
He, Z. P. Experimental Guidance on Chemical Control of Crops (Beijing Agricultural University Press, 1993).
Guo, Q. Q. Research on the relationship between protein accumulation and carbon metabolism in soybean grain. Master’s thesis, Northeast Agricultural University (2010).
Yu, X. X. Mechanism of MeJA enhancing salt tolerance of Glycyrrhizauralensis in seed germination and seedling growth stages. Master’s thesis, Ningxia Medical University Yinchuan (2019).
Loulakakis, K. A. & Roubelakis-Angelakis, K. A. Plant NAD (H)-Glutamate dehydrogenase consists of two subunit polypeptides and their participation in the seven isoenzymes occurs in an ordered ratio. Plant Physiol. 97, 104–111 (1991).
Chen, P. F. et al. Sucrose is involved in the regulation of iron deficiency responses in rice (Oryza sativa L.). Plant Cell Rep. 37, 789–798 (2018).
Fougère, F., Rudulier, D. L. & Streeter, J. G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 964, 1228–1236 (1991).
Kaplan, F. et al. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 1364, 4159–4168 (2004).
Rosa, M. et al. Soluble sugars-metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 45, 388 (2009).
Whittaker, A., Martinelli, T., Farrant, J. M., Bochicchio, A. & Vazzana, C. Sucrose phosphate synthase activity and the co-ordination of carbon partitioning during sucrose and amino acid accumulation in desiccation-tolerant leaf material of the C4 resurrection plant Sporobolusstapfianus during dehydration. J. Exp. Bot. 58, 3775–3787 (2007).
Wang, J. F. et al. Effects of nitrogen fertilizer and plant density on carbon metabolism, nitrogen metabolism and grain yield of two winter wheat varieties. Sci. Agric. Sin. 54, 4070–4083 (2021).
Wu, Y. W., Zhao, B., Du, L. J., Zhou, F. & Liu, B. X. Effects of low nitrogen stress on carbon-nitrogen metabolites and related enzyme activities in leaves of maize varieties with contrasting nitrogen efficiency at seedling stage. J. Maize Sci. 27, 74–83 (2019).
Wang, L. et al. Effects of exogenous glucose and sucrose on photosynthesis in triticale seedlings under salt stress. Photosynthetica 571, 286–294 (2019).
Gao, X. Y. Effect of saline-alkali stress on growth and physiological ecology of rice in different concentrations. Master’s thesis, Jilin Agricultural University (2014).
Arghavani, M., Zaeimzadeh, A., Savadkoohi, S. & Samiei, L. Tolerance of Kentucky bluegrass as affected by nitrogen fertilization. J. Agr. Sci. Tech. Iran. 19, 173–183 (2017).
Hossain, M. A., Piyatida, P., Teixeira, S., Jaime, A. & Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 872875 (2012).
Williams, A. P. et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 3, 292–297 (2013).
Katiyar, S. & Dubey, R. S. Influence of NaCl salinity on behaviours of nitrate reductase and nitrite reductase in rice seedlings differing in salt tolerance. Agron. Crop. Sci. 169, 289–297 (1992).
Lopez-Delacalle, M., Camejo, D. M., Garcia-Marti, M., Nortes, P. A. & Rivero, R. M. Using tomato recombinant lines to improve plant tolerance to stress combination through a more efficient nitrogen metabolism. Front. Plant Sci. 10, 1702 (2020).
Liu, Y. et al. Effects of saline-alkali stress on nitrogen metabolism activity and root yield and sugar content of suger beet. J. Nuclear Agric. Sci. 29, 397–404 (2015).
Cai, X. J., Guan, S. Y. & Guo, X. M. Effects of NaCl stress on antioxidant enzymes activities and soluble protein content in 3 kinds of sweet potatoes (Dioscorea esculenta). J. Anhui Agric. Sci. 40, 9228–9229 (2012).
Meng, X. H., Liu, Y. G., Zhang, Y. M., Lin, Q. & Zhang, Y. S. Effects of salt stress on photosynthetic characteristics and yield of different salt-tolerant wheat varieties. Chin. Agric. Sci. Bull. 31, 46–52 (2015).
Chen, X. J. et al. Effects of salt stress on root respiratory metabolism, antioxidant enzyme activities, and yield of oats. Chin. J. Ecol. 40, 2773–2782 (2021).
Zhang, R. Z. et al. Effect of saline-alkali stress on source-sink and yield of rice. Chin. J. Rice Sci. 20, 116–118 (2006).
Chu, G., Chen, T. T., Wang, Z. Q., Yang, J. C. & Zhang, J. H. Morphological and physiological traits of roots and their relationships with water productivity in water-saving and drought-resistant rice. Field Crops Res. 162, 108–119 (2014).
Hiroaki, S. et al. Root-shoot interaction as a limiting factor of biomass productivity in new tropical rice lines. Soil Sci. Plant Nutr. 50, 545–554 (2004).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32160510), Program of High-level Innovation Talents, Guizhou Province, China (QianKeHePingTaiRenCai-GCC [2022]024-1), the Science and Technology Support Plan of Guizhou Province, China (QianKeHeZhiCheng [2021] yiban 271), the Program of Scientific and Technology Innovation Team of Guizhou Education Department of China (Qianjiaoji [2022]011), and Guizhou Normal University Academic New Seedling Fund Project (Qianshixinmiao[2022]16).
Author information
Authors and Affiliations
Contributions
Conceptualization and writing—original draft, X.Y.Z., P.Y.H.,X.Y.H., and K.F.H.; funding acquisition, K.F.H. and X.Y.H; investigation and methodology, X.Y.Z., R.Y.G; writing—review and editing, X.Y.Z., P.Y.H., and K.F.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., He, P., Guo, R. et al. Effects of salt stress on root morphology, carbon and nitrogen metabolism, and yield of Tartary buckwheat. Sci Rep 13, 12483 (2023). https://doi.org/10.1038/s41598-023-39634-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-39634-0
This article is cited by
-
Brassinosteroids promote adventitious rooting in marigold via modulating rooting-related enzyme activities, plant hormone and nutrient contents
Horticulture, Environment, and Biotechnology (2025)
-
Effects of Schoenoplectus americanus (Pers.) Volkart Ex Schinz & R.Keller and Phragmites australis (Cav.) on the Water Quality Improvement of Moderately Saline Wastewater
Wetlands (2025)
-
Effects of Abscisic Acid and Strigolactone on the Yield and Economic Benefit of Tartary Buckwheat
Journal of Soil Science and Plant Nutrition (2025)
-
Strigolactones affect the yield of Tartary buckwheat by regulating endogenous hormone levels
BMC Plant Biology (2024)
-
The effect of exogenous gibberellin and its synthesis inhibitor treatments for morphological and physiological characteristics of Tartary buckwheat
Scientific Reports (2024)